Abstract
A variety of GTP-binding protein (G protein)-coupled receptors are expressed at the nerve terminals of central synapses and play modulatory roles in transmitter release. At the calyx of Held, a rat auditory brainstem synapse, activation of presynaptic γ-aminobutyric acid type B receptors (GABAB receptors) or metabotropic glutamate receptors inhibits presynaptic P/Q-type Ca2+ channel currents via activation of G proteins, thereby attenuating transmitter release. To identify the heterotrimeric G protein subunits involved in this presynaptic inhibition, we loaded G protein βγ subunits (Gβγ) directly into the calyceal nerve terminal through whole-cell patch pipettes. Gβγ slowed the activation of presynaptic Ca2+ currents (IpCa) and attenuated its amplitude in a manner similar to the externally applied baclofen, a GABAB receptor agonist. The effects of both Gβγ and baclofen were relieved after strong depolarization of the nerve terminal. In addition, Gβγ partially occluded the inhibitory effect of baclofen on IpCa. In contrast, guanosine 5′-O-(3-thiotriphosphate)-bound Goα loaded into the calyx had no effect. Immunocytochemical examination revealed that the subtype of G proteins Go, but not the Gi, subtype, is expressed in the calyceal nerve terminal. These results suggest that presynaptic inhibition mediated by G protein-coupled receptors occurs primarily by means of the direct interaction of Go βγ subunits with presynaptic Ca2+ channels.
In the central nervous system, synaptic transmission is regulated by presynaptic autoreceptors or heteroreceptors. These receptors are coupled by G proteins to various targets such as Ca2+ channels, K+ channels, or the exocytotic machinery downstream of Ca2+ influx (1). At a rat brainstem auditory synapse formed by a giant nerve terminal, the calyx of Held, metabotropic glutamate receptors (mGluRs), or γ-aminobutyric acid type B receptors (GABAB receptors) primarily inhibit P/Q-type Ca2+ channels (2–4). The inhibitory effect of the GABAB receptor agonist baclofen on presynaptic Ca2+ currents can be blocked by the GDP analog guanosine 5′-O-(2-thiodiphosphate) (GDP[βS]) and occluded by the nonhydrolyzable GTP analog guanosine 5′-O-(3-thiotriphosphate) (GTP[γS]), both loaded directly into the calyx, indicating that G proteins mediate the presynaptic inhibition (3). At the cell soma, heterotrimeric G proteins attenuate Ca2+ currents acting either directly via a membrane-delimited pathway involving G protein βγ subunits (Gβγ) or indirectly via second messengers (5, 6). At the nerve terminal, however, it is not known which mechanism underlies this presynaptic inhibition. At the calyx of Held, which can be visually identified in slice, it is possible to load various molecules into the nerve terminal through whole-cell recording pipette (3, 7, 8). Using this technique, we examined the effect of Gβγ on IpCa. Also, because of its large structure, it was possible to use immunocytochemistry to determine the subtype of G proteins expressed in the calyceal nerve terminal. Our results suggest that the βγ subunits of Go mediate the presynaptic inhibition at the calyx of Held synapse by directly interacting with presynaptic Ca2+ channels.
Methods
Preparations and Solutions.
All experiments were performed in accordance with the guidelines of the Physiological Society of Japan. Wistar rats (12–17 days old) were killed by decapitation under halothane anesthesia. The whole brain was immersed in an ice-cold cutting solution containing 250 mM sucrose, 2.5 mM KCl, 26 mM NaHCO3, 10 mM glucose, 1.25 mM NaH2PO4, 1 mM CaCl2, 4 mM MgCl2, 0.5 mM myo-inositol, 2 mM sodium pyruvate, and 0.5 mM ascorbate, pH 7.4, when saturated with 95% O2/5% CO2. A tissue block containing the brainstem and cerebellum was removed and glued onto the stage of a tissue slicer (Microslicer DTK-1000, Dosaka, Kyoto, Japan) and immersed in the cutting solution. Transverse slices (150 μm in thickness) containing the medial nucleus of the trapezoid body (MNTB) were cut and incubated at 36°C for 1 h in the artificial cerebrospinal fluid containing 125 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 10 mM glucose, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2, 0.5 mM myo-inositol, 2 mM sodium pyruvate, 0.5 mM ascorbate, pH 7.4, when saturated with 95% O2/5% CO2.
Recording and Data Analysis.
