Abstract
OBJECTIVE
To determine if vitamin D status is associated with recurrent preterm birth, and any interactions between Vitamin D levels and fish consumption.
DESIGN
A nested case-control study using data from a randomized trial of omega-3 fatty acid supplementation to prevent recurrent preterm birth.
SETTING
14 academic health centers in the U.S.
POPULATION
Women with prior spontaneous preterm birth.
METHODS
In 131 cases (preterm delivery less than 35 weeks) and 134 term controls, we measured serum 25-hydroxyvitamin D (25[OH]D) concentrations by liquid chromatography-tandem mass spectrometry (LC-MS) among samples collected at baseline (16–22 weeks gestation). Logistic regression models controlled for study center, maternal age, race/ethnicity, number of prior preterm deliveries, smoking status, body mass index, and treatment.
MAIN OUTCOME MEASURES
Recurrent preterm birth at <37, <32 weeks.
RESULTS
The median mid-gestation serum 25(OH)D concentration was 67 (nmol/L) and 27% had concentrations less than 50 nmol/L. Serum 25(OH)D concentration was not significantly associated with preterm birth (OR = 1.33, 95% confidence interval =0.48–3.70 for lowest vs. highest quartile). Likewise, comparing women with 25(OH)D concentrations of 50 nmol/L or higher with those with less than 50nmol/L generated an odds ratio of 0.80 (0.38, 1.69). Contrary to expected, a negative correlation was observed between fish consumption and serum 25(OH)D concentration (−0.18, P<0.01).
CONCLUSION
In a cohort of women with a prior preterm birth, vitamin D status at mid-pregnancy was not associated with recurrent preterm birth.
Keywords: preterm birth, vitamin D, perinatal nutrition
INTRODUCTION
Vitamin D has multiple functions that are critical in growth and development (1). The best marker of vitamin D status is the circulating concentration of its metabolite 25-hydroxyvitamin D (25[OH]D). When serum 25(OH)D concentrations have been measured in cohorts of pregnant women in the United States, many women from various ethnic groups living at different latitudes are found to have a low vitamin D status, regardless of the exact definition used (2). Low maternal concentrations of 25(OH)D have been associated with severe preeclampsia and low birth weight in some studies, but not others (3–9).
In a randomized trial of omega-3 fatty acid supplementation in pregnant women with a history of preterm birth, our group observed an overall recurrent preterm birth rate of 40% and found that although omega-3 fatty acid supplementation did not reduce the risk of recurrent preterm birth (relative risk 0.91, 95% confidence interval 0.77–1.07)(10), self-reported fish consumption was protective (11). This probably represents unmeasured confounding, as fish is the major dietary source of omega-3 fatty acids. Because fish is a major dietary source of vitamin D, we conducted a secondary analysis in this cohort to examine whether vitamin D status was associated with recurrent preterm birth. We then explored whether vitamin D status was correlated with fish consumption and mediated the protective association between fish consumption and recurrent preterm birth.
MATERIAL AND METHODS
This is an observational study, secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network randomized clinical trial of omega-3 long chain polyunsaturated fatty acid (LCPUFA) supplementation to prevent recurrent preterm birth. Trial investigators recruited women with a history of at least one previous spontaneous singleton preterm birth at 13 Network Centers from January, 2005 to October, 2006 (10). A total of 434 women were randomized to receive daily supplementation of 1200 mg eicosapentaenoic acid (EPA, 20:5n-3) and 800 mg of docosahexaenoic acid (DHA, 22:6n-3); while 418 were assigned to matching placebos, beginning at 16 to 21 6/7 weeks’ gestation and continuing until 36 6/7 weeks’ gestation or delivery, whichever occurred first. As part of the trial, all enrolled women also received weekly injections of 17 alpha-hydroxyprogesterone caproate. Women currently taking fish oil or omega-3 PUFA supplements were ineligible for the trial; detailed inclusion and exclusion criteria are reported elsewhere (10). The study (NCT00135902 at www.clinicaltrials.gov) was approved by the IRBs of the biostatistical coordinating center and all participating clinical centers and this secondary analysis was determined to be exempt from IRB review of the Office of Human Subjects by the University of North Carolina, Chapel Hill NC IRB office. All enrolled women gave written informed consent. The CONSORT flowsheet and checklist for the omega-3 trial can be found in the article in which we published the primary trial results(10).
