ABSTRACT
Cell-cell signaling in Xylella fastidiosa has been implicated in the coordination of traits enabling colonization in plant hosts as well as insect vectors. This cell density-dependent signaling has been attributed to a diffusible signaling factor (DSF) produced by the DSF synthase RpfF. DSF produced by related bacterial species are unsaturated fatty acids, but that of X. fastidiosa was thought to be different from those of other taxa. We describe here the isolation and characterization of an X. fastidiosa DSF (XfDSF) as 2(Z)-tetradecenoic acid. This compound was isolated both from recombinant Erwinia herbicola expressing X. fastidiosa rpfF and from an X. fastidiosa rpfC deletion mutant that overproduces DSF. Since an rpfF mutant is impaired in biofilm formation and underexpresses the hemagglutinin-like protein-encoding genes hxfA and hxfB, we demonstrate that these traits can be restored by ca. 0.5 µM XfDSF but not by myristic acid, the fully saturated tetradecenoic acid. A phoA-based X. fastidiosa biosensor that assesses DSF-dependent expression of hxfA or hxfB revealed a high level of molecular specificity of DSF signaling.
IMPORTANCE
X. fastidiosa causes diseases in many important plants, including grape, where it incites Pierce’s disease. Virulence of X. fastidiosa for grape is coordinated by cell-cell signaling molecules, designated DSF (Diffusible Signaling Factor). Mutants blocked in DSF production are hypervirulent for grape, suggesting that virulence is suppressed upon DSF accumulation and that disease could be controlled by artificial elevation of the DSF level in plants. In this work, we describe the isolation of the DSF produced by X. fastidiosa and the verification of its biological activity as an antivirulence factor. We also have developed X. fastidiosa DSF biosensors to evaluate the specificity of cell-cell signaling to be investigated.
Introduction
The xylem-limited plant pathogen Xylella fastidiosa causes serious diseases of several important agricultural crop plants, including Pierce’s disease (PD) of grapevine and variegated chlorosis in citrus (CVC). The pathogen is obligately transmitted from one plant to another by xylem sap-feeding insects (1, 2). Infected plants exhibit progressive leaf scorching or other foliar symptoms consistent with the water stress that is associated with the large numbers of xylem vessels that are occluded by bacterial cells. The virulence of X. fastidiosa is thus linked with its ability to migrate and proliferate within xylem vessels, and disease symptoms may largely be an inadvertent effect caused by successful colonization that interferes with xylem sap flow. X. fastidiosa, like the related pathogens Xanthomonas and Stenotrophomonas species, utilizes small molecules known as diffusible signaling factors (DSF) to regulate its behavior in a cell density-dependent manner (3–5). DSF is also implicated in interspecies signaling, where they exert antagonistic activity against competitors in a given niche (6) and regulate antimicrobial drug resistance in Pseudomonas aeruginosa (7).
In those taxa in which they have been characterized, DSF species are generally monounsaturated fatty acids of medium chain length containing a Z alkene α to the carboxylic acid. Pathogens synthesize and respond to DSF using genes in the rpf cluster (regulation of pathogenicity factor). In this cell-cell communication pathway, RpfF is the DSF synthase, annotated as a homologue of crotonase, the first enzyme in fatty acid degradation pathways (8). Upon reaching a threshold level outside the cell, DSF is sensed by the hybrid membrane sensor kinase RpfC that, in turn, phosphorylates the intracellular response regulator RpfG which then converts the intercellular signal into an intracellular signal through its cyclic di-GMP phosphodiesterase activity (9).
RpfF-dependent signaling involving DSF accumulation has been associated with regulation of motility, biofilm formation, and virulence in several Xanthomonas species and in X. fastidiosa (4, 9–14). While promoting the expression of genes encoding secreted hydrolytic enzymes, flagella, and chemotaxis in X. campestris pv. campestris (Xcc) and X. citri subsp. citri (Xac) (3, 10, 11), DSF accumulation leads to suppression of those genes in the vascular pathogen of rice X. oryzae pv. oryzae (Xoo). Instead, in this organism, accumulated DSF promotes the expression of genes contributing to biofilm formation (12). DSF-dependent gene expression patterns in X. fastidiosa share many similarities with that in X. oryzae pv. oryzae, perhaps because it also colonizes a vascular habitat. In X. fastidiosa, DSF suppresses genes involved in motility (e.g., pil genes encoding type IV pili) and in hydrolytic enzymes that disrupt pit membranes (e.g., pglA, which encodes a pectinase), enabling cell movement from one xylem vessel to another. Instead, DSF promotes the expression of genes involved in cell-cell aggregation and surface attachment (e.g., fimA encoding type I pili and hxfA and hxfB encoding hemagglutinin-like proteins) and those that drive biofilm formation (e.g., gum genes involved in exopolysaccharide synthesis) (13, 14). However, despite the similarities in DSF-dependent regulation of such genes, an rpfF mutant of X. oryzae pv. oryzae is attenuated in virulence to rice (15), while an rpfF mutant of X. fastidiosa is hypervirulent in grape (4). Since an rpfF mutant of X. fastidiosa was unable to colonize and to thus be transmitted by insect vectors, it has been hypothesized that DSF signaling is used as a context-dependent switch that enables a subset of X. fastidiosa cells to become adhesive and thus able to be acquired by insects, a phenotype incompatible with movement through the plant (16). The accumulation of DSF thus favors insect transmission but suppresses the virulence of X. fastidiosa. As such, modulation of the levels of X. fastidiosa DSF (here designated XfDSF) in plants could provide a control strategy for PD by altering the behavior of this pathogen.
Three different chemical species that function as DSFs in both X. campestris pv. campestris and X. oryzae pv. oryzae have been characterized: 2(Z)-11-methyldodecenoic acid (designated DSF) (17), 2(Z)-dodecenoic acid (BDSF), and (2Z, 5Z)-11-methyldodecadienoic acid (CDSF) (18). Eight different DSF-like molecules, including DSF and BDSF, were found in the human pathogen Stenotrophomonas maltophilia; of the other six, two were saturated fatty acids and the rest were 2(Z)-mono-unsaturated fatty acids (19). While the fatty acids found in these six chemical species differ in their carbon chain lengths (C12 to C14) and in the position of the branched methyl group, their exact structure and biological activity were not determined. The DSF produced by another human pathogen, Burkholderia cenocepacia, was also characterized as BDSF (6), and its production by RpfF involved a novel dual process consisting of the dehydration of a 3-hydroxydodecanoyl-ACP to 2(Z)-dodecenoyl-ACP and the cleavage of the thioester bond to yield a free acid (8). It has been reported that XfDSF isolated from the CVC 9a5c strain of X. fastidiosa is 12-methyltetradecanoic acid (20). However, while culture extracts of both a CVC and PD strain of X. fastidiosa were found to induce expression of DSF-responsive genes in X. campestris pv. campestris (4, 20, 21), 12-methyltetradecanoic acid was inactive when assayed with X. campestris pv. campestris-based DSF biosensors (data not shown). Since the putative XfDSF from the CVC strain of X. fastidiosa was characterized only by high-resolution gas chromatography-mass spectrometry (HR-GC-MC) and was not shown by this group to have bioactivity or to be RpfF dependent in its production, it may represent either a component of an orthogonal signaling system or a compound unlinked to DSF signaling. We thus explored the isolation and characterization of XfDSF from X. fastidiosa strain temecula1, the causative agent of PD, by performing bioassay-guided fractionation of culture supernatants using a previously reported X. campestris pv. campestris-based DSF biosensor strain (4). Further, we demonstrate that the XfDSF specie, 2(Z)-tetradecenoic acid (Fig. 1A), alters gene expression in newly developed Xylella DSF biosensor strains and affects biofilm phenotypes at concentrations as low as 500 nM.