For electrophysiological recordings, one slice was transferred to a superfusing chamber on a stage of upright microscope (Axioskop, Zeiss), and individual calyces in the MNTB were visualized with a 40× water-immersion objective lens (Zeiss) through a charge-coupled device camera. The recording chamber was continuously superfused with the artificial cerebrospinal fluid. Whole-cell presynaptic Ca2+ currents were recorded from calyces under voltage clamp being evoked with depolarizing steps from a holding potential of −80 mV. To isolate Ca2+ currents, 10 mM tetraethylammonium chloride and 1 μM tetrodotoxin were included in the artificial cerebrospinal fluid, and the presynaptic patch pipette was filled with a solution containing 110 mM CsCl, 40 mM Hepes, 0.5 mM EGTA, 1 mM MgCl2, 2 mM ATP, 0.5 mM GTP, 12 mM disodium phosphocreatine, and 10 mM tetraethylammonium chloride, with pH adjusted to 7.4 with CsOH. The electrode resistance was 5–10 MΩ, and series resistance was typically 10–30 MΩ and compensated by 70–80%. Whole-cell current recordings were made by using a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA). Records were low-pass filtered at 2 kHz and digitized at 5 kHz with a CED 1401 interface (Cambridge Electric Design). Leak currents were subtracted for presynaptic currents by a scaled pulse divided by n (P/N) protocol (3). Statistical significance was evaluated by paired t test unless otherwise noted. The liquid junction potential between the internal and external solutions was not corrected for.
Applications of Drugs and Proteins.
Baclofen was purchased from Research Biochemicals (Natick, MA). Purified bovine brain Gβγ and recombinant myristoylated rat G protein α subunit (Goα) were from Calbiochem. Gβγ was dissolved at 50 μg/ml in 50 mM Hepes/1 mM DTT/1 mM EDTA/0.1% (1.25 μM) Lubrol, pH 7.6, and stored at −80°C. To inactivate the protein, the stock solution was boiled at 95°C for 10 min. Boiled or nontreated Gβγ was diluted with the pipette solution added with 0.05% or 0.1% CHAPS {3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate} for the final Gβγ concentrations to be 100 or 200 nM. The stock solution for Goα (1.7 mg/ml) contained 20 mM Hepes, 100 mM NaCl, 3 mM MgCl2, and 1 mM EDTA (pH 8.0). To activate Goα, this stock solution was diluted 10-fold with a solution containing 50 μM GTP[γS], 100 mM NaCl, 25 mM MgCl2, 1 mM DTT, 1 mM Na3EDTA, and 20 mM Hepes (pH 8.0) and incubated at 37°C for 2 h. After incubation, unbound GTP[γS] was removed by gel filtration (using Bio-Gel P-6, Bio-Rad). The final effective concentration of Goα–GTP[γS] assessed by using GTP[γ35S] was 100 nM (total amount of Goα was 250 nM). The concentration of free GTP[γS] remaining after gel filtration was estimated to be less than 0.12 nM. G protein subunits were applied through whole-cell pipettes into calyces by diffusion. Baclofen (2 or 20 μM) was bath-applied by switching superfusates by using solenoid valves. Experiments were carried out at room temperature (24–27°C).
Immunocytochemistry.
Wistar rats (13–15 days old) were anesthetized with Nembutal and transcardially perfused with a fixative (4% paraformaldehyde and 0.2% picric acid in 0.1 M sodium phosphate, pH 7.4). After fixation, rats were killed by decapitation, and a tissue block of brainstem including the MNTB region was removed for postfixation for 1–2 days at 4°C. The fixed tissue was cryoprotected at 4°C in 0.1 M sodium phosphate with sucrose of graded concentrations: in 4% sucrose for 30 min, 10% for 2 h, 15% for 2 h, and 20% overnight. Transverse slices (25 μm in thickness) were cut with a cryostat (CM3050, Leica, Nussloch, Germany) at −21°C. The sections were then processed for immunocytochemistry as follows: (i) blocking and permeabilization in phosphate-buffer saline solution containing 4% skim milk and 0.3% (wt/vol) Triton X-100 for 5 h; (ii) application of primary antibodies in PBS containing 0.5% (wt/vol) BSA and 0.05% Triton X-100 for 2 days at 4°C; (iii) application of secondary antibodies in the same buffer as primary antibodies for overnight at 4°C; and (iv) mounting with ProLong antifade kit (Molecular Probes). The primary antibodies were rabbit polyclonal anti-Goα subunit, anti-Goα and Giα3 subunits, anti-Giα1 and Giα2 subunits, anti-Giα3 subunit (Calbiochem), and mouse monoclonal anti-synapsin I (Chemicon) diluted 1:100. The secondary antibodies were anti-mouse IgG conjugated with Alexa fluor 568 and anti-rabbit IgG conjugated with Alexa fluor 488 (Molecular Probes, diluted 1:200). Stained sections were viewed with a 100× oil-immersion objective (numerical aperture 1.35) or 100× water-immersion objective (numerical aperture 1.0) using a confocal laser scanning microscope (Fluoview FV300, Olympus Optical, Tokyo). Excitation wavelengths were 488 nm (argon laser) and 543 nm (He/Ne laser). Emission wavelengths were 510–530 nm (for green) and >610 nm (for red). All of the immunocytochemical procedures were carried out at room temperature (22–27°C) unless otherwise noted.
