Abstract
The aim of this pilot study was to assess the correlation between activation of the innate immune system and metabolic syndrome (MetSyn), independent of BMI, in a young population. We quantitatively measured both systemic pro-inflammatory cytokines and gene expression of Toll-like receptors and downstream cytokines in circulating monocytes obtained from nine adolescents with metabolic syndrome (Overwt-MetSyn) and 8 BMI-matched controls (Overwt-Healthy). The Overwt-MetSyn group demonstrated a significant elevation in expression of TLR2, TLR4, TNFα and IL6 in peripheral monocytes, and increased circulating levels of TNFα and IL6 when compared to the Overwt-Healthy group. TLR2 (r = 0.78, P < 0.001), TLR4 (r = 0.57, P < 0.01) and TNFα (r=0.61, P < 0.01) gene expression positively correlated with serum levels of TNFα. Our study suggests that activation of the innate immune pathway via TLRs may be partially responsible for the increased systemic inflammation seen in adolescents with MetSyn.
Keywords: innate immunity, adolescents, monocytes, inflammation, obesity, metabolic syndrome
Metabolic syndrome (MetSyn) is a collection of risk factors that are associated with increased risk for the development of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) (1,2). MetSyn affects approximately 30% of the 11 million U.S. adolescents who are overweight or obese (3,4). Obesity is characterized by increased levels of the pro-inflammatory cytokines tumor necrosis factor-α (TNFα) and interleukin-6 (IL6), which predict the development of T2DM [5,6]. Innate immune cells, particularly macrophages, are a major source of cytokine production and secretion [7-9]. However, recent studies suggest that activated peripheral blood monocytes (macrophage precursors) may contribute to systemic inflammation [10]. In these cells, activation of Toll-like receptor (TLR) signal transduction cascades leads to cytokine production and secretion. TLR2 and TLR4 play a key role in the pathogenesis of T2DM by virtue of their responsiveness to lipopolysaccharide and saturated fatty acids released from hypertrophied adipocytes [11,12]. Disruption of TLR2 and TLR4 signaling protects against high-fat diet-induced inflammation and insulin resistance in rodent models [13,14], suggesting that TLR2 and TLR4 are important mediators in diet induced metabolic diseases.
Several clinical studies provide evidence supporting a role of TLRs in adults with type 1 diabetes [15] and metabolic syndrome [16]; however, there is a lack of data examining the activity of TLRs in adolescents with obesity and metabolic syndrome. Importantly, whether enhanced TLR signaling is associated with MetSyn, or simply obesity, in adolescents is not completely understood. In this pilot study, we explore this question by examining the expression of TLRs and inflammatory markers in overweight adolescents with and without metabolic syndrome. We hypothesize that adolescents with metabolic syndrome will have a higher expression of TLRs and inflammatory markers than their overweight peers without metabolic syndrome.
Adolescents between the ages of 15-19 years were recruited from the University of Massachusetts Worcester and Boston campuses. Persons with a prior diagnosis of diabetes mellitus, inflammatory disease, thyroid disease, hypercortisolism and those receiving medications that may affect our outcome parameters (metformin, lipid lowering medications, anti-hyperglycemic agents, steroids) were excluded. Overweight was defined as body mass index (BMI) ≥ 85th percentile for age and gender according to the CDC guidelines [17]. Participants were classified as Overwt-MetSyn using a modified definition proposed by deFerranti et al. [4] as the presence of three or more of the following risk determinants: increased waist circumference (> 75th percentile for age, gender, ethnicity), low serum concentrations of fasting high density lipoprotein (HDL) cholesterol (< 50 mg/dL females, < 40 mg/dL males), elevated fasting glucose (>100 mg/dL), elevated systolic blood pressure (BP) (>90th percentile for age and gender) and elevated fasting triglycerides (>100 mg/dL). Overwt-Healthy participants had ≤ 2 components of MetSyn. All participants provided written informed consent (and assent when appropriate) and the study was approved by the University of Massachusetts Medical School and the University of Massachusetts Boston Institutional Review Boards.