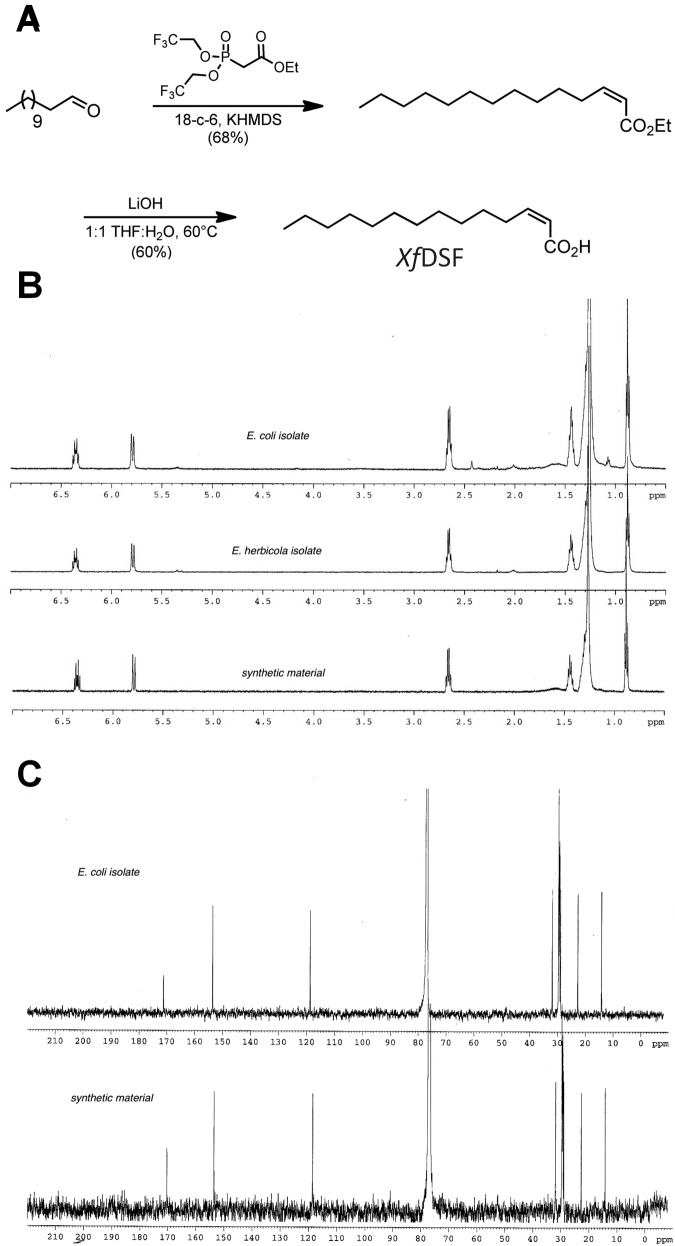
FIG 1.
Synthesis and structural confirmation of putative XfDSF. (A) Synthetic scheme for XfDSF featuring a Still-Gennari olefination followed by saponification to afford 2(Z)-tetradecenoic acid. (B and C) Comparative 1H (B) and 13C (C) NMR results determined for active isolates from recombinant expression compared to those determined for the synthetic acid.
RESULTS
XfDSF isolation and structural elucidation from recombinant E. herbicola and E. coli.
To obtain sufficient amounts of XfDSF for characterization, we introduced the X. fastidiosa rpfF gene from strain temecula1 under the control of the kan promoter into Escherichia coli and Erwinia herbicola on a high-copy-number plasmid. The recombinant bacteria released sufficient amounts of XfDSF into the culture medium to be readily detected by the X. campestris pv. campestris DSF (XccDSF) reporter. Since the cell yield of E. coli and E. herbicola was high, greater amounts of XfDSF were isolable from these strains than from X. fastidiosa cultures. These recombinant strains were thus used in initial isolation and characterization of XfDSF. After 36 h of growth, cells were pelleted from 3-liter cultures, and the supernatant was extracted with ethyl acetate (EtOAc). The crude isolate was purified by flash column chromatography (FCC), and fractions were purified using reverse-phase high-performance liquid chromatography (HPLC). Each fraction was assayed for DSF activity using the XccDSF reporter (4).
From both E. coli and E. herbicola cultures, we isolated and characterized a single active molecule. The 1H nuclear magnetic resonance (NMR) and 13C NMR spectra indicated that this compound was an enoic acid with 14 carbons (see Fig. S1A and S1B in the supplemental material). H-H correlation spectroscopy (COSY; see Fig. S1C in the supplemental material) of the active fraction showed that it contained a single compound where each peak in the spectrum correlated to at least one other signal. The proton spectrum of this acid (see Fig. S1A in the supplemental material) contains a triplet at 0.89 ppm that integrates into three protons consistent with a terminal methyl group. To determine whether the acid had any branch points on the chain, distortionless enhancement by polarization transfer (DEPT) NMR spectroscopy was used (see Fig. S2A in the supplemental material). This experiment revealed that the active compound had two CH signals corresponding to each olefinic carbon, 10 aliphatic CH2 carbons, and one CH3 signal correlating to the triplet at 0.89 ppm, consistent with a single terminal methyl group. There were no aliphatic quaternary carbons in the full 13C NMR spectrum. These results confirmed that the enoic acid did not contain any branching. The geometry of the olefin was determined using two-dimensional (2D) nuclear Overhauser effect spectroscopy (NOESY) (see Fig. S2B in the supplemental material), which showed a correlation between the olefinic protons. This observation is consistent with a Z olefin geometry. The spectral data indicated that the putative XfDSF isolated from the recombinant strains was 2(Z)-tetradecenoic acid, a straight-chained enoic acid with 14 carbons (Fig. 1A). The mass spectral data from the methyl ester of the isolated acid confirmed this assignment (See Figure S6).
The structure of the putative XfDSF was verified by its chemical synthesis (Fig. 1A). The compound was synthesized using a Still-Gennari olefination (22) followed by saponification of the ethyl ester to afford the enoic acid (see Materials and Methods in the supplemental material). The natural and synthetic 2(Z)-tetradecenoic acids were shown to be identical by spectroscopic methods (Fig. 1B and C; Table 1). See Figure S5 for NMR spectra of synthetic compounds.
TABLE 1.
Chemical shift data for synthetic 2(Z)-tetradecenoic acid versus the natural XfDSF isolate
|
1H NMR result(s) 500 MHz, CDCl3 |
13C NMR shifts 125 MHz, CDCl3 |
||
|---|---|---|---|
| Natural | Synthetic | Natural | Synthetic |
| 171.2 | 171.4 | ||
| 6.36, m, 1 H | 6.36, m, 1 H | 153.6 | 153.6 |
| 5.79, d (J = 11.5 Hz), 1 H | 5.79, d (J = 11.4 Hz), 1 H | 118.8 | 118.8 |
| 2.66, m, 2 H | 2.66, m, 2 H | 31.9 | 31.9 |
| 1.45, m, 2 H | 1.45, m, 2 H | 29.6 (2C) | 29.6 (2C) |
| 1.20–1.39, m, 16 H | 1.20–1.39, m, 16 H | 29.5 | 29.5 |
| 0.89, t (J = 6.9 Hz), 3 H | 0.89, t (J = 6.9 Hz), 3 H | 29.4 | 29.4 |
| 29.23 | 29.23 | ||
| 29.17 | 29.18 | ||
| 28.9 | 28.9 | ||
| 22.7 | 22.7 | ||
| 14.1 | 14.1 | ||
Isolation and structural elucidation of XfDSF from a Xylella fastidiosa rpfC deletion mutant.
To verify that 2(Z)-tetradecenoic acid is produced in X. fastidiosa itself and not only in surrogate hosts harboring an rpfF gene from this species, we also isolated active compounds from X. fastidiosa. Since it was shown previously that RpfC is a negative regulator of RpfF in X. fastidiosa (13), an rpfC mutant hyperexpresses rpfF and therefore overproduces XfDSF. An rpfC mutant of strain temecula1 was thus used for XfDSF isolation from X. fastidiosa. While growth of the rpfC mutant in periwinkle wilt broth medium did not result in appreciable yields of the XfDSF compound, substantial amounts could be recovered from 300 plates of this medium solidified with gelrite using the same extraction scheme used with surrogate hosts. From this round of isolation, we obtained 0.6 mg of an enoic acid. The 1H NMR spectrum of this acid aligned with that of the XfDSF isolated from E. herbicola (see Fig. 2SC in the supplemental material).