Results
Inhibitory Effect of Gβγ on Presynaptic Ca2+ Currents.
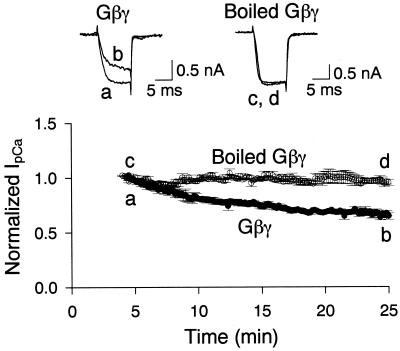
To examine the effect of Gβγ on IpCa, we loaded Gβγ (100 nM) directly into the calyceal nerve terminal through a whole-cell patch pipette by passive diffusion. After rupture of patch membrane, IpCa was evoked by a depolarizing pulse (10 ms) from a holding potential of −80 mV (Fig. 1). At the calyx of Held at this postnatal age (12–17 days old), IpCa has been pharmacologically characterized as the P/Q type (9, 10). After rupture with a pipette containing Gβγ, the activation rate of IpCa gradually became slower and its amplitude smaller (measured 2.5 ms after the onset of pulse). The slowness of the effects of Gβγ might, at least in part, be due to a slow rate of diffusion of Gβγ (about 40 kDa) into the calyx. The mean amplitude of IpCa 25 min after rupture was 65.4 ± 3.1% (n = 3) of the amplitude measured 5 min after rupture. In contrast, no appreciable change was observed for IpCa when Gβγ inactivated by boiling (100 nM) was loaded into calyces (96.2 ± 4.0%, n = 3). Thus, Gβγ specifically and significantly inhibited IpCa (P < 0.005, unpaired t test). This effect of Gβγ is similar to that obtained by externally applying the GABAB agonist baclofen (3, 4), the mGluR agonist L-2-amino-4-phosphonobutyrate (2) or by intracellularly infusing GTP[γS] (3, 7).
Figure 1.
The effect of Gβγ on IpCa. Untreated (traces a and b) or boiled (traces c and d) Gβγ (each 100 nM) was applied to calyceal nerve terminals by means of whole-cell pipettes. Here and in Fig. 3, abscissae indicate the time after the patch membrane rupture. IpCa was evoked by a 10-ms depolarizing pulse stepped from −80 mV to 10 mV every 10 s. Ordinates indicate the mean amplitude of IpCa (derived from three cells each) and normalized to the mean of the first five data points. The mean amplitude of IpCa was measured 2.5 ms after the command pulse onset shown here and in Figs. 2 and 3. Error bars represent SEM here and in Fig. 2. Sample records on top row indicate IpCa in calyces loaded with Gβγ (a and b) or boiled Gβγ (c and d), 5 min (a and c) and 25 min (b and d) after ruptures, respectively (superimposed).
Depolarization-Induced Unblock of IpCa Inhibitions by Gβγ and Baclofen.
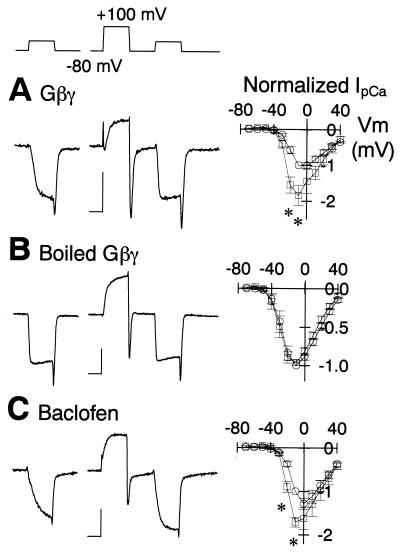
Inhibition of voltage-dependent Ca2+ channels by Gi or Go takes place via a membrane-delimited pathway and is transiently disinhibited after strong depolarization (11, 12). We examined whether this disinhibition might be observed for the effects of Gβγ on IpCa (Fig. 2). First, we evoked a series of IpCa by using 10-mV incremental depolarizing step pulses of a 10-ms duration to formulate a current–voltage relationship (9). We then applied a large conditioning depolarizing pulse (to +100 mV, 10-ms duration) and followed this with the same series of test pulses. When Gβγ (200 nM) was present in the recording pipette, the conditioning pulse increased the activation rate and amplitude of IpCa for a wide range of test potentials (Fig. 2A, significant differences at −20 mV and −10 mV, P < 0.02, n = 6 cells). The peak of IpCa in the current–voltage relationship showed a concomitant shift by about −10 mV, indicating that gating of the Ca2+ channels had shifted toward the “willing” state (13). Similar effects were observed when baclofen (20 μM) was present in the superfusate (Fig. 2C), as previously reported (4). In contrast, no such disinhibition was observed when boiled Gβγ (200 nM) was present in the recording pipette (Fig. 2B, n = 8) or before application of baclofen (not shown). Thus, Gβγ mimics the inhibitory effect of baclofen.