Clinical measurements including body weight, height, BP, and waist circumference were obtained in all participants using standard measurement protocols. A 20 ml sample of whole blood was obtained via venipuncture in the fasting state. Blood samples were collected in Cell Preparation Tubes (Becton Dickinson, New Jersey) which contain anti-coagulant. Plasma obtained without anti-coagulant served as a standard for determining the dilution produced by the anticoagulant. The lipid results were multiplied by a factor of 1.3 to correct for sample dilution. Complete blood count, lipid, lipoprotein profile, and glucose were assayed by standard laboratory techniques. Circulating concentrations of TNFα and IL6 were measured using a high sensitivity enzyme-linked immunosorbent assay (R&D Systems, Minneapolis). Blood mononuclear cells were isolated via centrifugation, and untouched primary monocytes were further isolated from the mononuclear fraction using a negative selection kit (Miltenyi Biotec, Germany).
RNA was isolated from monocytes according to the QIAGEN MiniPrep protocol. cDNA from total RNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). Real-time PCR to quantify expression of key inflammatory mediators (TLR2, TLR4, TNFα, IL6) was performed using SybrGreen assays according to the manufacturer’s instructions (Bio-Rad Laboratories). Expression of TLR5 was also measured to assess a receptor that is expressed on the monocyte but not involved in diet and metabolism. Expression of specific mRNAs was quantified in duplicate samples on an iCycler IQ Real-Time PCR detection system (Bio-Rad Laboratories) using the ΔΔCT method with normalization to cycle threshold measurements for glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Baseline differences between the Overwt-Healthy and Overwt-MetSyn groups were evaluated using the Student’s t-test for continuous variables and Fisher’s exact test for categorical values. Log transformations were used to normalize variables with positively skewed distributions. Bivariate analyses were performed using Pearson correlation coefficient. Using data from a prior study in adults (10) we determined that a sample size of nine participants per group would be needed to detect a significant difference in TLR gene expression of 80% (i.e. 1.8 fold increase in gene expression in monocytes from adolescents with MetSyn vs. adolescents without MetSyn ) with 90% power at the 5% level of probability. Data are presented as mean ± SEM unless otherwise specified. Statistical significance was defined as P < 0.05.
Seventeen adolescents (three boys and fourteen girls) participated in the study. Nine (53%) of the participants met the criteria for metabolic syndrome (Overwt-MetSyn) and the other 8 were healthy (Overwt-Healthy). There were no significant differences between the Overwt-MetSyn and Overwt-Healthy groups in age, gender, ethnicity, or BMI (Table). Total white blood cell counts, cholesterol, LDL, and glucose were similar between the 2 groups. As expected, participants in the Overwt-MetSyn group displayed higher levels of triglycerides when compared to their healthy weight-matched peers. The Overwt-MetSyn group had, on average, larger waist circumference and lower HDL levels than Overwt-Healthy subjects, but this difference did not reach statistical significance. They also exhibited a higher absolute number of monocytes, and increased circulating levels of TNFα and IL6.
TABLE.
