Abstract
Purpose
The robotic system offers potential technical advantages over laparoscopy for total mesorectal excision with radical lymphadenectomy for rectal cancer. However, the requirement for fixed docking limits its utility when the working volume is large or patient repositioning is required. The purpose of this study was to evaluate short-term outcomes associated with a novel setup to perform total mesorectal excision and radical lymphadenectomy for rectal cancer by the use of a “reverse” hybrid robotic-laparoscopic approach.
Methods
This is a prospective consecutive cohort observational study of patients who underwent robotic rectal cancer resection from January 2009 to March 2011. During the study period a technique of reverse-hybrid robotic-laparoscopic rectal resection with radical lymphadenectomy was developed. This technique involves reversal of the operative sequence with lymphovascular and rectal dissection to precede proximal colonic mobilization. This technique evolved from a conventional hybrid resection with laparoscopic vascular control, colonic mobilization, and robotic pelvic dissection. Perioperative, and short-term oncologic outcomes were analyzed.
Results
Thirty patients underwent reverse-hybrid resection. Median tumor location was 5 cm (interquartile range 3-9) from the anal verge. Median BMI was 27.6 (interquartile range 25.0-32.1 kg/m2). Twenty (66.7%) received neoadjuvant chemoradiation. There were no conversions. Median blood loss was 100 mL (interquartile range 75-200). Total operation time was a median 369 (interquartile range 306-410) minutes. Median docking time was 6 (interquartile range 5-8) minutes and console time was 98 (interquartile range 88-140) minutes. Resection was R0 in all patients with no patients had an incomplete mesorectal resection. Six patients (20%) underwent extended lymph node dissection or en bloc resection.
Conclusions
Reverse-hybrid robotic surgery for rectal cancer maximizes the therapeutic applicability of the robotic and conventional laparoscopic techniques for optimized application in minimally invasive rectal surgery.
Keywords: Robotic surgery, Rectal cancer, Laparoscopy
INTRODUCTION
The robotic platform for minimally invasive total mesorectal excision (TME) offers a number of technical advantages including improved operative precision for vascular dissection, 3-dimensional optics, and fixed retraction for sharp mesorectal dissection. On the basis of these advances, several approaches to robotic TME surgery, broadly categorized as hybrid laparoscopic-robotic or totally robotic, have been proposed. With the conventional-hybrid technique, the proximal lymphovascular dissection and colonic mobilization are first performed laparoscopically followed by robotic rectal dissection. 1 This approach, however, does not take full advantage of the benefits of the robotic interface, in particular, during lymphovascular dissection. The totally robotic approach more fully uses the robotic interface, but can require multiple dockings of the surgical cart increasing logistic complexity and operative time 2, 3, 4. Furthermore because the robot does not permit repositioning after docking, splenic flexure colonic mobilization can be more difficult. Indeed, reported series of the totally robotic approach with a single docking have rarely included splenic flexure mobilization 2, 4.
To more optimally take advantage of the operating characteristics of the robot, we developed a reverse-hybrid robotic-laparoscopic technique. This approach more fully incorporates the technical advances of the robotic interface without the fixed or multiple docking limitations of the totally robotic approach.
METHODS
Patient Identification and Selection
Patients with histologically confirmed adenocarcinoma who underwent robotic rectal resection between January 2009 and March 2011 were included. Patients underwent robotic rectal surgery, and their outcomes, including evaluation of the quality of mesorectal excision,5 were assessed under an institutional review board approved prospective data-monitoring protocol.
Reverse-hybrid technique
All procedures were performed using the 4-arm da Vinci S surgical system (Intuitive Surgical, Sunnyvale, CA) and conventional laparoscopic instrumentation. Conventional-hybrid laparoscopic-robotic and totally robotic techniques have been previously described4, 6.
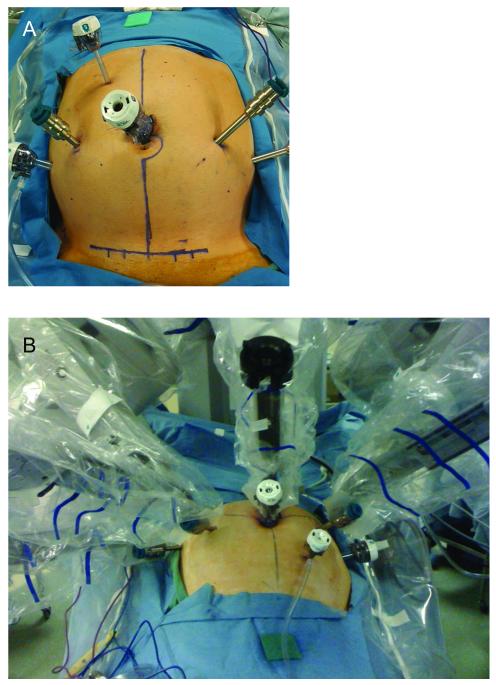
We developed a 6-port reverse-hybrid technique incorporating the robotic interface early during the lymphovascular and pelvic dissection steps (Fig. 1). After laparoscopic exploration, the patient is placed in 5° to 10° right-side down Trendelenburg position, and the robot is docked between the legs.