The current analysis is a nested case-control study in which patients that delivered at or beyond 37 weeks’ gestation were selected as controls and matched on race/ethnicity and study site in an approximate 1:1 ratio to cases, defined as delivery before 35 weeks’ gestation. The cut point of 35 weeks was chosen as an inclusion criterion to enrich the total number of preterm births at less than 37 and 32 weeks in the clinical trial. The reader should note as they read our results that the gestational age limit defining the inclusion criteria differed from the outcome. This analysis is restricted to patients that consented to the use of their blood for future research on prematurity and other pregnancy complications. Outcomes assessed included all preterm births (<37 weeks) and very early preterm birth (<32 weeks) in the subsequent pregnancy.
To characterize the women’s vitamin D status at trial enrollment, we measured 25(OH)D concentrations using liquid chromatography-tandem mass spectrometry (LC-MS) from serum collected at the baseline randomization visit (16–22 weeks’ gestation) in 131 cases and 134 controls. Serum 25(OH)D was also measured in follow-up samples collected at 25–28 weeks’ gestation in a subset of 80 cases and 88 controls. The method used is an isotope dilution, LC-MS assay optimized in MGH laboratory based on published procedures (12). The limit of detection is 5 nmol/L for D2 and 7.5 nmol/L for D3. The between-run CV for a quality control serum containing a total vitamin D concentration of 57 nmol/L is 7.5%. 25(OH)D measurements are robust even when frozen and not altered by exposure to light. For the purpose of this analysis, 25(OH)D concentrations were examined as quartiles (based on the distribution of controls) to assess dose response and dichotomized at 50 nmol/L, below which is considered “inadequate” by the Institute of Medicine (13).
The exact date of the solstice or equinox in each given year was used to define the season (winter, spring, summer, fall) when the serum was collected for 25(OH)D. Fish consumption was categorized as none, 1–2 times per week or 3 or more times per week and was based on self-reported baseline intake during the current pregnancy of dark-meat fish, canned tuna, other fish, and shellfish.
To examine the association between baseline 25(OH)D concentration and recurrent preterm birth (yes/no), we used conditional logistic regression models to control for race/ethnicity, study center, maternal age, number of prior preterm deliveries, smoking status, BMI, season when measurement was made, and treatment group, which were chosen a priori based on clinical relevance. A locally weighted scatterplot smoothing technique (loess) was used to assess the full range of 25(OH)D concentration against the logit of preterm birth. The point biserial correlation was used to assess the relationship between serum 25(OH)D concentration and the number of fish servings per week. Two-sided P values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics are presented in Table 1. The median 25(OH)D concentration was 67 nmol/L at 16–22 weeks’ gestation and 76 nmol/L at 25–28 weeks’ gestation. Only 22% of participants had 25(OH)D concentrations less than 50 nmol/L at mid-gestation. 160 subjects had vitamin D levels measured at both time points. Using a paired t-test to compare means at the different times, vitamin D levels were higher at the second visit (mean difference 5.7 nmol/L, p<0.0001). We also compared medians at the different time points for the whole sample (acknowledging that some women delivered prior to the second visit and thus could bias results), the difference in medians was not significant (p=0.053). Table 2 shows the baseline characteristics by mid-gestation serum 25(OH)D nmol/L dichotomized at 50 nmol/L. The correlation between serum 25(OH)D concentration and fish consumption was −0.18 (P<0.01); therefore a formal mediation analysis was not conducted.
Table 1.
