Abstract
Background
Previous research shows that numerous child, parent, and procedural variables affect children’s distress responses to procedures. Cognitive-behavioral interventions such as distraction are effective in reducing pain and distress for many children undergoing these procedures.
Objectives
The purpose of this report was to examine child, parent, and procedural variables that explain child distress during a scheduled intravenous insertion when parents are distraction coaches for their children.
Methods
A total of 542 children, between 4 and 10 years of age, and their parents participated. Child age, gender, diagnosis, and ethnicity were measured by questions developed for this study. Standardized instruments were used to measure child experience with procedures, temperament, ability to attend, anxiety, coping style, and pain sensitivity. Questions were developed to measure parent variables, including ethnicity, gender, previous experiences, and expectations, and procedural variables, including use of topical anesthetics and difficulty of procedure. Standardized instruments were used to measure parenting style and parent anxiety, whereas a new instrument was developed to measure parent performance of distraction. Children’s distress responses were measured with the Observation Scale of Behavioral Distress–Revised (behavioral), salivary cortisol (biological), Oucher Pain Scale (self-report), and parent report of child distress (parent report). Regression methods were used for data analyses.
Results
Variables explaining behavioral, child-report and parent-report measures include child age, typical coping response, and parent expectation of distress (p < .01). Level of parents’ distraction coaching explained a significant portion of behavioral, biological, and parent-report distress measures (p < .05). Child impulsivity and special assistance at school also significantly explained child self-report of pain (p < .05). Additional variables explaining cortisol response were child’s distress in the morning before clinic, diagnoses of attention deficit hyperactivity disorder or anxiety disorder, and timing of preparation for the clinic visit.
Discussion
The findings can be used to identify children at risk for high distress during procedures. This is the first study to find a relationship between child behavioral distress and level of parent distraction coaching.
Keywords: children, cortisol, distress, distraction, pain
Management of children’s health includes medical procedures that may be painful or, at a minimum, stressful to the child (Weisman, Bernstein, & Schechter, 1998). Children who experience inadequate pain control during medical procedures can suffer immediate and long-term negative sequelae (von Baeyer, Marche, Rocha, & Salmon, 2004; Zempsky & Schechter, 2003). There is now sufficient evidence to conclude that cognitive-behavioral interventions are effective in reducing pain and distress for many children undergoing these procedures (Kleiber & Harper, 1999; Uman, Chambers, McGrath, & Kisely, 2008).
Distraction is a cognitive-behavioral intervention that diverts attention from a negative, uncomfortable stimulus and focuses the individual’s attention on nonstressful, pleasant stimuli. Immediate benefits are decreased distress behaviors and increased ease of the procedure (Kleiber & Harper, 1999; Uman et al., 2008). In addition, evidence suggests that distraction buffers children’s memories of painful procedures; they may remember less of the negative aspects of the procedure, which affects their response to later painful events and interventions (Brown et al., 1999; Cohen et al., 2001; Salmon & Pereira, 2002; Salmon, Price, & Pereira, 2002). Previous research has identified numerous variables that influence children’s responses to painful procedures. Identifying which of these factors best explain children’s risk for distress will assist in determining the level of distraction intervention that will best decrease an individual child’s distress.
A randomized controlled trial (RCT) was carried out to study the impact of parent-provided distraction on the distress responses of children, 4 to 10 years of age. The purpose of this RCT was to identify factors that explain which children benefit from a cognitive-behavioral intervention (distraction) when parents coach their children to alleviate the child’s distress during a medical procedure (intravenous insertion). The RCT had two specific aims. The first aim was to determine the effectiveness of training parents to be distraction coaches during a medical procedure (intravenous insertion) by comparing the responses of parents and children who receive the training (intervention group) with those who did not receive the training (control group). Families were assigned randomly to groups at three participating sites. The RCT Aim 1 results found that children in the intervention and control groups did not differ on three of four measures of child distress. However, contamination between groups occurred; some parents in the intervention group were unable to provide distraction, and some parents in the control group provided distraction without receiving the training. The quality and the quantity of parent distraction coaching were then evaluated, and parent and child dyads were regrouped on the basis of the level of parent distraction coaching. Children of parents who provided the highest level of distraction coaching displayed less behavioral distress (p = .017), less cortisol responsivity (p = .003), and lower parent reports of distress (p = .092; McCarthy et al., 2010).