Figure 1.
Port placement for hybrid robotic laparoscopic surgery A. Port placement for reverse-hybrid rectal resection B. Fan orientation of robotic arms maximizes instrument range of motion
Robotic dissection
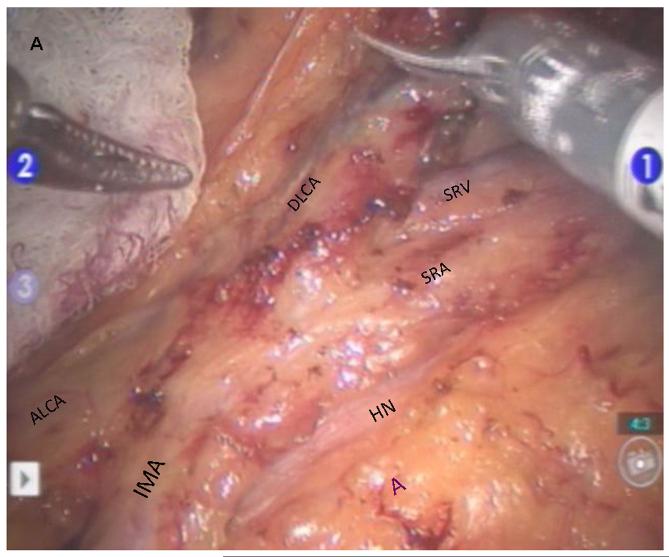
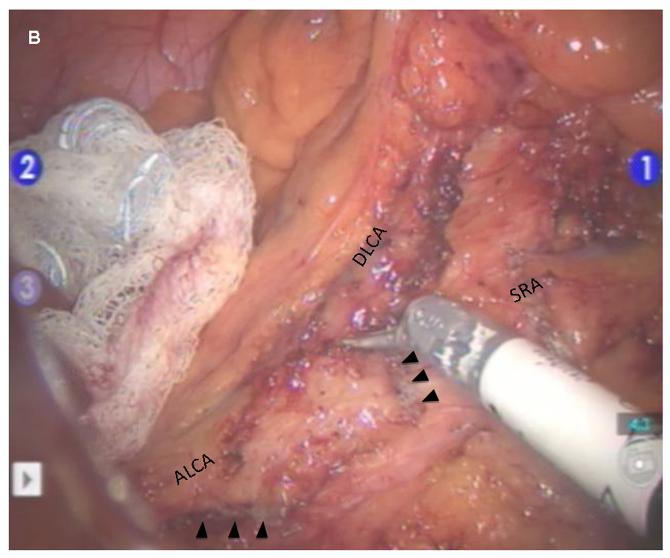
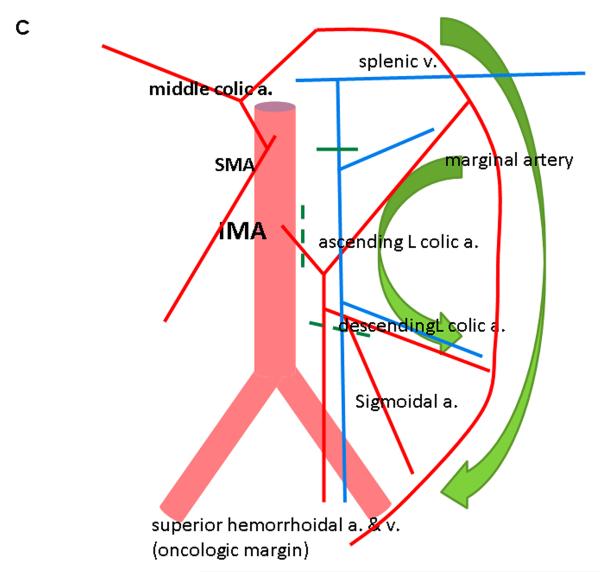
Robotic dissection is initiated at the superior rectal vessels overlying the sacral promontory and the mobilization is carried up along the aorta to isolate the inferior mesenteric artery (IMA) at its origin while visualizing the sympathetic nerve fibers. The IMA lymph node bundle is dissected and resected with the superior rectal vessels (Figure 2). The ascending left colic artery to the splenic flexure and the descending left colic artery to the descending-sigmoid colon junction are dissected. The IMA is divided at its origin, and the superior rectal artery is divided just distal to the descending left colic artery. This preserves an accessory collateral blood supply to the descending colon. The medial-to-lateral mobilization of the sigmoid mesocolon followed by lateral sigmoid mobilization is then performed.