Anthropometric and laboratory data of study participants
| Overwt MetSyn (n=9) |
Overwt Healthy (n=8) |
|
|---|---|---|
| Females/males | 9/0 | 5/3 |
| Age (yr) | 16.4 ± 0.4 | 16.8 ± 0.4 |
| Ethnic group, No. (%) | ||
| African American | 5 (56) | 6 (76) |
| Caucasian | 1 (11) | 1 (12) |
| Hispanic | 3 (33) | 1 (12) |
| BMI (kg/m2) | 37 ± 3 | 31 ± 2 |
| BMI % | 97 ± 1 | 94 ± 2 |
| Waist circumference | 111 ± 7 | 95 ± 5 |
| Systolic BP (mm Hg) | 124 ± 3 | 116 ± 4 |
| Diastolic BP (mm Hg) | 75 ± 4 | 72 ± 3 |
| White blood cell counts (k/uL) | 8 ± 1 | 6 ± 1 |
| Monocytes (%) | 9 ± 1 | 8 ± 1 |
| Absolute monocytes (th/mm3) | 0.6 ± 0.1 a | 0.5 ± 0 |
| Cholesterol (mg/dL) | 146 ± 7 | 148 ± 11 |
| Triglyceride (mg/dL) | 96 ± 9 a | 45 ± 8 |
| HDL (mg/dL) | 46 ± 3 | 57 ± 4 |
| LDL (mg/dL) | 81 ± 6 | 83 ± 8 |
| Fasting glucose (mg/dL) | 97 ± 5 | 91 ± 5 |
| TNFa (pg/mL) | 1.8 ± 0.6a | 0.4 ± 0.1 |
| IL6 (pg/mL) | 3 ± 1a | 1.1 ± 0.3 |
Data presented as mean ± SEM
P < 0.05 compared with overweight healthy
We performed quantitative PCR analysis of TLRs and pro-inflammatory gene expression in circulating monocytes. Monocyte mRNA levels of TLR2 (P <0.01), TLR4 (P < 0.01), TNFα (P < 0.05) and IL6 (P < 0.05) were all significantly elevated in monocytes from the Overwt-MetSyn group when compared to the Overwt-Healthy group (Figure). Expression of TLR5 was similar among the Overwt-Healthy and Overwt-MetSyn groups (data not shown).
FIGURE.
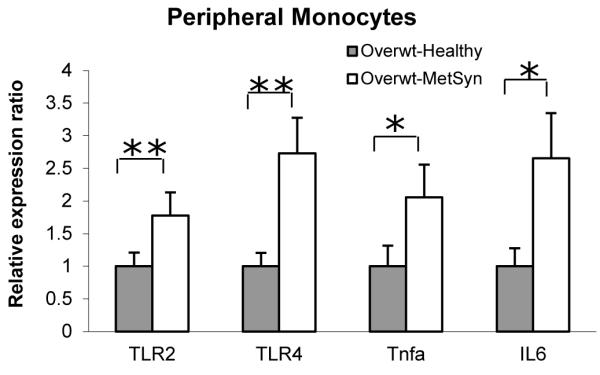
mRNA expression in monocytes of Overwt-Healthy (gray bars) vs Overwt-MetSyn (white bars). Mean value of the relative gene expression of Overwt-Healthy group was taken as 1. Data are represented as mean ± SEM. (*, P < 0.05; **, P < 0.01).
Next, we evaluated whether serum levels of pro-inflammatory cytokines and metabolic parameters correlated with monocyte gene expression. Serum levels of TNFα positively correlated with gene expression of TNFα (r = 0.61, P < 0.01), TLR2 (r = 0.78, P < 0.001) and TLR4 (r = 0.57, P < 0.01) in circulating monocytes. Furthermore, serum triglyceride levels positively correlated with gene expression of TNFα (r = 0.48, P < 0.05). There was also a significant correlation between serum levels of IL6 and TLR4 expression (r = 0.68, P < 0.005). There were no significant correlations between monocyte expression of inflammatory markers and waist circumference (data not shown).
Our pilot study suggests that metabolic syndrome in adolescents is associated with increased activation of the TLR signaling pathway within monocytes. Our correlational analyses revealed a positive association between serum cytokine levels, and the expression of TLR2 and TLR4 as well as TNFα in monocytes from adolescents with metabolic syndrome. These findings suggest that monocytes may be a source of the circulating cytokines that are frequently elevated in obesity and contribute to associated co-morbidities, even in a young population.
Previous studies have described a positive association between TLR signaling and metabolic dysfunction in adults. Patients with metabolic syndrome and newly diagnosed T2DM demonstrate significantly increased TLR2 and TLR4 gene expression in monocytes compared with healthy human subjects [10, 16]. Even monocytes from normal weight adults with central obesity have increased expression of TNFα and IL6 compared to normal weight adults with normal fat distribution [18]. While most studies exploring innate immunity, obesity and metabolic dysfunction have been conducted in adults, one recent study conducted in children validates a positive correlation between the expression of adipokine genes in adipose tissue and cytokines in peripheral mononuclear blood cells [19]. Our observations are consistent with prior studies and add to the existing literature by identifying the presence of TLR activation in a young pre-diabetic population.