Unfortunately, we were unable to obtain a 13C spectrum of the X. fastidiosa isolate due to the paucity of material. Instead, we turned to electron ionization low-resolution MS (EI LRMS) to validate our structural assignment of the putative XfDSF isolated from X. fastidiosa. We used EI because this is a relatively harsh ionization technique that promotes a large amount of fragmentation from the molecular ion. Consequently, EI LRMS spectra have complex fragmentation patterns with a unique signature for the analyte being tested. The EI LRMS spectrum for the isolate from X. fastidiosa displayed a molecular ion peak for the acid at 226 m/z. However, there was an additional peak at 270 m/z which was not associated with the analyte of interest. High-sensitivity mass spectrometry as an analytical technique can exaggerate trace impurities, since the ion intensity does not necessarily correlate with quantity in the analyte. Since there were baseline peaks in the 1H NMR spectrum of the X. fastidiosa isolate, we did not attribute the peak at 270 m/z to our compound. The rest of the LRMS spectrum had a fragmentation pattern identical to that of the synthetic 2-(Z)-tetradecenoic acid (see Fig. S2D in the supplemental material). The calculated mass is 226.1933 for an empirical formula of C14H26O2. An EI high-resolution MS (HRMS) analysis of the molecular ion at 226 m/z found a mass of 226.1929. These data strongly suggested that heterologous expression did yield the RpfF protein product and that XfDSF was 2-(Z)-tetradecenoic acid. We did not isolate 2(Z)-tetradecenoic acid from extracts of an rpfF mutant of X. fastidiosa, verifying the function of RpfF as the XfDSF synthase (see Fig. S3A in the supplemental material).
Construction of X. fastidiosa-based DSF reporters as a secondary assay.
To characterize the biological activity of putative DSF species, we developed two X. fastidiosa bioreporter strains that respond to XfDSF (which we refer to here as XfDSF reporters). Since there was evidence that the X. campestris pv. campestris-based DSF reporter strain in which an rpfF mutant strain harbors an engXCA′::gfp transcriptional fusion was not very responsive to XfDSF (4), we produced an X. fastidiosa bioreporter in which known DSF-responsive genes were linked to a promoterless phoA reporter gene. The low-codon-usage bias of X. fastidiosa (23) reflects an atypical ratio of tRNA abundances and thus altered translational parameters (24), leading to the apparently low efficiency of production of heterologous proteins. Previous attempts to express transcriptional fusions with gfp and inaZ reporter genes in X. fastidiosa failed (data not shown). The endogenous phoA was an effective reporter gene in X. fastidiosa since it conferred substantial alkaline phosphatase (AP) activity when driven by the E. coli lacZ promoter upon induction with isopropyl-β-d-thio-galactoside (IPTG) (data not shown). An X. fastidiosa phoA mutant was constructed by allelic replacement, and the phoA gene of X. fastidiosa was cloned into the broad host vector pBBR1MCS-2 (25). Promoters of the DSF-dependent genes encoding the hemagglutinin-like proteins HxfA and HxfB (13, 14) were then cloned upstream of a promoterless phoA and introduced into the phoA mutant to yield the resultant XfDSF reporters termed XfHA and XfHB, respectively. The rrsH promoter driving the expression of one of the two 16S rRNA-encoding genes (PD0133) was also fused with a promoterless phoA to yield a constitutive AP control termed XfR. The XfDSF reporters all harbored a wild-type rpfF since DSF-responsive transcription in X. fastidiosa was found to be dependent on the presence of the DSF synthase RpfF by mechanisms to be described in a subsequent report.
DSF produced by X. fastidiosa induces the hxfA and hxfB promoters.
To assess the temporal patterns of DSF production in X. fastidiosa and to determine the appropriate incubation times to assess DSF-dependent gene expression from exogenous application, the AP activity of XfHA and XfHB as well as XfR and a promoterless control was assayed over 5 days of growth on PD3 plates. The relative levels of AP activity of the various bioreporters at a given time differed greatly (Fig. 2A). While little AP activity was observed in XfHA or XfHB for about 30 h after inoculation, activity increased substantially after this time; the activity exhibited by XfHA was always about 4-fold greater than that of XfHB. XfR, reflecting the expression of the rRNA genes, exhibited earlier and stronger activation for up to 60 h, but its AP activity decreased greatly after this time as cells stopped multiplying. The strain harboring only a promoterless phoA fusion exhibited no detectable AP activity, confirming that the phoA knockout was complete and that no transcriptional activity derived from other promoters present on the plasmid induced expression of the phoA reporter gene. Assuming that expression of the hxfA and hxfB promoters is induced only by DSF, endogenous XfDSF accumulates to a detectable level only after ca. 24 h of cell growth, yielding an amply long time during which the effect of exogenous XfDSF on their expression can be assessed (see Fig. S4A in the supplemental material).
FIG 2.
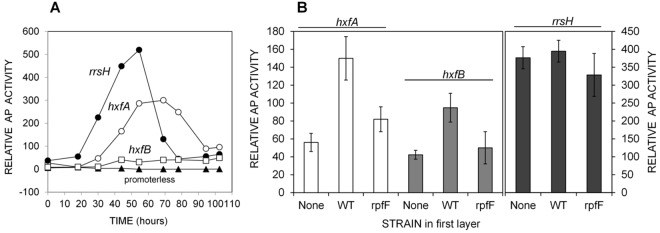
(A) Activity of hxfA, hxfB, and rrsH-phoA transcriptional fusions (designated XfHA, XfHB, and XfR) during growth of X. fastidiosa on solid PD3 medium. (B) Induction of XfHA and XfHB in response to XfDSF. X. fastidiosa wild-type (WT) cells or an rpfF mutant were grown for 5 days on PD3 plates, and then the plates were overlaid with a fresh PD3 layer onto which the bioreporter strains were inoculated. AP (alkaline phosphatase) activity was measured after 24 to 48 h.
The specificity of the response of the XfDSF reporters to the signal molecules produced by X. fastidiosa RpfF was assayed. The XfHA or XfHB bioreporters were grown on media that were overlaid on cultures of either the wild-type X. fastidiosa strain or an rpfF mutant. AP activity would be associated with compounds from the producing strains that had diffused through the medium. The AP activity of both XfHA and XfHB was about 2-fold higher in overlays of the wild-type culture than in overlays of cultures of either an rpfF mutant or uninoculated plates (Fig. 2B), indicating that the increased AP activity of the bioreporters cultured over that of the wild-type strain was solely attributable to excreted XfDSF. The levels of AP activity of XfR were similar under all conditions, suggesting that XfDSF did not greatly affect translation in this species.
2(Z)-Tetradecenoic acid induces hxfA and hxfB promoter activity.