Figure 2.
Robotic dissection of inferior mesenteric artery A. Clear identification of IMA and its branches to the left colon and rectum B. Complete lymphovascular dissection around IMA. Arrows indicate stapled-cut end of IMA. C. Diagram showing process of reverse hybrid robotic-laparoscopic rectal resection. IMA, inferior mesenteric artery; SRA, superior rectal artery; SRV, superior rectal vein; ALCA, ascending left colic artery; DLCA, descending left colic artery; HN, hypogastric nerve; A, aorta.
The circumferential rectal mobilization is then performed along the mesorectal plane with the use of a gauze tied around the proximal rectum to facilitate retraction. The dissection is performed with a nerve-sparing, sharp technique. Once the mesorectal dissection is complete, the robotic procedure is terminated.
Laparoscopic splenic flexure mobilization
After the robotic procedure, a laparoscopic medial to lateral dissection of the inferior mesenteric vein and descending mesocolon is performed, and the lesser sac is entered along the anterior aspect of the pancreas. The complete splenic flexure mobilization is performed with the same ports used during the robotic steps, but with the advantage of dynamic table positioning to facilitate exposure. In cases of abdominoperineal resection, flexure mobilization is omitted, but laparoscopic omental flap mobilization is performed.
Rectal transaction and anastomosis
Rectal transection is completed by the use of a transecting TA stapler through a Pfannenstiel incision after undocking, or transanally depending on tumor location. When indicated, frozen-section evaluation for distal margin status is performed. The anastomosis is then constructed followed by direct endoscopic evaluation of the anastomosis.
Results
Conventional-hybrid (n = 6), totally robotic (n = 4), or reverse-hybrid (n = 30) robotic resections were performed. The operative technique evolved from conventional-hybrid and totally robotic to the reverse-hybrid technique depending on the indication.
Operative parameters and short-term outcomes
Operations were sphincter-sparing in 80%, most commonly ultra-low anterior resection or coloanal reconstruction (63%). Six (20%) patients required a combined procedure, eg, patients with lateral pelvic or retroperitoneal lymph node resection (n = 5) or with adjacent organ resection (n = 1) patients. Median operative time was 369 minutes (interquartile range 306-410 min) and the console time contributed to a median 30.9% of the total operation time. There were no intraoperative complications or conversions. Operative parameters are summarized in Table 1. Postoperative complications included small-bowel obstruction (n = 1), anastomotic leak (n = 1, following 1 year systemic chemotherapy for metastases), and bleeding from the vessel-sealed IMA stump (n = 1, previous history of retroperitoneal radiotherapy) (Table 2).
Table 1.
Clinical, tumor characteristics and operative parameters of patients who underwent reverse hybrid surgery
| Clinical Variables | n | (%) or [IQR] |
|---|---|---|
| Sex | ||
| M:F | 16:14 | (53.3:46.7) |
| Age, yr* | 58 | [46-64] |
| Body mass index, kg/m2 * | 27.6 | [25.0-32.0] |
| > 30 | 13 | (43.3) |
| ASA | ||
| II | 12 | (40) |
| III | 18 | (60) |
| History of abdominal surgery | 9 | (30) |
| Neoadjuvant chemoradiotherapy | 20 | (66.7) |
| Tumor location | ||
| Upper rectum (> 11cm) | 8 | (26.7) |
| Mid rectum (6-10cm) | 7 | (23.3) |
| Lower rectum (≤ 5cm) | 15 | (50) |
| Operation | ||
| Low anterior resection | 5 | (16.7) |
| Ultralow anterior resection or coloanal anastomosis |
19 | (63.3) |
| Abdominoperineal resection | 6 | (20.0) |
| Total operative time, min * | 369 | [306-410] |
| Surgeon console time, min * | 98 | [88-140] |
| Docking time, min * | 6 | [5-8] |
| Estimated blood loss, mL * | 100 | [75-200] |
Median values with interquartile range [IQR]
Table 2.