Baseline characteristics
| Cases (< 35 weeks) | Controls (≥ 37 weeks) | P-Value | |
|---|---|---|---|
| n=131 | n=134 | ||
| Age (years) | 26.8 ± 5.5 | 27.3 ± 5.6 | 0.54 |
| Race/ethnicity | 0.73 | ||
| Black | 53 (40%) | 50 (37%) | |
| Hispanic | 16 (12%) | 14 (10%) | |
| White | 62 (47%) | 70 (52%) | |
| Number of prior preterm deliveries | <0.0001 | ||
| 1 | 79 (60%) | 108 (81%) | |
| 2 | 39 (30%) | 24 (18%) | |
| 3 or more | 13 (10%) | 2 (1%) | |
| Smoking during pregnancy | 32 (24%) | 13 (10%) | 0.002 |
| Pre-pregnancy BMI (kg/m2) | 26.8 ± 7.2 | 26.4 ± 6.1 | 0.91 |
| Study center region | 1.0 | ||
| Northern US* | 96 (73%) | 99 (74%) | |
| Southern US† | 35 (27%) | 35 (26%) | |
| Season of blood draw for 25(OH)D | 0.34 | ||
| Winter | 15 (11%) | 26 (19%) | |
| Spring | 50 (38%) | 45 (34%) | |
| Summer | 41 (31%) | 41 (31%) | |
| Fall | 25 (19%) | 22 (16%) | |
| Number of times fish eaten/week | 0.008 | ||
| None | 53 (40%) | 33 (25%) | |
| 1–2 | 58 (44%) | 84 (63%) | |
| 3+ | 20 (15%) | 17 (13%) | |
| 25(OH)D concentration (nmol/L) | 70.7 ± 30.7 | 72.7 ± 32.6 | 0.61 |
| Assigned to Omega-3 group | 63 (48%) | 72 (54%) | 0.40 |
Continuous variables are presented as mean ± standard deviation, and the P-values reported from the Wilcoxon test. Categorical variables are presented as frequencies, and the P-values reported from Fisher’s Exact test.
Table 2.
Baseline characteristics of women by mid-gestation serum 25(OH)D nmol/L
| 25(OH)D < 50 nmol/L | 25(OH)D ≥ 50 nmol/L | P-Value | |
|---|---|---|---|
| n=71 | n=194 | ||
| Age (years) | 24.7 ± 5.0 | 27.9 ± 5.5 | <0.0001 |
| Race/ethnicity | |||
| Black | 52 (73%) | 51 (26%) | <0.0001 |
| Hispanic | 14 (20%) | 16 (8%) | |
| White | 5 (7%) | 127 (65%) | |
| Number of prior preterm deliveries | |||
| 1 | 54 (76%) | 133 (69%) | 0.26 |
| 2 | 12 (17%) | 51 (26%) | |
| 3 or more | 5 (7%) | 10 (5%) | |
| Smoking during pregnancy | 17 (24%) | 28 (14%) | 0.095 |
| Pre-pregnancy BMI (kg/m2) | 29.6 ± 8.3 | 25.5 ± 5.6 | 0.0002 |
| Study center region | <0.0001 | ||
| Northern US* | 38 (54%) | 157 (81%) | |
| Southern US† | 33 (46%) | 37 (19%) | |
| Season of blood draw for 25(OH)D | 0.0316 | ||
| Winter | 17 (24%) | 24 (12%) | |
| Spring | 28 (39%) | 67 (35%) | |
| Summer | 14 (20%) | 68 (35%) | |
| Fall | 12 (17%) | 35 (18%) | |
| Number of times fish eaten/week | 0.0090 | ||
| None | 13 (18%) | 73 (38%) | |
| 1–2 | 45 (63%) | 97 (50%) | |
| 3+ | 13 (18%) | 24 (12%) | |
| 25(OH)D concentration (nmol/L) | 34.1 ± 10.3 | 85.5 ± 24.9 | <0.0001 |
| Assigned to Omega-3 group | 34 (48%) | 101 (52%) | 0.58 |
Northern sites include: Utah, Illinois, Michigan, Ohio, Pennsylvania, New York, and Rhode Island
Southern sites include: North Carolina, Alabama, and Texas
Low vitamin D status at 16–22 weeks’ gestation was not associated with recurrent preterm birth (Table 3 and Figure). In the univariate relationship with preterm birth (Table 1), number of prior pre-term deliveries, smoking status, and fish intake were found to be significantly associated. These remained significant in the fully adjusted model. Similar null findings were observed for Vitamin D status at 25–28 weeks’ gestation (data not shown). Analyses were repeated for the 70 preterm births <32 weeks and again there was no association between low vitamin D status and very early preterm birth.