TLRs are a family of pattern recognition receptors expressed on macrophages and monocytes whose activation initiates a signaling cascade resulting in downstream cytokine production. Our correlative studies suggest that the amplified inflammation common in metabolic syndrome may be partially mediated by activation of TLR pathways on monocytes, providing supporting rationale for studying monocytes in this population. TLRs, particularly TLR2 and TLR4, have multiple endogenous ligands including endotoxin and free fatty acids. Identification of the specific ligand responsible for the TLR activation seen in our population requires further study.
The present study has several limitations including small sample size, lack of data on insulin sensitivity and inability to identify a direct causal relationship between TLR expression and elevated levels of circulating cytokines. The major strength of our study is the inclusion of BMI-matched overweight healthy subjects. This is significant since it allows for the identification of altered pathways that are directly related to metabolic dysfunction and not simply a feature of the weight status. We were also able to study the influence of a pure population of monocytes separate from other mononuclear cells.
In conclusion, our data show that activation of the innate immune pathway via TLRs may be partially responsible for the increased systemic inflammation seen in MetSyn. The identification of TLR signaling activation in monocytes of adolescents with metabolic syndrome advances our understanding of the molecular pathways responsible for metabolic dysfunction in the early stages of obesity. A comprehensive analysis of the TLR signaling pathway and downstream products warrants further studies in this population.
Acknowledgments
This project was supported by the UMass Center for Clinical and Translational Science (Award Number UL1RR031982) from the National Center for Research Resources (NCRR), the Life Sciences Moment Fund, and a pilot Grant supported by the Diabetes Endocrinology Research Center grant DK32520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the National Institutes of Health.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002 Dec 4;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 2.Hanson RL, Imperatore G, Bennett PH, et al. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002 Oct;51(10):3120–7. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 3.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004 Feb;4(1):53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- 4.de Ferranti SD, Gauvreau K, Ludwig DS, et al. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004 Oct 19;110(16):2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 5.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001 Jul 18;286(3):327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 6.Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008 Aug;93(8):3165–72. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006 Dec 14;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003 Dec;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasu MR, Devaraj S, Park S, et al. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010 Apr;33(4):861–8. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. PMID: 17082484. [DOI] [PubMed] [Google Scholar]
- 12.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007 Jun 8;100(11):1589–96. doi: 10.1161/CIRCRESAHA.106.142851. PMID: 17478729. [DOI] [PubMed] [Google Scholar]
- 13.Davis JE, Braucher DR, Walker-Daniels J, et al. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem. 2011 Feb;22(2):136–41. doi: 10.1016/j.jnutbio.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006 Nov;116(11):3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaraj S, Dasu MR, Rockwood J, et al. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008 Feb;93(2):578–83. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jialal I, Huet BA, Kaur H, et al. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012 Apr;35(4):900–4. doi: 10.2337/dc11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National Center for Health Statistics CDC growth charts: United States. 2000 May 30; http://www.cdc.gov/growthcharts/ Web. August 01, 2011.
- 18.Hermsdorff HH, Puchau B, Zulet MA, et al. Association of body fat distribution with proinflammatory gene expression in peripheral blood mononuclear cells from young adult subjects. OMICS. 2010 Jun;14(3):297–307. doi: 10.1089/omi.2009.0125. [DOI] [PubMed] [Google Scholar]
- 19.Dedoussis GV, Kapiri A, Samara A, et al. Expression of inflammatory molecules and associations with BMI in children. Eur J Clin Invest. 2010 May;40(5):388–92. doi: 10.1111/j.1365-2362.2010.02277.x. [DOI] [PubMed] [Google Scholar]