Postoperative outcomes (%)
| Outcome Variables | n | (%) or [IQR] |
|---|---|---|
| Length of hospital stay, days a | 4 | [3-6] |
| Postoperative complications | ||
| Anastomotic leak | 1 | (4.2) |
| Pelvic abscess | 1 | (3.3) |
| Hemorrhage | 1 | (3.3) |
| Small bowel obstruction | 1 | (3.3) |
| Wound infection c | 4 | (13.3) |
| Urinary retention (transient) | 3 | (10.0) |
| Functional outcomes | ||
| Erectile dysfunction | 0 | (0) |
| Ejaculatory dysfunction | 1 | (6.3) c |
| Urinary dysfunction | 0 | (0) |
| Mortality | 0 |
Median values with interquartile range [IQR]
Includes abdominal (n = 1) and perineal wound (n = 3)
Among 16 male patients
All patients underwent total or tumor-specific mesorectal excision. Pathologic specimen grading was performed to assess the completeness of the mesorectal dissection. There were no cases of incomplete mesorectal excision or of positive circumferential resection margin (Table 3).
Table 3.
Pathologic results
| Operative Variables | n | (%) or [IQR] |
|---|---|---|
| [y] pTNM stage | ||
| 0 | 6 | (20) |
| I | 7 | (23.3) |
| II | 4 | (13.3) |
| III | 10 | (33.3) |
| IV | 3 | (10) |
| Number of examined lymph nodes a | 20 | [14-25] |
| Status of CRM+ | ||
| Negative | 30 | (100) |
| CRM distance mm b | 11 | [5-20] |
| TME Integrity | ||
| Complete | 25 | (83.3) |
| Nearly Complete c | 5 | (16.7) |
| Incomplete d | 0 | (0) |
Median values with interquartile range [IQR]
Quantitative distance available for 26 patients (not applicable for patients with pathologic complete response)
Presence of ,5-mm areas of disruption of the mesorectal envelope with no loss of fat.
A >5-mm defect with loss of fat or with coning.
Discussion
We have developed a reverse-hybrid robotic-laparoscopic approach to TME to optimally utilize the robotic interface for minimally invasive rectal cancer surgery. In comparison with the conventional-hybrid approach, the procedure is “reversed”; robotic lymphovascular and pelvic dissection is performed before laparoscopic descending colon and splenic flexure mobilization. The robot is therefore used where it provides the greatest advantage to standard laparoscopy— during radical lymphovascular and pelvic dissection where the working volume is small, the precision requirements are high, and there is no need for table repositioning or redocking. This approach may be suitable for a wide range of patients, including those with high BMI, distal cancers within a deep narrow pelvis, or lateral pelvic nodal metastases.
Several advantages of the robot make it an appealing tool for minimally invasive surgery 7, 8, 9. However, there are challenges associated with using the robot for rectal surgery that result from the large working volume, inability to reposition the table to facilitate colonic mobilization once the robotic cart has been docked and the lack of effective instrumentation for bowel handling.
These characteristics resulted in the initial appeal of conventional-hybrid techniques during which the robot is used only during pelvic dissection after laparoscopic lymphovascular dissection has been completed 1, 4, 6, 10. The conventional-hybrid technique, however, does not take full advantage of the robotic capabilities.
The more recent “totally” robotic approach uses the robotic interface for both the lymphovascular and pelvic dissection 2, 4, 11. A major purported advantage of this approach is the use of the robot to perform a safe and complete lymphadenectomy and vascular dissection. The robot is most commonly docked over the left hip, and most of the reported procedures have been performed without splenic flexure mobilization 2, 4. However, in our US population of patients with predominantly distal rectal cancers, complete splenic flexure mobilization is routinely necessary. In particular for taller or heavier patients, it is not possible to adequately mobilize the splenic flexure or complete the distal rectal dissection by the use of the over-the-leg docked robot without a second docking,3 and the limitations of totally robotic approach to splenic flexure mobilization with over-the-leg docking have thus been recognized 2, 11.
We have therefore combined the lessons learned from conventional-hybrid and totally robotic surgery to develop the reverse-hybrid technique. It combines the advantages of a totally robotic procedure without requiring multiple dockings or being subject to the working volume limitations of the robotic system, particularly during splenic flexure mobilization. Laparoscopic flexure mobilization permits dynamic use of gravity and operating table repositioning without the fixed-docking limitation. All robotic steps are possible with a single between-the-legs docking optimizing the robot’s mechanical operating characteristics. Then the inferior mesenteric vein division and splenic flexure mobilization can be efficiently performed with the use of conventional laparoscopy.