Figure 2.
Depolarization-induced disinhibition of IpCa. IpCa was evoked by a depolarizing test pulse from a holding potential of −80 mV to different membrane potentials by 10-mV steps with (□) or without (○) a prior conditioning depolarizing pulse (to +100 mV, duration 10 ms). (Left) Experimental paradigm (Top) and sample records of IpCa evoked by depolarizing steps to −10 mV. Calibrations are 5 ms and 0.5 nA. (Right) In the current–voltage relationship, the IpCa amplitude was normalized to that at −10 mV without conditioning. Recording pipettes contained Gβγ (A) or boiled Gβγ (B) (200 nM each), or none of them (C). In C, baclofen (20 μM) was present in superfusates. Mean amplitudes and SEM were derived from 6 (A), 8 (B), and 4 (C) calyces, respectively. Asterisks indicate data points of significant difference (P < 0.02).
Occlusion of Gβγ with Baclofen in the Inhibitory Effect on IpCa.
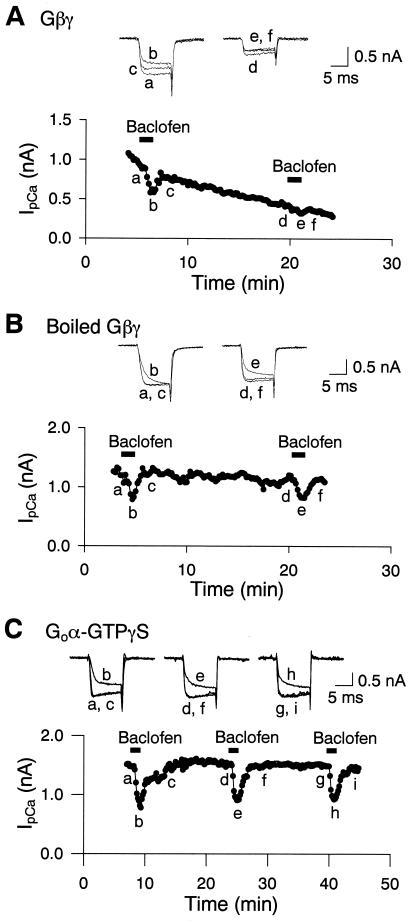
If Gβγ mediates the baclofen-induced inhibition of IpCa, intracellular loading of Gβγ would be expected to occlude the effect of baclofen. In the presence of Gβγ in the presynaptic pipette (200 nM), the first application of baclofen (2 μM) just after rupture (<6 min) clearly inhibited IpCa (Fig. 3A). However, the second application of baclofen 15–20 min later inhibited IpCa amplitude to a significantly lesser extent (66 ± 0.4%, n = 8, P < 0.00002). In contrast, when heat-inactivated Gβγ was included in the presynaptic patch pipette, the second application of baclofen inhibited IpCa to a similar extent as the first application (Fig. 2B, 94 ± 3%, n = 7, P > 0.1). Thus, Gβγ occluded the inhibitory effect of baclofen on IpCa. The occluding effect of Gβγ on baclofen-induced inhibition of IpCa was weaker than that obtained with GTP[γS] (3). This may be due to insufficient presynaptic concentrations of Gβγ because of its slow diffusion, inadequate conditions for its full potency, or the involvement of additional subunits or second messengers.
Figure 3.
Gβγ occluded the baclofen-induced inhibition of IpCa, whereas Goα–GTP[γS] did not. Whole-cell recording pipettes contained Gβγ (A) or boiled Gβγ (B) (200 nM each). Baclofen (2 μM) applied 3–5 min after rupture inhibited the IpCa amplitude by 43.1 ± 2.2% (n = 8). Baclofen-induced IpCa inhibition was attenuated by Gβγ in the second application after a 15- to 20-min interval (A). Boiled Gβγ had no effect (B). (C) Goα–GTP[γS] included in the presynaptic pipette (100 nM; total Goα, 250 nM) had no effect on the baclofen-induced IpCa inhibition.