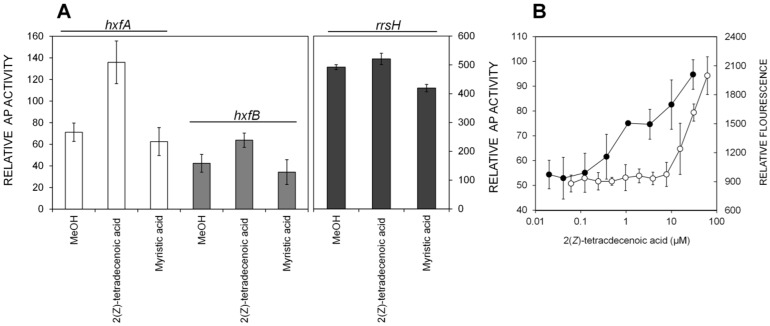
Since the isolation of the putative XfDSF was guided by the XccDSF reporter, the biological activity of 2(Z)-tetradecenoic acid in X. fastidiosa was verified by using the newly developed XfDSF reporters. Substantial induction of AP activity was observed within 24 h after exposure of XfHA and XfHB to 10 µM synthetic 2(Z)-tetradecenoic acid on solid PD3 media (Fig. 3A). As observed for crude extracts, XfHA exhibited higher AP activity than did XfHB upon exposure to 2(Z)-tetradecenoic acid. A similar concentration of myristic acid did not induce any AP activity in these bioreporters. Likewise, XfR exhibited similar levels of AP activity irrespective of the presence or absence of these molecules. XfHA exhibited a dose-dependent increase in AP activity in PD3 broth containing more than about 0.5 µM 2(Z)-tetradecenoic acid (Fig. 3B). In contrast, the green fluorescent protein (GFP) fluorescence exhibited by the XccDSF reporter, induced by its cognate DSF (11-methyl-cis-2-dodecenoic acid) at concentrations as low as 0.5 µM (17), was induced only by concentrations of 2(Z)-tetradecenoic acid higher than about 10 µM (Fig. 3B), suggesting that the Rpf system of X. fastidiosa is much more sensitive to 2(Z)-tetradecenoic acid than that of X. campestris. The putative XfDSF reported in the CVC strain of X. fastidiosa (20) 2-methyl-tetradecenoic acid did not induce any detectable AP activity in either the XfHA or XfHB bioreporter (see Fig. S4C in the supplemental material) or induce GFP fluorescence in the XccDSF reporter strain.
FIG 3.
(A) Alkaline phosphatase activity exhibited by XfHA, XfHB, and XfR in response to 10 µM 2(Z)-tetradecenoic acid or myristic acid. AP activity was measured after 24 to 48 h of growth on PD3 plates. (B) Alkaline phosphatase activity exhibited by XfHA (filled circles) and GFP fluorescence exhibited by the XccDSF reporter (open circles) in response to increasing amounts of 2(Z)-tetradecenoic acid. XfHA was assayed in a 48-well microtiter plate after 24 to 48 h of growth in PD3 broth.
2(Z)-Tetradecenoic acid induces biofilm formation in X. fastidiosa.
To further test the biological activity of 2(Z)-tetradecenoic acid in X. fastidiosa, its ability to induce biofilm formation at the liquid-air interface of shake cultures in glass tubes was quantified by crystal violet staining. rpfF mutants of X. fastidiosa are substantially impaired in the formation of biofilm, and thus exogenous XfDSF should induce this trait. Crude culture extracts from the wild-type strain do induce biofilm formation in this assay. Biofilm formation in a wild-type strain, temecula1, was induced in a dose-dependent manner within 24 h by addition of 2(Z)-tetradecenoic at concentrations greater than about 1.0 µM (Fig. 4A).
FIG 4.
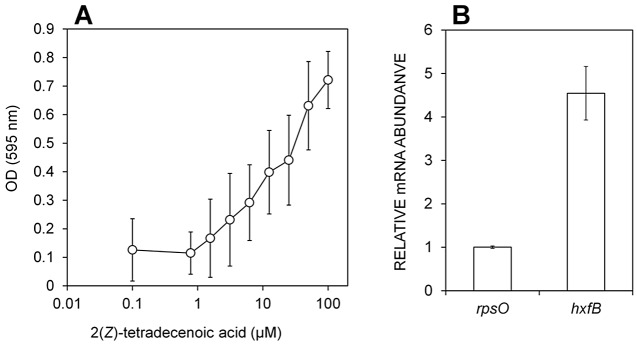
(A) 2(Z)-Tetradecanoic acid dose-dependent increase in biofilm formation in the liquid-air interface of a wild-type X. fastidiosa strain grown in a shaken broth culture. Biomass attached to the glass wall of a culture tube was measured after 24 to 48 h by crystal violet staining. (B) qPCR analysis of the relative levels of expression of hxfB in X. fastidiosa wild-type cells, in a shaken broth culture in the presence of 10 µM 2(Z)-tetradecanoic acid or MeOH only. RNA was extracted after 24 to 48 h; rpsO and rpoD served as endogenous controls.
To more directly link XfDSF exposure with changes in gene expression in X. fastidiosa, the abundance of transcripts of hxfB was assessed by real-time PCR analysis in cells grown with and without 10 µM 2(Z)-tetradecenoic acid. In the presence of 2(Z)-tetradecenoic acid, transcripts of hxfB were 4.5-fold higher than the transcript of rpsO, which was invariant. Relative transcription levels were normalized to rpoD transcript levels (Fig. 4B). This demonstrates that XfDSF-mediated increases in biofilm formation are accompanied by increases in the expression of hxfB.
2(Z)-Tetradecenoic acid accounts for only part of the induction of hxfA in culture extracts of X. fastidiosa.
Since several related chemical species with possible DSF activity are produced by Xanthomonas and Stenotrophomonas species (18, 19), we explored the extent to which 2(Z)-tetradecenoic acid could account for the induction of AP activity in the XfHA bioreporter by crude culture extracts of X. fastidiosa. XfHA was thus exposed to serial dilutions of crude extract of plate cultures added to PD3 broth in a microtiter well plate assay. A concentration-dependent increase in AP activity was observed in samples containing as little as 10% of a culture plate from which the extracts were made (Fig. 5; see also Fig. S4B in the supplemental material). Extracts containing 10-fold more than this threshold amount of material conferred apparent toxicity, resulting in decreased growth of the bioreporter, and reduced or complete suppression of AP activity was observed. Quantitative HPLC (using synthetic XfDSF as a standard) was used to estimate the concentration of 2(Z)-tetradecenoic acid in the extract. The concentration of 2(Z)-tetradecenoic acid in samples in which AP activity of the XfHA bioreporter was maximally induced was only about 100 nM (Fig. 5). These concentrations of 2(Z)-tetradecenoic acid are lower than the minimum concentration required for detection by XfHA (ca. 500 nM; Fig. 3B), suggesting that other active molecules that can induce hxfA were also present in the extract or that other compounds that can synergize with 2(Z)-tetradecenoic acid, thereby enhancing signal, were present in the cell extracts.
FIG 5.
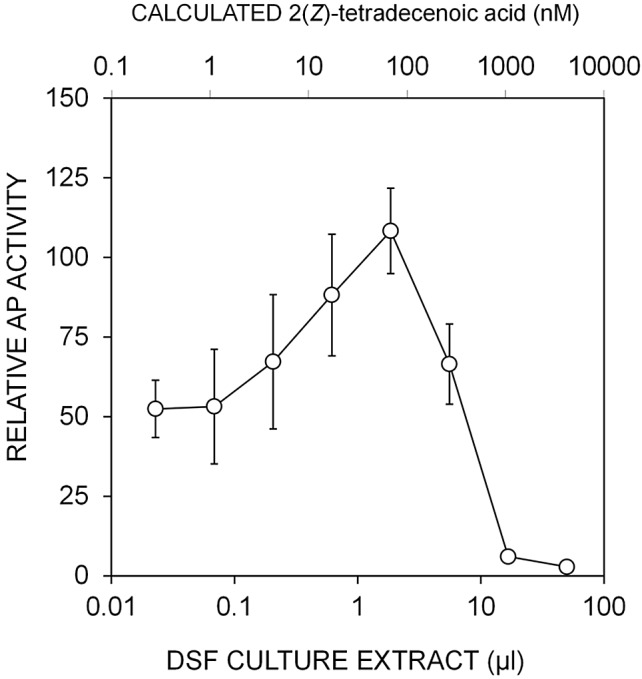
Alkaline phosphatase activity exhibited by XfHA harboring an hxfA′::phoA transcriptional fusion incubated in PD3 broth containing increasing amounts of DSF-containing culture extract of PD3 plate cultures of a wild-type X. fastidiosa strain. The concentration of 2(Z)-tetradecenoic acid in the extract determined by HPLC (high-performance liquid chromatography) is shown by the upper x axis.