Table 3.
Association between mid-gestation serum 25(OH)D concentrations and recurrent preterm birth
| Quartiles of 25(OH)D measured at 16–22 weeks’ gestation | 25(OH)D concentration at 16–22 weeks’ gestation | |||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | < 50 nmol/L | ≥ 50 nmol/L | |
| n | 63 | 72 | 62 | 68 | 71 | 194 |
| Median 25(OH)D concentration (nmol/L) | 35 | 57 | 82 | 110 | 35 | 82 |
| Range of 25(OH)D concentrations (nmol/L) | (10, 45) | (47, 67) | (70, 95) | (97, 167) | (10, 47) | (50, 167) |
| Preterm birth, n (%) | 33 (52%) | 37 (51%) | 31 (50%) | 30 (44%) | 35 (49%) | 96 (49%) |
| Model 1* odds ratio (95% CL) | 1.43 (0.60, 3.41) | 1.38 (0.66, 2.91) | 1.27 (0.62, 2.60) | 1.00 (referent) | 0.87 (0.46, 1.66) | 1.00 (referent) |
| Model 2† odds ratio (95% CL) | 1.28 (0.47, 3.49) | 1.23 (0.53, 2.87) | 1.30 (0.60, 2.84) | 1.00 (referent) | 0.82 (0.40, 1.70) | 1.00 (referent) |
| Model 3†† odds ratio (95% CL) | 1.33 (0.48, 3.70) | 1.25 (0.53, 2.97) | 1.31 (0.60, 2.87) | 1.00 (referent) | 0.80 (0.38, 1.69) | 1.00 (referent) |
From conditional logistic regression, controlling for the matching variables only (race/ethnicity, study center)
From conditional logistic regression, controlling for the matching variables (race/ethnicity, study center) and maternal age, number of prior preterm deliveries, smoking status, BMI, season when blood drawn, and treatment group
From conditional logistic regression, controlling for all variables in Model 2 plus fish intake
Figure 1.
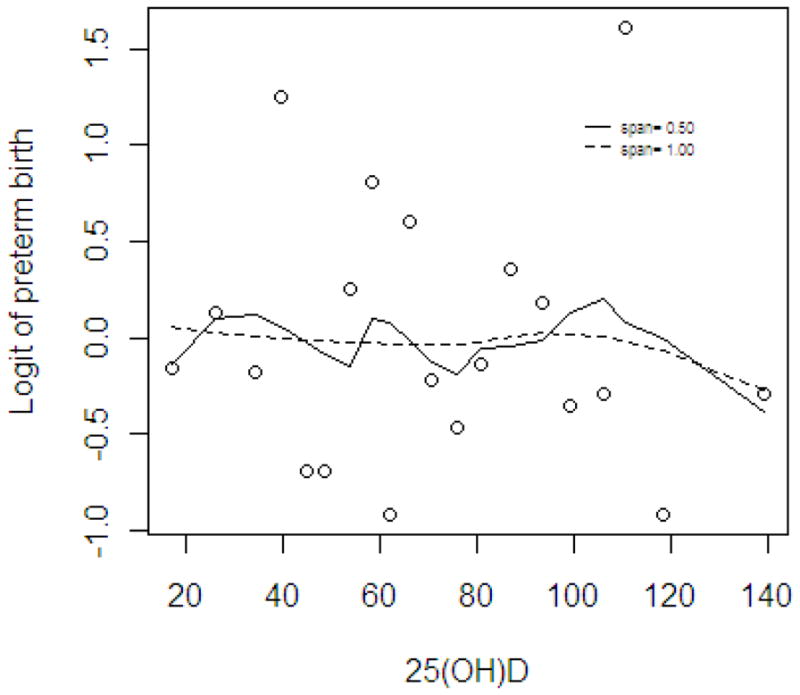
Relationship between mid-gestation serum 25(OH)D concentration and repeat preterm birth, using loess smoother.