The purpose of this article is to present the results of Aim 2 from this RCT to examine concurrently child, parent, and procedural variables that explain children’s distress responses to a medical procedure. Children’s distress is measured from multiple perspectives, including behavioral, biological, self-report, and parent report. The goal of this program of research is to develop a clinically useful instrument that quickly identifies the appropriate intervention needed by a child about to undergo a medical procedure.
Background
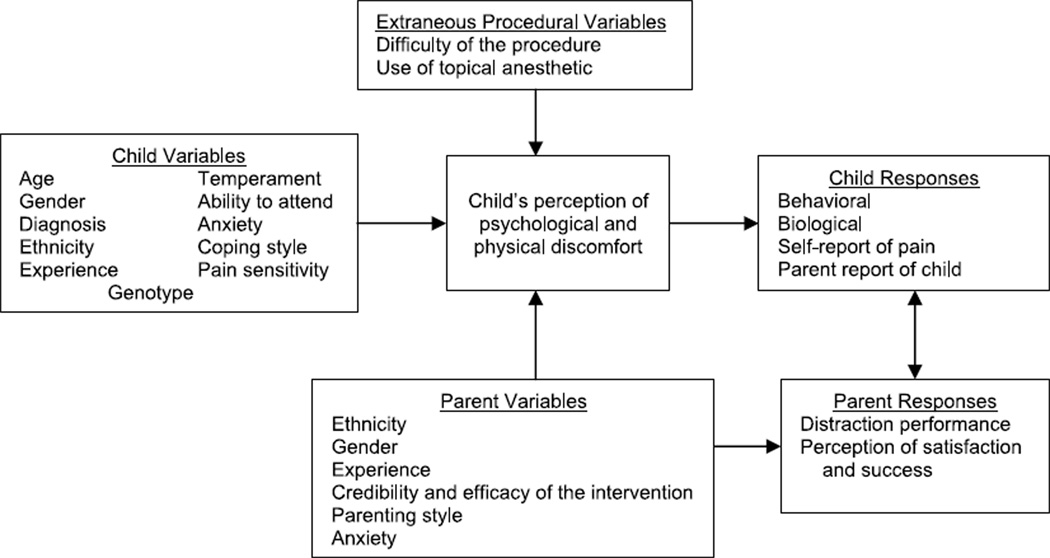
Numerous studies were found examining the effects of one or two factors shown to affect children’s responses to painful procedures, such as child age and gender, but few studies had a sample size adequate to examine multiple variables. This study was planned to measure all potential explanatory variables concurrently to determine the best combination of variables that explain child distress. The conceptual model, Child Response to Medical Procedures When Distraction Is Provided by a Parent (Figure 1), includes child, parent, and procedural variables reported in the literature as associated with child distress (McCarthy & Kleiber, 2006).
FIGURE 1.
Model for child response to medical procedures when distraction is provided by a parent. Measures are described in SDC 1. From “A conceptual model of factors influencing children’s responses to a painful procedure when parents are distraction coaches” by A. M. McCarthy and C. Kleiber, 2006, Journal of Pediatric Nursing, 21(2), p. 89. Copyright 2006 by the Journal of Pediatric Nursing. Adapted with permission of the author.
Child Variables
The original conceptual model was built on research studies identifying child age, gender, experience, temperament, anxiety, coping style, pain sensitivity, and genotype as influencing response to a medical procedure. Younger children display more behavioral distress and report more pain with medical procedures (Bournaki, 1997; Gagliese & Katz, 2003; Goodenough et al., 1999). Girls display more crying and clinging behavior (Rudolph, Dennig, & Weisz, 1995) and more dependent coping with support seeking and less stoicism (Walker, Baber, Garber, & Smith, 2008). The quality of previous painful experiences rather than the quantity is the more important determinate of future response to procedures (von Baeyer et al., 2004). Children who have a history of being very distressed with previous procedures display increased distress behaviors during a subsequent procedure (Frank, Blount, Smith, Manimala, & Martin, 1995; Kleiber, Craft-Rosenberg, & Harper, 2001). Difficult temperament (Broome, Rehwaldt, & Fogg, 1998; Corbo-Richert, 1994; Lee & White-Traut, 1996; Merritt, Ornstein, & Spicker, 1994) and elevated preprocedural state anxiety (Claar, Walker, & Smith, 2002; Kleiber, Sorenson, Whiteside, Gronstal, & Tannous, 2002) also have been associated with increased distress during a painful procedure in children.