Effects of Goα on IpCa.
We examined whether Goα subunits are also involved in the baclofen-induced presynaptic inhibition by loading calyces with constitutively active Goα containing bound GTP[γS]. In the presence of Goα–GTP[γS] in the presynaptic whole-cell pipette (100 nM), IpCa remained unchanged for at least 40 min after rupture, and the first and second applications of baclofen (2 μM), separated by 15- to 20-min intervals, inhibited IpCa to similar extents (Fig. 3C, 103 ± 4.8%, n = 5, P > 0.5). Goα–GTP[γS] has been reported to be effective in attenuating Ca2+ currents in chick dorsal rot ganglia in culture at lower concentrations (<20 nM) (14). Thus, Goα does not seem to mediate baclofen-induced IpCa inhibition.
Effects of Cyclic Nucleotides on the Baclofen-Induced IpCa Inhibition.
Our present results are consistent with a membrane-delimited, G protein-dependent mechanism for the baclofen effect. However, it has been reported that cyclic (c) GMP is responsible for mGluR-mediated presynaptic inhibition (15) and somatostatin-induced inhibition of Ca2+ currents (16). We examined further the possible contribution of adenylyl cyclase or guanylyl cyclase to presynaptic inhibition by loading calyces with cAMP or cGMP at high concentrations (200 μM). This concentration of cyclic nucleotides is three orders of magnitude higher than their resting concentrations (17). In the presence of 200 μM cAMP, baclofen (20 μm) attenuated IpCa by 37.7 ± 3.5% (n = 4). Similarly, in the presence of 200 μM cGMP, baclofen attenuated IpCa by 39.5 ± 2.8% (n = 5). The magnitudes of inhibition of IpCa by baclofen are comparable with that observed in their absence (38.0 ± 3.8%, n = 6). These results suggest that the presynaptic inhibitory effect of baclofen is mediated mainly by a membrane-delimited pathway, rather than via intracellular cyclic nucleotides.
Effects of Botulinum Toxin C1 on the Baclofen-Induced IpCa Inhibition.
It has been reported that cleaving syntaxin 1A with botulinum toxin C1 blocks (18) or attenuates (19) G protein modulation of N-type Ca2+ channels. We examined whether botulinum toxin C1 might affect the baclofen-induced inhibition of IpCa. Preincubation of slices with botulinum toxin C1 (332 nM) for 3–4 h had no significant effect on the magnitude of inhibition of IpCa by baclofen (20 μM, 31.8 ± 5.6%, n = 6, data not shown). When botulinum toxin C1 was included in whole-cell pipette (286 nM), no appreciable change was observed for the amplitude and kinetics of IpCa, and baclofen inhibited IpCa to a similar extent for at least 40 min after rupture. Thus, it remains undetermined whether syntaxin plays a role in G protein modulation of presynaptic P/Q-type Ca2+ channels.
Presence of G Proteins at the Calyx of Held Nerve Terminal.
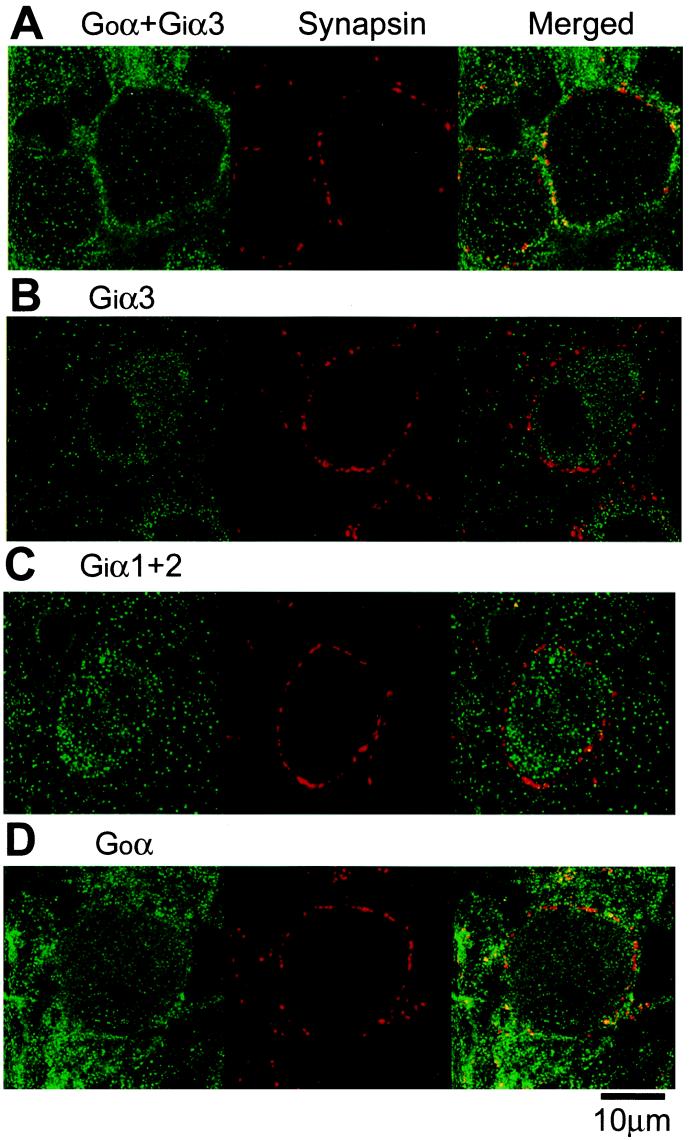