Circles represent each median of 20 groups ranked by 25(OH).
Our study participants had a marked racial disparity in vitamin D deficiency (<50nmol/L) at 16–22 weeks. Fifty-two percent of self-reported African Americans were vitamin D deficient versus only 27% of the total cohort. We did a subgroup analysis looking only at recurrent preterm birth in African American women and found no association with vitamin D deficiency (Table 4).
Table 4.
Association between mid-gestation serum 25(OH)D concentrations and recurrent preterm birth in the African American subgroup only.
| Quartiles of 25(OH)D measured at 16–22 weeks’ | 25(OH)D concentration at gestation 16–22 weeks’ gestation | |||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | < 50 nmol/L | ≥ 50 nmol/L | |
| n | 21 | 31 | 15 | 36 | 52 | 51 |
| Median 25(OH)D concentration (nmol/L) | 22 | 40 | 55 | 75 | 35 | 67 |
| Range of 25(OH)D concentrations (nmol/L) | (10, 30) | (32, 47) | (50, 65) | (62, 112) | (10, 47) | (50, 112) |
| Preterm birth, n (%) | 9 (43%) | 18 (58%) | 7 (47%) | 19 (53%) | 27 (52%) | 26 (51%) |
| Model 1* odds ratio (95% CL) | 0.64 (0.21, 2.02) | 1.17 (0.44, 3.12) | 0.70 (0.20, 2.45) | 1.00 (referent) | 1.06 (0.49, 2.30) | 1.00 (referent) |
| Model 2† odds ratio (95% CL) | 0.54 (0.14, 2.00) | 1.01 (0.32, 3.14) | 0.46 (0.11, .97) | 1.00 (referent) | 1.07 (0.44, 2.61) | 1.00 (referent) |
| Model 3†† odds ratio (95% CL) | .54 (0.14, 2.04) | 0.98 (0.31, 3.07) | 0.42 (0.09, 1.89) | 1.00 (referent) | 1.09 (0.45, 2.67) | 1.00 (referent) |
From conditional logistic regression, controlling for the matching variables only (study center)
From conditional logistic regression, controlling for the matching variables (study center) and maternal age, number of prior preterm deliveries, smoking status, BMI, season when blood drawn, and treatment group
From conditional logistic regression, controlling for all variables in Model 2 plus fish intake
DISCUSSION
Given the mixture of positive and negative associations previously reported between vitamin D status and poor pregnancy outcomes (e.g., severe pre-eclampsia)(1, 3–9), we wanted to explore the hypothesis that low vitamin D status would be associated with recurrent preterm birth. We found no evidence of such a relationship. While our null findings may reflect the underlying biology in this high-risk population, one should keep in mind that the majority of our mothers did not have serum 25(OH)D concentrations less than 50 nmol/L and prior spontaneous preterm birth was an entry criterion for the omega-3 supplementation trial. That entry criterion would select against women with histories of preeclampsia (another pathway to early delivery), the perinatal condition to which low vitamin D status is most often is linked (5).
Our results differ from prior observational studies of vitamin D status in pregnancy, which have reported that low vitamin D status was common (1); we found that participants in this clinical trial to prevent recurrent preterm birth had, for the most part, concentrations of 50 nmol/L or higher. Because the human fetus is entirely dependent on the maternal pool of vitamin D, these previous reports of hypovitaminosis D have caused public health officials and nutritionists to question whether recommendations about vitamin D intake in pregnancy should be increased (1). Our results from a diverse population of women cared for in US academic health centers should reduce concerns about widespread low vitamin D status in reproductive age women. Indeed, our median 25(OH) D concentration was quite similar to the mean value of women in a nationwide sample of pregnant women (2): 67nmol/L and 65nmol/L, respectively. The health benefits and risks of raising 25(OH)D concentrations in pregnant women to higher concentrations (e.g., >100 nmol/L) are not clear and merit further investigation (14).