Child preparation for the procedure and a pain-sensitive temperament may influence child response to a procedure. Although the timing of preparation has not been explored thoroughly, a review by Jaaniste, Hayes, and von Baeyer (2007) suggests that young children do not remember information given too far in advance, but giving information too close to an event does not allow the child time to process the information. There is some evidence that sensitivity to physical stimulation, such as being bothered by bright light or loud noise, contributes to children’s report of pain intensity of pain postoperatively (Kleiber, Suwanraj, Dolan, Berg, & Kleese, 2007) and during invasive procedures (Chen, Craske, Katz, Schwartz, & Zeltzer, 2000). Further, genetic research on pain as a complex trait is beginning to identify the role of an individual’s genotype on pain perception and response (Mogil, 2004).
In addition to those variables identified from the previous studies, we collected information on the child’s ethnicity, medical diagnoses, history of invasive procedures, and timing of child preparation for the procedure. Because the success of the distraction intervention depends upon capturing and maintaining the child’s attention, we also measured children’s ability to attend to a task.
Parent Variables
Research findings are mixed on how characteristics of parents affect the child’s response to medical procedures. Some researchers reported a gender interaction in how parents respond to their child in distress (Kankkunen, Vehviläinen-Julkunen, Pietilä, & Halonen, 2003), but others found no such interaction (Moon et al., 2008). Parents’ expectations about painful procedures, including their beliefs about the effectiveness of cognitive-behavioral interventions such as distraction, may affect how the child responds (Liossi, White, Franck, & Hatira, 2007). Parenting style may influence child response during a medical procedure. Dahlquist, Power, Cox, and Fernbach (1994) reported that the children of parents who set fewer rules or were less consistent or organized were more anxious during bone marrow or lumbar puncture. Children with chronic pain, whose parents either discounted their symptoms or were overprotective or critical, had more somatic symptoms (Claar, Simons, & Logan, 2008). Lastly, parent trait anxiety may be associated with child distress during a medical procedure (Jacobsen et al., 1990). In addition to variables identified from previous studies, we collected information on the parent’s ethnicity and experience with using distraction.
Parent Response: Use of Distraction
Although not discussed or measured in other studies, preliminary work by this research team suggested that parents’ performance of distraction coaching for their children may influence child distress. Therefore, an instrument, the Distraction Coaching Index (DCI), was developed for this study to measure the quality and quantity of parent distraction (Kleiber, McCarthy, Hanrahan, Myers, & Weathers, 2007) and included as an explanatory variable in this aim.
Extraneous Procedural Variables
Children who are pretreated with a topical anesthetic, such as EMLA (AstraZeneca, Wilmington, DE) or LMX 4 (Ferndale Laboratories, Inc., Ferndale, MI), before a needle stick generally display less distress and report less discomfort (Taddio, Soin, Schuh, Koren, & Scolnik, 2005). For some children, however, topical anesthetics fail to provide a pain-free needle stick, even when the duration of the application is well controlled (Kleiber et al., 2002). Although no research was found on the topic, difficulty with cannulating the vein is likely to influence child distress. Although the cutaneous tissue might be anesthetized, prolonged maneuvering with the intravenous needle is most likely stressful for the child.
Method
Design
This explanatory study used data from a cross-sectional multisite RCT that tested the impact of training parents in the use of distraction on children’s distress responses (McCarthy et al., 2010). Data from the control and the intervention groups were collapsed for this explanatory analysis. Genotype data were not included in these analyses. Although genetic variability likely contributes to a child’s distress response, it is not yet feasible to incorporate this information into a predictive profile. Genotype–phenotype analyses will be presented in a separate article.
Participants
English-speaking families were recruited from three Midwestern Children’s Hospitals in the United States. Each family had a developmentally typical child 4 to 10 years of age undergoing a scheduled intravenous insertion for a diagnostic medical procedure. One parent or guardian from each family agreed to participate as the child’s support person. The institutional review boards (IRBs) at each data collection site approved this study.