Baclofen-induced presynaptic inhibition can be blocked by externally applied N-ethylmaleimide (4). When N-ethylmaleimide (1 mM) was included in pipettes, baclofen (20 μm) only marginally inhibited IpCa (by 5.2 ± 1.2%, n = 4, data not shown). These suggest that the Gi or Go mediates the presynaptic inhibitory effect of baclofen. However, it is not known which type of G protein is expressed at the nerve terminal. The large size of this nerve terminal is advantageous for the immunocytochemical identification of the presynaptic molecules expressed there. We stained the calyces with antibodies specific for different G protein subtypes together with antibody against synapsin I as a nerve terminal marker. As shown in Fig. 4A, an antibody that binds both Goα and Giα3 stained calyces and postsynaptic MNTB cells, with its immunofluorescence substantially overlapped with that of an anti-synapsin I antibody. By contrast, an antibody specific for Giα3 stained mainly postsynaptic MNTB cells, with little overlap with the synapsin immunoreactivity (Fig. 4B). Similarly, antibody that recognizes both Giα1 and Giα2 stained only the postsynaptic cells sparing calyces (Fig. 4C). In contrast, an antibody specific for Goα clearly stained both calyces and postsynaptic cells (Fig. 4D). These results indicate that the main Gi family protein expressed at the calyx of Held nerve terminal is the Go subtype, whereas both Gi and Go are expressed at the postsynaptic MNTB cell.
Figure 4.
Immunocytochemical localization of Gi family at the calyx of Held nerve terminal. Distributions of immunoreactivities for Gi and/or Go subtypes (labeled green with Alexa fluor 488) and the presynaptic protein synapsin (labeled red with Alexa fluor 568) and overlaid (yellow). Immunofluorescence stainings were made with anti-[Goα + Giα3] (A), anti-Giα3 (B), anti-[Giα1 + α2] (C), and anti-Goα (D) antibodies.
Discussion
The observation that GABA depresses Ca2+ currents in chick dorsal root ganglion cells suggested that a similar mechanism might operate at the nerve terminal (20). Subsequent studies have been carried out on transmitter-induced modulation of Ca2+ channels expressed in the cell soma. In soma of sympathetic neurons, a membrane-delimited mechanism was shown to be the major pathway for the inhibition of N-type Ca2+ channel currents by heterotrimeric G proteins (5). In experiments using overexpressed Gβγ (21–24), Gβγ-binding peptide (23, 25), or Gα (26) in culture cells, this mechanism has been shown to involve Gβγ subunits. Similarly, in culture cells, recombinant P/Q-type Ca2+ channel currents can be attenuated by injection or overexpression of Gβγ subunits (21), and their inhibition by GTP[γS] can be reversed by Gβγ-binding peptides (27). Despite the wealth of information accumulated on somatic Ca2+ currents, there had been no direct evidence indicating that subunits of heterotrimeric G proteins attenuate presynaptic Ca2+ currents. In the present study, we loaded heterotrimeric G protein subunits directly into a mammalian giant nerve terminal, the calyx of Held, and demonstrated that Gβγ attenuates presynaptic Ca2+ currents. GTP[γS]-bound Goα subunit similarly loaded into the nerve terminal had no effect. This effect of Gβγ mimicked and occluded the effects of the GABAB receptor agonist baclofen. At this synapse, baclofen inhibits presynaptic Ca2+ currents (3, 4), and this effect fully explains its inhibitory effect on transmitter release (3), despite other mechanisms postulated at other synapses (1). Our present results indicate that presynaptic inhibition via GABAB receptor is mediated mainly by Gβγ at this mammalian central synapse.