DISCUSSION
While X. fastidiosa is one of several plant-pathogenic bacteria that coordinate their gene expression in a density-dependent fashion using small signaling molecules known as DSF, its signal molecule apparently differs from those of the other taxa. Here we isolated and characterized by XccDSF reporter-guided fractionation a novel DSF molecule, 2(Z)-tetradecenoic acid, from the PD strain of X. fastidiosa that both enhances the expression of genes contributing to biofilm formation (hxfA and hxfB) and promotes biofilm formation. This result provides further support for the idea that a clear structural specificity exists in various bacteria that conduct DSF-based signaling. Studies have shown that an XccDSF reporter reacts differentially to DSF extracts from other X. campestris pv. campestris strains and from the closely related pathogen X. oryzae pv. oryzae (17), suggesting that these strains produce different chemical species, or different ratios of a common group of related DSF molecules. In addition, the X. campestris pv. campestris-based DSF bioreporter responded quite differently to structurally diverse DSF-like fatty acids; a strong response to XccDSF was seen at concentrations as low as 0.5 µM whereas the minimum inducing concentrations of the E and the saturated isomers were 100 µM and 10 mM, respectively (17). Similarly, the 12-carbon, unbranched molecule with a 2(Z)-double bond (BDSF) had a minimum inducing concentration of 30 µM (17). Even within X. oryzae pv. oryzae, the three forms of DSF apparently have different biological activities (18). The response to such molecules appears species specific, since DSF and BDSF both restored exopolysaccharide (EPS) production and xylanase activity to an rpfF mutant of X. oryzae pv. oryzae at a concentration of ca. 1 µM (18). Likewise, in S. maltophilia, DSF and its saturated isomer appeared to be equally effective in the stimulation of flagellum-independent translocation (19). Curiously, a CVC strain of X. fastidiosa was reported to produce a potential DSF-like molecule (12-methyl-tetradecanoic acid) (20). However, while the DSFs from X. campestris pv. campestris and the PD strain of X. fastidiosa were verified through demonstration of the activity of synthetic material in their respective bioreporters (17, 18), this was not shown for the putative DSF from the CVC strain. Moreover, we have not been able to observe activity of this compound in the XfHA or XfHB bioreporters or in the X. campestris pv. campestris GFP reporter strains. This diversity of responses to related molecules within this group of bacteria suggests that even though they are closely related, their cognate signaling molecules (to which they are most responsive) can differ. Also, though the RpfC sensor machinery is conserved across taxa and can sense DSFs from related organisms, there is specificity associated with the sensor that gives differential responses of DSF signals in the DSF bioreporters. Therefore, although the putative XfDSF described in a CVC strain of X. fastidiosa was unable to activate either the XfHA bioreporter or the XccDSF reporter, it is possible that it could differ from that of the PD strain. For similar reasons, it is not surprising that our XccDSF reporter was not as proficient at sensing XfDSF in culture extracts (4) or synthetic 2(Z)-tetradecenoic acid (Fig. 3B).
XfDSF accumulation has been strongly linked with the induction of genes associated with attachment and biofilm formation. These processes, in turn, are associated with upregulation of several adhesins, including the hemagglutinin-like protein-encoding genes hxfA and hxfB that are involved in cell-cell aggregation and surface adhesion (13). Upregulation of these factors apparently attenuates the virulence of X. fastidiosa by increasing its adhesiveness, reducing its ability to spread throughout the plant (26). We have shown that 2(Z)-tetradecenoic acid is sufficient to upregulate the expression of these genes (Fig. 3). More importantly, the relatively low concentrations of 2(Z)-tetradecenoic acid that are sufficient to both induce gene expression and modulate biofilm formation (500 nM) support the idea of this molecule as being a biologically relevant signal molecule, since it is as active in the X. fastidiosa system as the cognate DSF species tested in Xanthomonas species (17). Although its DSF-dependent modulation of gene expression operates through an RpfCG phosphorelay pathway in X. fastidiosa, as in those other species (27), the genes that are regulated and their modes of regulation appear to differ in these taxa, reflecting the coordination of different lifestyles of these pathogens in their various environmental niches by similar regulatory mechanisms and structurally similar signal molecules (27). It remains to be seen if the distinctive DSF species produced by X. fastidiosa reflects a functional difference in its ability to act as an extracellular signal molecule in the high-flow, vascular environments that it colonizes or whether it might avoid cross-talk with other taxa that might co-occur in these settings.
Our finding that X. fastidiosa can sense exogenous DSF and alter gene expression and group behavior within a relatively short time of exposure is noteworthy in that it seems to differ from findings determined with other species that use DSF as a signaling molecule. Unlike the results seen in X. fastidiosa, DSF-mediated cell-cell signaling could not be induced in X. campestris pv. campestris in low-density cells by the addition of exogenous DSF (3). It was suggested that an additional factor(s) needs to accumulate to a functional level in order to allow cell-cell signaling to occur. Likewise, in some species such as Pseudomonas aeruginosa that utilize acyl homoserine lactones (AHLs) as signaling molecules, induction of expression at least some of the quorum-regulated genes is dependent on the expression of rpoS, and thus on the growth phase, in addition to accumulation of AHLs (28). The expression of hxfA, hxfB, and apparently other genes needed for biofilm formation is induced rapidly upon the addition of 2(Z)-tetradecenoic acid to cultures, independently of their cell concentration (Fig. 2 to 5). Since DSF accumulation tends to suppress the virulence of X. fastidiosa (27), it might be possible to alter the temporal patterns by which the pathogen would experience DSF in plants by applying this signal molecule to plants before or shortly after inoculation by the sharpshooter vector. Such cells would be expected to increase their adhesiveness and suppress their motility and secretion of extracellular enzymes, thereby reducing their ability to move and multiply within the plant. Therefore, the pathogen should aggregate before ever producing a quorum and would then be blocked in its ability to colonize the plant and cause disease. Disease control through this mechanism, termed “pathogen confusion,” would be facilitated by the cell density-independent response to DSF.
In this study, DSF identification in X. fastidiosa was initially guided by an XccDSF reporter. The isolated XfDSF molecule, 2(Z)-tetradecenoic acid, is thus one that can be sensed by the XccDSF reporter. While this molecule can modulate rpfF-dependent traits in both X. campestris pv. campestris and X. fastidiosa, it clearly is more active in X. fastidiosa, suggesting that other compounds that show an even higher preferential activity in X. fastidiosa might exist that may have been overlooked by the Xcc-based primary screen. There is evidence for the existence of such compounds, given that there is more biological activity sensed using the XfHA bioreporter than can be accounted for by the amount of 2(Z)-tetradecenoic acid isolated by HPLC (Fig. 5). The novel XfDSF reporter strains described here should be very useful in identification of any other DSF-like molecules produced by X. fastidiosa.
MATERIALS AND METHODS
Analytical methods.
Unless otherwise noted, starting materials and analytical grade solvents were obtained from commercial suppliers and used without further purification. Tetrahydrofuran (THF) was freshly distilled from sodium using benzophenone as an indicator. Both reaction mixtures and chromatography fractions were analyzed on Merck silica gel 60 F254 TLC plates. Flash column chromatography was carried out with ICN SiliTech silica gel (32 to 63 D, 60 Å). Fourier transform infrared (FT-IR) spectra were obtained as thin films on NaCl plates with an ATI Mattson Gemini spectrometer. Preparative reverse-phase HPLC purification was conducted using Agilent 1100 series preparative HPLC with an Alltech Econosil C18 column (250 mm by 22 mm) with acetonitrile and water and with 0.1% formic acid as the eluent. Proton and carbon NMR spectra (1H-NMR and 13C-NMR) were recorded on a Bruker DRX-500 spectrometer and calibrated to the residual solvent peak. Unless otherwise noted, spectra were recorded at room temperature (294.9 K). Multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, m = multiplet, dd = doublet of doublet. The prefix b stands for broad signals. Low-resolution mass spectra were recorded using a VGProSpec (EI) mass spectrometer. Unless otherwise noted, reactions were conducted with oven-dried glassware under an inert N2 atmosphere.
Construction of E. coli and E. herbicola strains for heterologous expression.