Instruments
Standardized instruments and questionnaires developed for this study were used to collect data on child, parent, and procedural variables and four outcome measures of child distress. Pilot testing of the instruments is described elsewhere (Kleiber & McCarthy, 2006). The instruments used to measure each potential explanatory variable in the model, Child Response to Medical Procedures When Distraction Is Provided by a Parent, are listed below (see Table, Supplemental Digital Content 1, which provides detailed descriptions, http://links.lww.com/NRES/A36).
Potential Explanatory Variables
The instruments used to measure child variables are as follows: Child Demographic Questionnaire (e.g., age, gender, diagnosis, and ethnicity), Severity of Illness Scale, Perception of Procedures Questionnaire (PPQ), questions ascertaining the number of painful medical procedures and timing of preparation for the procedure, Dimensions of Temperament Scale–Revised, Pediatric Behavior Scale, Children’s Anxiety Meter, Child Behavior Style Scale, Typical Coping, Preferred Coping Style, and Sensitivity Temperament Inventory for Pain–Child version. Parent variables were measured with the Parent Demographic Questionnaire (e.g., ethnicity, gender, and parent experience and expectations), the Parenting Dimensions Inventory, and the State-Trait Anxiety Inventory. Parent use of distraction (i.e., quantity and quality of parent distraction) was measured using the DCI. Dwell time of the topical anesthetic and procedural difficulty questions were used to measure extraneous procedural variables.
Outcome Measures
Child responses to the intravenous insertion were evaluated with four measures: a behavioral assessment of distress (Observation Scale of Behavioral Distress–Revised [OSBD-R]), a biological measure of distress (salivary cortisol), a child self-report of pain (Oucher Pain Scale), and a parent report of child distress (PRCD). Parent responses as an outcome are not included in this analysis.
The OSBD-R was developed and tested by Jay and Elliott (1986) as a behavioral measure of child distress during painful procedures. The scale consists of eight behavioral categories indicative of anxiety or pain in children (e.g., cry, scream, flail, restrain). Time samplings of the child’s behavior are recorded, and each behavioral category is weighted to indicate the intensity of distress (e.g., scream is weighted more than cry). A total distress score is calculated by adding together the weighted values of each of the behaviors at each interval, with higher scores reflecting more distress. Jay and Elliott reported an internal consistency of 0.72; internal consistency for this study was 0.76. In this study, coders trained in the use of the OSBD-R achieved an interrater reliability of 99.3% (intraclass correlation, 95% confidence interval = 0.968–0.999).
Salivary cortisol was used to measure the biological response of the child to the intravenous insertion. Salivary cortisol and serum cortisol measures have demonstrated strong correlations (r = .71 to .96; Kirschbaum & Hellhammer, 1994). Four salivary cortisol samples were obtained from each child to reflect baseline levels and to measure responsivity to the intravenous procedure. Detailed collection procedures and methods for salivary cortisol analysis used in this study are described elsewhere (Hanrahan, McCarthy, Kleiber, Lutgendorf, & Tsalikian, 2006; McCarthy et al., 2009). The coefficient of variation for the data set of 1,120 duplicate samples was 8.18%. Control samples were included in 9 of 10 batches analyzed. The coefficient of variation between controls in each batch was <0.1% to 8.71% and between batches was 13.9% to 15.2%.
The Oucher Pain Scale has been used extensively to assess pain intensity in children ages 3 to 12 years and includes two variations, a numeric 0 to 10 scale and ethnically sensitive picture scales (Aradine, Beyer, & Tompkins, 1988). Correlations with the visual analogue scale (.89; p < .01) and the Hospital Fears Rating and Scare Scale (Gamma coefficients = .003 and .075) have been demonstrated (Aradine et al., 1988; Beyer & Aradine, 1986). In this study, the Oucher Pain Scale was administered to children immediately after the intravenous was taped in place.
The PRCD was measured with one item from the revised PPQ, developed by Kazak, Penati, Waibel, and Blackall (1996). This one-item Likert-type question: “How distressed was your child today during the intravenous procedure?” is anchored on a 7-point scale, with 1 = not at all and 7 = extremely distressed.