At the calyx-type nerve terminal in chick ciliary ganglia, both the Go and Gi subclasses are expressed together with other G proteins (28). Surprisingly, however, the calyx of Held terminal expressed only Go, whereas the postsynaptic cell expressed both Go and Gi. These results suggest that GABAB receptors are coupled with Goα (but not Giα) and that its partner, Goβγ, inhibits presynaptic P/Q-type Ca2+ channels at this synapse. This is consistent with the hypothesis that Go-type G proteins (but not Gi-type) are involved in the membrane-delimited inhibition of Ca2+ currents (29–31). It has been reported that Giα (not Goα) inhibits adenylyl cyclase (6). Our results also indicate that cyclic nucleotides are not involved in the baclofen-induced inhibition of presynaptic Ca2+ currents. It has previously been reported that Gi (but not Go) activates the G protein-activated inwardly rectifying potassium current (GIRK) (32). At the calyx of Held synapse, baclofen does not activate GIRK, but photo-released GTP[γS] from caged compound can activate it (3). It is possible that Goα fully activated by GTP[γS] might activate GIRK. It is also possible that Giα is expressed to a small extent at the calyx terminal and can activate GIRK after strong stimulation by GTP[γS], although it cannot couple GABAB receptors with GIRK.
An involvement of a phospholipase C-dependent pathway has been suggested for the inhibition of P/Q-type Ca2+ currents by mGluR7 in cerebellar granule cell soma (33). The calyx of Held terminal expresses mGluR4 and also mGluR7 at later stages of development (34), and presynaptic inhibition by an mGluR agonist is mediated by inhibition of presynaptic P/Q-type Ca2+ currents (2). This effect of mGluR agonist can be completely occluded by baclofen (Y.K. and T.T., unpublished observation), suggesting that the presynaptic inhibitory mechanism via mGluRs and GABAB receptors may be identical. Also, at this synapse, activation of protein kinase C by phorbol ester has no effect on presynaptic Ca2+ currents (8). Thus, the Goβγ seems to play essential roles in autoreceptor- and heteroreceptor-mediated presynaptic inhibition at the calyx of Held synapse. At this synapse, a general block of G protein activity using GDP[βS] slows the rate of recovery from activity-dependent synaptic depression by blocking vesicle trafficking (7). It remains to be seen whether Goβγ is involved in the vesicular replenishment at this mammalian nerve terminal.
Inhibition of Ca2+ currents by G proteins is characterized by a slowing in the rate of activation and the relief of inhibition following large depolarization, which has been explained by proposing a shift of Ca2+ channel gating between “reluctant” and “willing” states (13). These properties were also observed for the inhibitory effects of Gβγ and baclofen on presynaptic P/Q-type Ca2+ currents. It is possible that presynaptic inhibition by means of G protein-coupled receptors is relieved during high-frequency transmission because of presynaptic action potentials (35). At the calyx of Held synapse, presynaptic Ca2+ currents undergo facilitation during repetitive activation (36, 37). This facilitation depends on Ca2+ influx and can be induced by a 1-ms depolarizing conditioning pulse from −80 mV to −10 mV but is not affected by GDP[βS] or GTP[γS], excluding an involvement of G proteins in its mechanism (36). In fact, much stronger depolarization is required for relieving IpCa from G protein-dependent inhibition. Thus, the physiological significance of the depolarization-induced relief of Ca2+ currents from G protein-dependent inhibition remains to be determined.
Acknowledgments
We thank Bertil Hille, Katsunori Kobayashi, David Saffen, and Tetsuhiro Tsujimoto for comments on the manuscript. This work was supported by the Research for the Future Program by The Japan Society for the Promotion of Sciences.