The rpfF gene from X. fastidiosa strain temecula1 (4, 29, 30) (see Table S1 in the supplemental material) was expressed from the promoter of the kanamycin resistance gene, kan2. rpfF was amplified from the X. fastidiosa genome by PCR using the rpfF primers (see Table S1 in the supplemental material). The rpfF ribosomal binding site (RBS) was included in the forward primer to enable translation. The Pkan promoter (137 bp) was amplified from the transposon kan2 Tn5 (Epicenter) with the kanPromoter primers (see Table S1 in the supplemental material). The rpfF forward and kanPromoter reverse primers included a HindIII restriction site, while the rpfF reverse and the kanPromoter forward primers included an EcoRI site. The two PCR fragments were digested with HindIII and then ligated to each other and subsequently amplified with the primers kanPromoter forward and rpfF reverse, gel purified, and then cloned into PCR2.1 (Invitrogen). The insertion was then subcloned into the EcoRI site of pVSP61 to yield pVSP61-rpfF. DSF production in E. coli (pVSP61-rpfF) was confirmed using the XccDSF reporter strain (see below) and pVSP61-rpfF transformed also into E. herbicola 299R electrocompetent cells; transformants were selected on Kirby-Bauer (KB) plates (31) supplemented with 50 µg/ml kanamycin and verified for DSF production as before.
Isolation of XfDSF from heterologous expression.
E. coli or E. herbicola harboring pVSP61-rpfF was cultured in 3 liters of KB broth (31) containing kanamycin. Cultures were incubated at 28°C for 36 h. Cells were formed into pellets, and the supernatant was extracted three times with equal volumes of EtOAc. The combined organics were washed once with brine (500 ml) and dried over Na2SO4, and the volatiles were removed in vacuo. The crude extract was purified using flash column chromatography (10:1 → 5:1 → 1:1 → 1:5 hexane:EtOAc → EtOAc). The active fraction from elution with 5:1 hexane:EtOAc was further purified with preparative reverse-phase HPLC on an Alltech Econosil C18 column (250 mm by 22 mm) with the gradient depicted in Table S2 in the supplemental material.
Isolation from rpfC mutant of X. fastidiosa.
The inoculum of the rpfC mutant was harvested from PWG Periwinkle Gelrite (PWG) plates into 10 mM phosphate buffer (optical density at 600 nm [OD600] of ~1), and then 50 µl was plated onto each of 300 PWG plates. Cells were allowed to grow for 12 days at 28°C, and the medium containing the cells was then sliced into 2-mm2 cubes and sonicated in an equal volume of EtOAc for 2 h. The EtOAc was decanted, and the volatiles were removed in vacuo. The crude extract was purified using flash column chromatography (10:1 → 5:1 → 1:1 → 1:5 hexane:EtOAc → EtOAc). The active fraction from elution with 5:1 hexane:EtOAc was further purified with preparative reverse-phase HPLC on an Alltech Econosil C18 column (250 mm by 22 mm) with the gradient depicted in Table S2 in the supplemental material. Using this method, 0.6 mg of putative XfDSF was isolated.
XccDSF reporter assay.
rpfF mutant 8523 of X. campestris pv. campestris (3) harboring an engXCA′::gfp transcriptional fusion (highly stable plasmid pKLN55; here designated the XccDSF reporter) (4) was employed for assays of DSF. A 2-day-old culture grown on KB plates without antibiotics was suspended in 10 mM phosphate buffer (pH 7.4) to an OD600 of 0.1. Cells were then sprayed on a KB plate onto which a DSF-producing strain was spotted at the center. The plates were incubated at 28°C for 36 h, and relative DSF abundance was estimated from the radius of cells exhibiting GFP fluorescence.
Alkaline phosphatase (AP) assays.
AP activity was quantified after cells were permeabilized and disrupted (32). Cells were grown on either PD3 plates or PD3 broth supplemented with 15 µg/ml gentamicin; much lower AP activity was seen in cells grown on PWG plates. Cells of XfHA, XfHB, XfR, or XfPL were grown for 5 to 6 days at 28°C on PWG plates supplemented with 15 µg/ml gentamicin and 50 µg/ml kanamycin prior to inoculation into the assay plates. When the assays were performed using solid media, cells were incubated either on PD3 plates supplemented with 10 µM 2(Z)-tetradecenoic acid (added from a stock of 100 mM dissolved in MeOH directly to the medium; MeOH alone was used as a control) or on a fresh layer of PD3 applied over 5-day cultures of various strains in PD3. Uncultured plates were overlaid as well for a negative control. To enable harvest of cells after only short incubation times, a high initial cell concentration (>109 cells/ml) was used. XfDSF reporter strains (10 μl) were plated side by side on a single plate, and cells were collected and assayed for AP activity after 24 to 48 h of incubation. Cells were collected from assay plates using a loop and suspended in 1 ml of 10 mM Tris-Base (pH 8.0) containing 10 mM MgSO4 in a 2-ml Eppendorf tube. The cells were formed into pellets by centrifugation for 4 min at 10,000 × g and resuspended in 2 ml 1 M Tris-Base (pH 8.0). The samples were transferred to glass assay tubes, and cell density (measured as OD600) was adjusted to ca. 0.15. Then, 1 ml of 1 M Tris-Base (pH 8.0) containing 0.1 mM ZnCl2 was added to each tube and the final OD600 was recorded. The cells were then disrupted by adding 50 µl of 0.1% SDS and 50 µl of chloroform followed by vigorous mixing by vortexing and incubated for 5 min at room temperature, and 3 µl of a 10 mM fluorescein diphosphate (Sigma) stock solution was then added to the tube and mixed briefly by vortexing. Fluorescence measurements (excitation, 485 nm; emission, 515 nm) were taken at 2-min intervals for 30 min using TD-700 fluorimeter (Turner Design). Enzyme activity was calculated as the rate of fluorescence signal accumulation with time divided by the cell density. For assays using 48-well Falcon microtiter plates (Becton Dickenson), PD3 broth was supplemented with either DSF analogs or DSF-containing crude extract (in MeOH) or an equal volume of MeOH. The two solutions (with and without DSF) were then mixed to produce various concentrations of DSF, and 400 µl was distributed into wells with six replicates. The XfHA reporter was collected into PD3 broth, and 400 µl of the suspension was applied to each well (final OD600 = ~0.05). At various time intervals, the AP activity of the microtiter plate was determined; the plate was centrifuged for 10 min at 4,000 rpm (Eppendorf), the growth medium was removed by aspiration, the cells were resuspended in 400 µl of 10 mM Tris-Base (pH 8.0) containing 10 mM MgSO4, and AP activity was then measured using a method similar to that described above.
Biofilm formation assays.
The effect of XfDSF on the biofilm formation capacity of the wild-type X. fastidiosa strain was determined in PIM6 broth (a medium developed in the laboratory of Michele Igo, University of California, Davis; personal communication) in glass culture tubes at 28°C and 200 rpm. Cells were grown on PWG plates for 7 days, resuspended in PIM6 broth to an OD600 = 0.2, and added to PIM6 broth containing either 10 µM 2(Z)-tetradecenoic acid (from a 100 mM MeOH stock solution) or an equal volume of MeOH only (0.1% [vol/vol] final concentration). The tubes were incubated for 24 to 48 h, during which a visible biomass was observed at the liquid-air interface. The medium, containing unattached cells, was then removed by aspiration, and the biomass attached to the glass wall was quantified by staining with 1% crystal violet for 10 min.
Quantitative PCR (qPCR).
hxfB expression was assessed in cultures of the wild-type strains grown in PIM6 broth containing either 10 µM 2(Z)-tetradecenoic or an equal volume of MeOH only. Total RNA was isolated using an RNeasy RNA extraction kit (Qiagen); DNA was eliminated using an on-column RNase-Free DNase kit (Qiagen). RNA samples were stored at −80°C, and 1-µg aliquots were used to initiate cDNA synthesis using 3 µg of random hexamer primers and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions.