In interpreting our previous finding that fish consumption was protective against recurrent preterm birth (12), we hypothesized that the protective effects may have been mediated via differences in vitamin D status, as fatty fish and fish liver oils are the main natural dietary sources for vitamin D. Contrary to expected, we observed a negative correlation between fish consumption and serum 25(OH)D concentration (i.e., higher intake of fish was associated with lower 25(OH)D concentrations). This would point to other sources of vitamin D in this group, ultraviolet irradiation of the skin, fortified foods (such as milk, cereal, or orange juice), or vitamin D supplements. We did not ascertain the usage of prenatal vitamins, and vitamin D amounts in prenatal vitamins vary from 200–800 IU.
Potential limitations of our report include the following. First, we only measured 25(OH)D at two time points in mid-pregnancy, with the second in a significantly smaller number of participants. Multiple measures across pregnancy may have demonstrated different outcomes. Also, and as stated earlier, we studied a group of mothers in which fewer than anticipated had serum 25(OH)D concentrations less than 50 nmol/L and thus, our results may not be generalizable to women who enter pregnancy with a low vitamin D status. Strengths include prospective design, subjects from multiple centers across the U.S., and high prevalence of recurrent PTB.
In conclusion, mid-gestation serum 25(OH)D concentrations of less than 50 nmol/L occurred less often than we anticipated and was not associated with recurrent preterm birth. Several clinical trials have failed to show any effect of vitamin D supplementation on the risk of preterm birth. These trials have been small and lacked adequate power to conclusively answer the important question (12–15). Kovacs et al. have published a comprehensive review article that thoroughly addresses the biology, toxicology, and epidemiology of this important nutrient in pregnancy and lactation (16). While the health benefits of vitamin D supplementation remain unclear (3–9, 15), these data do not support its routine use for the prevention of recurrent preterm birth.
Acknowledgments
The author thanks the following MFMU Network members who participated in protocol development and coordination between clinical research centers (Karen Dorman, RN, MS), protocol/data management and statistical analysis (Madeline Rice, PhD and Elizabeth Thom, PhD), and protocol development and oversight (Catherine Y. Spong, MD).
FUNDING:
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD27860, HD27917, HD40560, HD34208, HD40485, HD21410, HD27915, HD40500, HD40512, HD40544, MO1-RR-000080, HD34136, HD27869, HD40545, HD36801, HD19897] and does not necessarily represent the official views of the NICHD or NIH.
Dr Camargo was supported, in part, by the Massachusetts General Hospital Center for D-receptor Activation Research (Boston, MA).
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network are as follows:
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Dorman, E. Prata, K. Hamden
Wake Forest University Health Sciences, Winston-Salem, NC – P. Meis, M. Swain, B. Scott, C. Leftwich
Wayne State University, Detroit, MI – G. Norman, D. Driscoll, C. Sudz, L. Wynn, S. Blackwell
University of Utah Health Sciences Center, Salt Lake City, UT – K. Anderson (University of Utah Health Sciences Center), S. Bonnemort (McKay-Dee Hospital), D. Lund (University of Utah Health Sciences Center), J. Russell (LDS Hospital), J. Parsons (Utah Valley Regional Medical Center)
Columbia University, New York, NY – S. Bousleiman, S. South, V. Carmona, H. Husami, C. Lankford, C. Perez
University of Pittsburgh, Pittsburgh, PA – M. Luce, M. Cotroneo
The Ohio State University, Columbus, OH – F. Johnson, M. Landon, D. Cline, H. Walker
Women and Infants Hospital, Brown University, Providence, RI – D. Allard, J. Tillinghast
Northwestern University, Chicago, IL – M. Dinsmoor (NorthShore University HealthSystem), P.J. Simon, M. Huntley, C. Whitaker-Carr, M. Ramos-Brinson, G. Mallett
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH – C. Milluzzi, J. Hunter, W. Dalton, H. Ehrenberg, B. Stetzer
Drexel University College of Medicine – M. Hoffman, M. Talucci, C. Tocci, S. Wilson, M. Lake
University of Alabama at Birmingham, Birmingham, AL – W.W. Andrews, A. Northen, M. Parks, P. Blake Files
The University of Texas Health Science Center at Houston, Houston, TX – L.C. Gilstrap, B. Glenn-Cole, K. Cannon
The George Washington University Biostatistics Center – E. Thom, M. Rice, J. Zachary, R. Palugod, L. Leuchtenburg
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa
Footnotes
DISCLOSURES:
None.