Abbreviations
- GABAB receptor
γ-aminobutyric acid type B receptor
- GDP[γS]
guanosine 5′-O-(2-thiodiphosphate)
- GTP[γS]
guanosine 5′-O-(3-thiotriphosphate)
- Gβγ
G protein βγ subunits
- MNTB
medial nucleus of the trapezoid body
- GIRK
G protein-activated inwardly rectifying potassium current
- mGluR
metabotropic glutamate receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wu L-G, Saggau P. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Forsythe I D, Tsujimoto T, Barnes-Davies M, Onodera K. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Kajikawa Y, Tsujimoto T. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaacson J S. J Neurophysiol. 1998;80:1571–1576. doi: 10.1152/jn.1998.80.3.1571. [DOI] [PubMed] [Google Scholar]
- 5.Hille B. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 6.Wickman K, Clapham D E. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Hori T, Kajikawa Y, Tsujimoto T. Science. 2000;289:460–463. doi: 10.1126/science.289.5478.460. [DOI] [PubMed] [Google Scholar]
- 8.Hori T, Takai Y, Takahashi T. J Neurosci. 1999;19:7262–7267. doi: 10.1523/JNEUROSCI.19-17-07262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsythe I D, Tsujimoto T, Barnes-Davies M, Cuttle M F, Takahashi T. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki S, Takahashi T. J Physiol (London) 2000;509:419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda S R. J Physiol (London) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai H. J Physiol (London) 1992;448:189–209. doi: 10.1113/jphysiol.1992.sp019036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bean B P. Nature (London) 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 14.Diverse-Pierluissi M, Remmers A E, Neubig R R, Dunlap K. Proc Natl Acad Sci USA. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaum S R, Miller R J. Neuropharmacology. 1995;34:953–964. doi: 10.1016/0028-3908(95)00076-i. [DOI] [PubMed] [Google Scholar]
- 16.Meriney S D, Gray D B, Pilar G R. Nature (London) 1994;369:336–339. doi: 10.1038/369336a0. [DOI] [PubMed] [Google Scholar]
- 17.Adams S R, Harootunian A T, Buechler Y J, Taylor S S, Tsien R Y. Nature (London) 1991;349:694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- 18.Stanley E F, Mitroznik R R. Nature (London) 1997;385:340–343. doi: 10.1038/385340a0. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis S E, Magga J M, Beedle A M, Braun J E A, Zamponi G W. J Biol Chem. 2000;275:6388–6394. doi: 10.1074/jbc.275.9.6388. [DOI] [PubMed] [Google Scholar]
- 20.Dunlap K, Fischbach G D. J Physiol (London) 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herlitz S, Garcia D E, Mackie K, Hille B, Scheuer T, Catterall W A. Nature (London) 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda S R. Nature (London) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 23.Delmas P, Brown D A, Dayrell M, Abogadie F C, Caulfield M P, Buckley N J. J Physiol (London) 1998;506:319–329. doi: 10.1111/j.1469-7793.1998.319bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Velasco V, Ikeda S R. J Neurosci. 2000;20:2183–2191. doi: 10.1523/JNEUROSCI.20-06-02183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delmas P, Abogadie F C, Dayrell M, Haley J E, Milligan G, Caulfield M P, Brown D A, Buckley N J. Eur J Neurosci. 1998;10:1654–1666. doi: 10.1046/j.1460-9568.1998.00170.x. [DOI] [PubMed] [Google Scholar]
- 26.Jeong S-W, Ikeda S R. J Neurosci. 1999;19:4755–4761. doi: 10.1523/JNEUROSCI.19-12-04755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herlitze S, Hockerman G H, Scheuer T, Catterall W A. Proc Natl Acad Sci USA. 1997;94:1512–1516. doi: 10.1073/pnas.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirotznik R R, Zheng X, Stanley E F. J Neurosci. 2000;20:7614–7621. doi: 10.1523/JNEUROSCI.20-20-07614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Nature (London) 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 30.Campbell V, Berrow N, Dolphin A C. J Physiol (London) 1993;470:1–11. doi: 10.1113/jphysiol.1993.sp019842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caulfield M P, Jones S, Vallis Y, Buckley N J, Kim G-D, Milligan G, Brown D A. J Physiol (London) 1994;477:415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takano K, Yasufuku-Takano J, Kozasa T, Nakajima S, Nakajima Y. J Physiol (London) 1997;502:559–567. doi: 10.1111/j.1469-7793.1997.559bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perroy J, Prezeau L, De Waard M, Shigemoto R, Bockaert J, Fagni L. J Neurosci. 2000;20:7896–7904. doi: 10.1523/JNEUROSCI.20-21-07896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elezgarai I, Benitez R, Mateos J M, Lazaro E, Osorio A, Azkue J J, Bilbao A, Lingenhoehl K, van der Putten H, Hampson D R, et al. J Comp Neurol. 1999;411:431–440. doi: 10.1002/(sici)1096-9861(19990830)411:3<431::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 35.Bertram R, Behan M. J Comput Neurosci. 1999;7:197–211. doi: 10.1023/a:1008976129832. [DOI] [PubMed] [Google Scholar]
- 36.Cuttle M F, Tsujimoto T, Forsythe I D, Takahashi T. J Physiol (London) 1998;512:723–729. doi: 10.1111/j.1469-7793.1998.723bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borst J G G, Sakmann B. J Physiol (London) 1998;513:149–155. doi: 10.1111/j.1469-7793.1998.149by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]