Quantitative PCR was performed in an ABI Prism 7100 sequence detector system (Applied Biosystems), and primers listed in Table S1 in the supplemental material. PCR products were detected by measuring the increase in fluorescence produced upon binding of SYBR green dye (Qiagen) to double-stranded DNA (33). Two endogenous control genes (rpoD and rpsO) were employed to normalize gene expression. To ensure that the threshold cycle (Ct) values obtained were from a single PCR product, melting curve analysis was run at the end of each expression analysis. Relative expression (RQ) was calculated from the threshold cycle (Ct) as follows (33): dCt = Ct (target gene) − Ct (endogenous control); ddCt = dCt (treatment) − dCt (reference); RQ = 2(−ddCt).
Additional Material and Methods are described in the supplemental material in Text S1.
SUPPLEMENTAL MATERIAL
Supplemental Materials and Methods. Download
Isolation of putative XfDSF from heterologous expression in recombinant E. herbicola strain expressing RpfF from X. fastidiosa strain temecula1. (A) 1H and (B) 13C NMR of active isolate from recombinant expression. (C) Putative structure of XfDSF from recombinant expression and COSY spectrum confirming the presence of a single compound in the active fraction. 1H-1H COSY spectra show correlation peaks for protons that transfer magnetization through bonds in a process termed coupling. Coupled protons must be separated by only 3 to 4 bonds. In the COSY spectrum of XfDSF, the olefinic protons couple at 2.65 ppm to one other proton signal, a methylene unit, suggesting that the enoic acid has a Δ2.3 site of unsaturation. Download
(A) Distortionless enhancement polarization transfer (DEPT) 135 spectrum of XfDSF from heterologous expression of RpfF in E. herbicola. In this method, positive peaks represent CH and CH3 carbons whereas CH2 carbons are displayed as negative. Quaternary carbons are not observed in this type of spectroscopy, but they can be determined as the resonances present in the full 13C spectrum that are absent in the DEPT spectra. This spectrum showed that, in addition to the two positive peaks for the olefinic CH carbons from the DEPT 90 spectrum, there was a third positive resonance which could indicate a CH3 at the terminus of the acid chain. In the proton spectrum of this acid (see Fig. S1A in the supplemental material), there is a triplet at 0.89 ppm that integrates to three protons consistent with a terminal methyl group. The remaining carbon resonances were inverted in the DEPT 135 spectrum. There were no aliphatic quaternary carbons in the full 13C NMR spectrum (see Fig. S1B in the supplemental material). These data indicated that the putative XfDSF was a straight-chained enoic acid with 14 carbons. (B) Two-dimensional (2D) nuclear Overhauser effect spectroscopy (NOESY) of XfDSF from heterologous expression of RpfF in E. herbicola. The geometry of the olefin was determined using 2D NOESY. These spectra have correlation peaks for magnetic nuclei that transfer magnetization through space. The typical NOESY spectrum shows correlation peaks for magnetic nuclei that are within 5 Å of each other. In the 2D NOESY spectrum, the alkene protons Ha and Hb have correlation peaks, suggesting the Z olefin geometry. In an E configuration, those two protons would not be near enough in space to transfer magnetization. In addition, there was no correlation peak between Ha and Hc, which should be present if the olefin had an E configuration. These data, in conjunction with the spectra described above, support the assignment of XfDSF as 2(Z)-tetradecenoic acid. (C and D) Comparative spectra for the XfDSF isolate from strain temecula1. (C) The 1H NMR data determined for the X. fastidiosa isolate from heterologous expression of RpfF in E. herbicola match those of the active isolate. (D) LRMS EI spectrum for the X. fastidiosa isolate compared to that of the synthetic XfDSF. The fragmentation pattern is a match for that of the X. fastidiosa isolate, with the exception of the spurious peak at 270 m/z. In this fragmentation method, the peak height does not necessarily correlate to compound abundance, and this ion could correspond to a compound that contributes to the background signals in the 1H NMR of the X. fastidiosa isolate. Download
(A) HPLC chromatogram for isolation from the rpfF mutant of X. fastidiosa. (B) HPLC chromatogram for isolation of XfDSF from the rpfC mutant of X. fastidiosa. (C) HPLC chromatogram of synthetic XfDSF. Download
Kinetics of an hxfA′::phoA activity in response to (A) supplemental exogenous 30 µM XfDSF and (B) various doses of culture crude extract at 30 h and 36 h postculturing. An increase in background activity with time is due to accumulation of endogenous DSF produced by the cells which are RpfF positive (RpfF+). (C) hxfA′::phoA activity in response to 12-methyltetradecanoic acid (the DSF-like molecule isolated from the X. fastidiosa CVC strain) (20). Download
(A) 1H NMR of enoic ester. (B) 13C NMR of enoic ester. (C) 1H NMR of synthetic XfDSF. (D) 13C NMR of synthetic XfDSF. Download
GC-MS spectrum of methyl ester of XfDSF isolated from heterologous expression in E. herbicola. XfDSF was treated with trimethylsilyldiazonmethane (TMS-diazomethane) to form the methyl ester. The GC-MS data for this sample showed a molecular ion with m/z of 240, corresponding to a C14 acid with a single site of unsaturation. Download
Strains, plasmids, and primers used in this study.
Preparative HPLC gradient method for DSF isolation and separation of E and Z isomers of 2-tetradecenoic acid.
ACKNOWLEDGMENTS
This research was supported by Vaadia-Bard Postdoctoral Fellowship award FI-427-09 from BARD, the United States-Israel Binational Agricultural Research and Development Fund, and by the Pierce’s Disease Control Program (PDCP), California Department of Food and Agriculture (CDFA).
Footnotes
Citation Beaulieu ED et al. 2013. Characterization of a diffusible signaling factor from Xylella fastidiosa. mBio 4(1):e00539-12. doi:10.1128/mBio.00539-12.