With a higher proportion of distal tumors, and higher median BMI (27.6) than most previous reports, the total operative time in this study was in line with other highly experienced centers 12,13. The median robotic-at-console time was 30.8% (IQR 24.3-34.8%) of total time, also in line with previous reports 2, 4, 6. The remaining time was for additional procedures such as fresh pathology evaluation with frozen section of margins, pedicled flap omentoplasty (abdominoperineal resection), endoscopic anastomotic interrogation, or diverting ileostomy; technical issues related to distal bowel transection, J-pouch creation, or handsewn anastomoses. Before incorporation of the robotic technique, patients who required extended resections or extramesorectal pelvic lymph node resection, or who had a high BMI, would not have been eligible for minimally invasive resection.
Notwithstanding the small sample size, the functional outcomes following robotic rectal surgery were acceptable despite the relatively advanced and distal disease within the cohort. The real impact of the minimally invasive approach to rectal cancer will be more clearly elucidated by the ongoing American College of Surgeons Oncology Group phase III randomized trial comparing minimally invasive (laparoscopic or robotic) TME with open TME to which eligible patients were also recruited.
Conclusions
The reverse-hybrid technique for robotic rectal resection optimally incorporates the advantages of the robotic interface for the performance of minimally invasive rectal cancer surgery, while avoiding the working volume, gravity, and target organ limitations, thus improving the applicability of minimally invasive surgery to patients with a wider range of tumor characteristics and body habitus.
Acknowledgments
Financial Disclosure: Supported in part by an American Society of Clinical Oncology Foundation Career Development Award and a National Cancer Institute K07-CA133187 research grant research grant (to GJC).
References
- 1.Hellan M, Anderson C, Ellenhorn JD, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol. 2007;14:3168–73. doi: 10.1245/s10434-007-9544-z. [DOI] [PubMed] [Google Scholar]
- 2.Choi DJ, Kim SH, Lee PJ, Kim J, Woo SU. Single-stage totally robotic dissection for rectal cancer surgery: technique and short-term outcome in 50 consecutive patients. Dis Colon Rectum. 2009;52:1824–30. doi: 10.1007/DCR.0b013e3181b13536. [DOI] [PubMed] [Google Scholar]
- 3.Spinoglio G, Summa M, Priora F, Quarati R, Testa S. Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum. 2008;51:1627–32. doi: 10.1007/s10350-008-9334-0. [DOI] [PubMed] [Google Scholar]
- 4.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 17:3195–202. doi: 10.1245/s10434-010-1162-5. [DOI] [PubMed] [Google Scholar]
- 5.Washinton MKBJ, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–51. doi: 10.5858/133.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480–7. doi: 10.1245/s10434-009-0435-3. [DOI] [PubMed] [Google Scholar]
- 7.Ou YC, Wang J, Cheng CL, Patel VR. Robotic-assisted laparoscopic radical prostatectomy: learning curve of first 100 cases. INt J Urol. 2010;17:635–40. doi: 10.1111/j.1442-2042.2010.02546.x. YC. [DOI] [PubMed] [Google Scholar]
- 8.SJ Robotically assisted laparoscopic prostatectomy: an assessment of its contemporary role in the surgical management of localized prostate cancer. Am J Surg. 2004;188:63S–7S. doi: 10.1016/j.amjsurg.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Marchal FRP, Vandromme J, et al. Telerobotic-assisted laparoscopic hysterectomy for benign and oncologic pathologies: initial clinical experience with 30 patients. Surg Endosc. 2005;19:826–31. doi: 10.1007/s00464-004-9122-4. [DOI] [PubMed] [Google Scholar]
- 10.D’Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, Guidolin D. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum. 2004;47:2162–8. doi: 10.1007/s10350-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 11.Luca F, Cenciarelli S, Valvo M, Pozzi S, Faso FL, Ravizza D, Zampino G, Sonzogni A, Biffi R. Full robotic left colon and rectal cancer resection: technique and early outcome. Ann Surg Oncol. 2009;16:1274–8. doi: 10.1245/s10434-009-0366-z. [DOI] [PubMed] [Google Scholar]
- 12.Leong QMSD, Cho JS, et al. Robot-assisted intersphincteric resection for low rectal cancer: technique and short-term outcome for 29 consecutive patients. Surg Endosc. 2011 doi: 10.1007/s00464-011-1657-6. [DOI] [PubMed] [Google Scholar]
- 13.Kang JHH, Min BS, Lee KY, Kim NK. Robotic Coloanal Anastomosis with or without Intersphincteric Resection for Low Rectal Cancer: Starting with the Perianal Approach Followed by Robotic Procedure. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1952-4. [DOI] [PubMed] [Google Scholar]