CONTRIBUTION TO AUTHORSHIP:
All authors listed were significant contributors to the design and implementation of the study. The data was analyzed by KM, the lab analysis was done by CAC. All authors reviewed the manuscript and contributed to the final submission.
DETAILS OF ETHIC APPROVAL:
The study (NCT00135902 at www.clinicaltrials.gov) was approved by the IRBs of the biostatistical coordinating center and all participating clinical centers and this secondary analysis was determined to be exempt from IRB review of the Office of Human Subjects by the University of North Carolina, Chapel Hill NC IRB office. All enrolled women gave written informed consent.
References
- 1.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutrition Reviews. 2010;68:465–77. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 2.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. American Journal of Obstetrics and Gynecology. 2010 May;202(5):436.e1–8. doi: 10.1016/j.ajog.2009.11.036. Epub 2010 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. British Medical Journal. 1980;280:751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 5.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–5109. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata M, Suzuki A, Sekiya T, Sekiguchi S, Asano S, et al. High prevalence of hypovataminosis D in pregnant Japanese women with threatened premature delivery. J Bone Miner Metab. 2011;29(5):615–20. doi: 10.1007/s00774-011-0264-x. [DOI] [PubMed] [Google Scholar]
- 7.Baker Arthur M, Haeri Sina, Camargo Carlos A, Stuebe Alison M, Boggess Kim A. A Nested Case-Control Study of First-Trimester Maternal Vitamin D Status and Risk for Spontaneous Preterm Birth. Am J Perinatol. 2011;28:667–72. doi: 10.1055/s-0031-1276731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, Hertzmark E, Msamanga GI, Fawzi WW. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009 Oct 1;200(7):1022–30. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010 Dec;117(13):1593–8. doi: 10.1111/j.1471–0528.2010.02742.x. Epub 2010 Oct 13. [DOI] [PubMed] [Google Scholar]
- 10.Harper M, Thom E, Klebanoff MA, Thorp J, Jr, Sorokin Y, Varner MW, et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth; a randomized controlled trial. Obstetrics and Gynecology. 2010;115:234–42. doi: 10.1097/AOG.0b013e3181cbd60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klebanoff MA, Harper M, Lai Y, Thorp J, Jr, Sorokin Y, Varner MW, Wapner RJ, et al. Fish consumption, erythrocyte fatty acids, and preterm birth. Obstetrics and Gynecology. 2011;117:1071–1077. doi: 10.1097/AOG.0b013e31821645dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh RJ, Taylor RL, Reddy GS, Grebe SKG. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complication accurate measurement and interpretation of vitamin D status. Journal of Clinical Endocrinology and Metabolism. 2006;91:3055–61. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 13.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–34. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine (IOM) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 15.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011 Oct;26(10):2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecol Obstet Invest. 1987;24(1):38–42. doi: 10.1159/000298772. [DOI] [PubMed] [Google Scholar]
- 17.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980 Mar 15;280(6216):751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, Adhikari RK, Sommer A, West KP., Jr Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003 Mar 15;326(7389):571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs CS. The Role of Vitamin D in Pregnancy and Lactation: Insights from Animal Models and Clinical Studies. Ann Rev Nutr. 2012;32:9.1–9.27. doi: 10.1146/annurev-nutr-071811-150742. [DOI] [PubMed] [Google Scholar]
- 20.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J New Zealand Asthma and Allergy Cohort Study Group. Cord blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]