REFERENCES
- 1. Purcell AH, Finlay AH, MacLean DL. 1979. Pierce’s disease bacterium: mechanism of transmission by leafhopper vectors. Science 206:839–841 [DOI] [PubMed] [Google Scholar]
- 2. Purcell AH, Hopkins DL. 1996. Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34:131–151 [DOI] [PubMed] [Google Scholar]
- 3. Barber CE, et al. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555–566 [DOI] [PubMed] [Google Scholar]
- 4. Newman KL, Almeida RP, Purcell AH, Lindow SE. 2004. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. U. S. A. 101:1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fouhy Y, et al. 2007. Diffusible signal factor-dependent cell-cell signaling and virulence in the nosocomial pathogen Stenotrophomonas maltophilia. J. Bacteriol. 189:4964–4968 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Boon C, et al. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2:27–36 [DOI] [PubMed] [Google Scholar]
- 7. Ryan RP, et al. 2008. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 68:75–86 [DOI] [PubMed] [Google Scholar]
- 8. Bi H, Christensen QH, Feng Y, Wang H, Cronan JE. 2012. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol. Microbiol. 83:840–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryan RP, et al. 2006. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc. Natl. Acad. Sci. U. S. A. 103:6712–6717 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. He YW, et al. 2006. Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell-cell communication-dependent genes and functions. Mol. Microbiol. 59:610–622 [DOI] [PubMed] [Google Scholar]
- 11. Guo Y, Zhang Y, Li JL, Wang N. 2012. Diffusible signal factor-mediated quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri. Mol. Plant Microbe Interact. 25:165–179 [DOI] [PubMed] [Google Scholar]
- 12. Rai R, Ranjan M, Pradhan BB, Chatterjee S. 2012. Atypical regulation of virulence-associated functions by a diffusible signal factor in Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 25:789–801 [DOI] [PubMed] [Google Scholar]
- 13. Chatterjee S, Wistrom C, Lindow SE. 2008. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. U. S. A. 105:2670–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang N, Li JL, Lindow SE. 2012. RpfF-dependent regulon of Xylella fastidiosa. Phytopathology 102:1045–1053 [DOI] [PubMed] [Google Scholar]
- 15. Chatterjee S, Sonti RV. 2002. rpfF mutants of Xanthomonas oryzae pv. oryzae are deficient for virulence and growth under low iron conditions. Mol. Plant Microbe Interact. 15:463–471 [DOI] [PubMed] [Google Scholar]
- 16. Chatterjee S, Newman KL, Lindow SE. 2008. Cell to cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant Microbe Interact. 21:1309–1315 [DOI] [PubMed] [Google Scholar]
- 17. Wang LH, et al. 2004. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 51:903–912 [DOI] [PubMed] [Google Scholar]
- 18. He YW, Wu J, Cha JS, Zhang LH. 2010. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol. 10:187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang TP, Wong AC. 2007. A cyclic AMP receptor protein-regulated cell-cell communication system mediates expression of a FecA homologue in Stenotrophomonas maltophilia. Appl. Environ. Microbiol. 73:5034–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colnaghi Simionato AV, da Silva DS, Lambais MR, Carrilho E. 2007. Characterization of a putative Xylella fastidiosa diffusible signal factor by HRGC-EI-MS. J. Mass Spectrom. 42:1375–1381 [DOI] [PubMed] [Google Scholar]
- 21. Scarpari LM, Lambais MR, Silva DS, Carraro DM, Carrer H. 2003. Expression of putative pathogenicity-related genes in Xylella fastidiosa grown at low and high cell density conditions in vitro. FEMS Microbiol. Lett. 222:83–92 [DOI] [PubMed] [Google Scholar]
- 22. Still WC, Gennari C. 1983. Direct synthesis of Z-unsaturated esters. A useful modification of the Horner-emmons olefination. Tetrahedron Lett. 24:4405–4408 [Google Scholar]
- 23. Smolka MB, et al. 2003. Proteome analysis of the plant pathogen Xylella fastidiosa reveals major cellular and extracellular proteins and a peculiar codon bias distribution. Proteomics 3:224–237 [DOI] [PubMed] [Google Scholar]
- 24. Ikemura T. 1985. Codon usage and tRNA content in unicellular and multicellular organisms. Mol. Biol. Evol. 2:13–34 [DOI] [PubMed] [Google Scholar]
- 25. Kovach ME, et al. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 26. Guilhabert MR, Kirkpatrick BC. 2005. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to biofilm maturation to X. fastidiosa and colonization and attenuate virulence. Mol. Plant Microbe Interact. 18:856–868 [DOI] [PubMed] [Google Scholar]
- 27. Chatterjee S, Almeida RP, Lindow S. 2008. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 46:243–271 [DOI] [PubMed] [Google Scholar]
- 28. Schuster M, Hawkins AC, Harwood CS, Greenberg EP. 2004. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol. Microbiol. 51:973–985 [DOI] [PubMed] [Google Scholar]
- 29. Loper JE, Lindow SE. 1994. A biological sensor for iron available to bacteria in their habitats on plant surfaces. Appl. Environ. Microbiol. 60:1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brandl M, Clark EM, Lindow SE. 1996. Characterization of the indole-3-acetic acid (IAA) biosynthetic pathway in an epiphytic strain of Erwinia herbicola and IAA production in vitro. Can. J. Microbiol. 42:586–592 [Google Scholar]
- 31. King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301–307 [PubMed] [Google Scholar]
- 32. Torriani A. 1968. Alkaline Please confirm that "218i" is correct for the page number range in reference 32 or correct it.phosphatase of Escherichia coli, p. 212–218 In Grossman L, Moldave K, Methods in enzymology, vol. 12 Academic Press, New York, NY. [Google Scholar]
- 33. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods. Download
Isolation of putative XfDSF from heterologous expression in recombinant E. herbicola strain expressing RpfF from X. fastidiosa strain temecula1. (A) 1H and (B) 13C NMR of active isolate from recombinant expression. (C) Putative structure of XfDSF from recombinant expression and COSY spectrum confirming the presence of a single compound in the active fraction. 1H-1H COSY spectra show correlation peaks for protons that transfer magnetization through bonds in a process termed coupling. Coupled protons must be separated by only 3 to 4 bonds. In the COSY spectrum of XfDSF, the olefinic protons couple at 2.65 ppm to one other proton signal, a methylene unit, suggesting that the enoic acid has a Δ2.3 site of unsaturation. Download
(A) Distortionless enhancement polarization transfer (DEPT) 135 spectrum of XfDSF from heterologous expression of RpfF in E. herbicola. In this method, positive peaks represent CH and CH3 carbons whereas CH2 carbons are displayed as negative. Quaternary carbons are not observed in this type of spectroscopy, but they can be determined as the resonances present in the full 13C spectrum that are absent in the DEPT spectra. This spectrum showed that, in addition to the two positive peaks for the olefinic CH carbons from the DEPT 90 spectrum, there was a third positive resonance which could indicate a CH3 at the terminus of the acid chain. In the proton spectrum of this acid (see Fig. S1A in the supplemental material), there is a triplet at 0.89 ppm that integrates to three protons consistent with a terminal methyl group. The remaining carbon resonances were inverted in the DEPT 135 spectrum. There were no aliphatic quaternary carbons in the full 13C NMR spectrum (see Fig. S1B in the supplemental material). These data indicated that the putative XfDSF was a straight-chained enoic acid with 14 carbons. (B) Two-dimensional (2D) nuclear Overhauser effect spectroscopy (NOESY) of XfDSF from heterologous expression of RpfF in E. herbicola. The geometry of the olefin was determined using 2D NOESY. These spectra have correlation peaks for magnetic nuclei that transfer magnetization through space. The typical NOESY spectrum shows correlation peaks for magnetic nuclei that are within 5 Å of each other. In the 2D NOESY spectrum, the alkene protons Ha and Hb have correlation peaks, suggesting the Z olefin geometry. In an E configuration, those two protons would not be near enough in space to transfer magnetization. In addition, there was no correlation peak between Ha and Hc, which should be present if the olefin had an E configuration. These data, in conjunction with the spectra described above, support the assignment of XfDSF as 2(Z)-tetradecenoic acid. (C and D) Comparative spectra for the XfDSF isolate from strain temecula1. (C) The 1H NMR data determined for the X. fastidiosa isolate from heterologous expression of RpfF in E. herbicola match those of the active isolate. (D) LRMS EI spectrum for the X. fastidiosa isolate compared to that of the synthetic XfDSF. The fragmentation pattern is a match for that of the X. fastidiosa isolate, with the exception of the spurious peak at 270 m/z. In this fragmentation method, the peak height does not necessarily correlate to compound abundance, and this ion could correspond to a compound that contributes to the background signals in the 1H NMR of the X. fastidiosa isolate. Download
(A) HPLC chromatogram for isolation from the rpfF mutant of X. fastidiosa. (B) HPLC chromatogram for isolation of XfDSF from the rpfC mutant of X. fastidiosa. (C) HPLC chromatogram of synthetic XfDSF. Download
Kinetics of an hxfA′::phoA activity in response to (A) supplemental exogenous 30 µM XfDSF and (B) various doses of culture crude extract at 30 h and 36 h postculturing. An increase in background activity with time is due to accumulation of endogenous DSF produced by the cells which are RpfF positive (RpfF+). (C) hxfA′::phoA activity in response to 12-methyltetradecanoic acid (the DSF-like molecule isolated from the X. fastidiosa CVC strain) (20). Download
(A) 1H NMR of enoic ester. (B) 13C NMR of enoic ester. (C) 1H NMR of synthetic XfDSF. (D) 13C NMR of synthetic XfDSF. Download
GC-MS spectrum of methyl ester of XfDSF isolated from heterologous expression in E. herbicola. XfDSF was treated with trimethylsilyldiazonmethane (TMS-diazomethane) to form the methyl ester. The GC-MS data for this sample showed a molecular ion with m/z of 240, corresponding to a C14 acid with a single site of unsaturation. Download
Strains, plasmids, and primers used in this study.
Preparative HPLC gradient method for DSF isolation and separation of E and Z isomers of 2-tetradecenoic acid.