Procedures
Children with scheduled appointments requiring an intravenous insertion in an ambulatory care clinic were identified and enrolled according to procedures approved by the IRBs and described elsewhere (McCarthy et al., 2010). Before the clinic visit, a letter explaining the study was sent to eligible families. At the time of the clinic visit, parent consent and child assent for children 7 years of age or older were obtained according to IRB guidelines. A parent or a child could choose to not participate in the videotaping, genetics, and/or cortisol portions of the study.
After consent/assent, children provided a saliva sample for a measure of cortisol. Clinic staff applied a topical lidocaine anesthetic cream to two potential intravenous sites on the child according to the clinic protocol. Parents and children answered study questions during the topical anesthetic dwell time. All children had at least one family member present for the procedure. A basket of developmentally appropriate distraction items was within reach of the family member during the procedure. Parents had varied experiences with distraction coaching, ranging from formal training, to modeling by healthcare providers, to no experience or training at all.
The intravenous insertion procedure took place as usual in the clinic setting. Clinic personnel were requested to allow the parent to support their child during the procedure and to not interfere by providing other interventions. The procedure was videotaped from the time the child was placed on the examination table to the time the clinic staff indicated that the intravenous catheter was secured in place.
After intravenous insertion, the child and the parent were asked to complete the remaining instruments. Children provided a second salivary cortisol sample 20 to 30 minutes after the intravenous insertion. Families were given collection material to obtain the child’s baseline cortisol samples at home. Each family received $30 compensation for participating in the study.
Data Management and Analysis
A data management system using Microsoft Access was developed. Data were double entered and stored on a secured server. Only unique subject identifiers were used to label samples and research materials (including videotapes) to protect subject’s privacy. Limited personal identifiers (name, address, and birth date) were collected and maintained in a separate database. Cortisol samples were processed and sent to a commercial laboratory for analysis. Videotapes were transferred to digital media for secured storage and then returned to the primary site for behavioral analysis. A second consent was obtained from some willing participants to use videotapes for additional educational purposes. Videotapes will be destroyed at an unspecified time, when all data analyses are completed, in accordance with the Data Management Plan approved by the IRB.
Explanatory variables for OSBD-R and cortisol were identified using multifactor regression analysis. However, the distribution of the Oucher Pain Scale and the PRCD data was skewed, so these data were divided into categories. For the Oucher Pain Scale, 60% responded in the 0 to 3 range, 20% in the 4 to 6 range, and 20% in the 7 to 10 range. With small frequency in the mid and high ranges, the Oucher Pain Scale was divided into low (0–3) versus moderate–high (4–10), and logistic regression analysis was performed to identify factors associated with the low or moderate–high Oucher Pain Scale response. For the PRCD, more than 40% of the subjects had distress levels 1 and 2. The scale was categorized into three levels, low (1 and 2), moderate (3–5), and high (6 and 7) distress, with explanatory variables determined by the generalized logit model.
Candidate explanatory variables for possible inclusion in the models for OSBD-R, cortisol, Oucher Pain Scale, and PRCD were child, parent, and procedural variables. Initial screening of variables for inclusion in the regression model was performed using bivariate methods to test for the association between a single explanatory variable and each distress measure. A Spearman or a Pearson correlation was completed to test the association between two continuous variables, a two-sample t-test or a one-way analysis of variance for the association between a categorical and a continuous variable, and a Pearson chi-square to test association between two categorical variables. Variables with p < .20 on these tests of association were included as independent variables in the stepwise variable selection in the linear regression analysis for OSBD-R and cortisol and logistic regression analysis for the PRCD and the Oucher Pain Scale. At each step of the stepwise selection analysis, the independent variables were evaluated using p < .15 for entry into the model and p > .15 for removal from the model. The variables identified by the stepwise selection analysis were included in the final model.
Results
Participants
Over 3 years, 720 families were recruited and 542 (75%) participated, including 262 girls (48%) and 280 boys (52%), with a mean age of 6.95 years (SD = 1.90 years). Participants were primarily White (84% of children and 89% of parents). Mothers (n = 476, 88%), fathers (n = 62, 12%), or a guardian (n = 4, <1%) acted as the distraction coach. Four families (<1%) were withdrawn from the study for the following reasons: a parent spoke English well enough to converse and consent but was unable to understand and answer the study questions, a child refused participation after the initial assent, a medical care provider did not allow a parent into the treatment room, and the procedure was so rushed that there was not time for data collection. Families that declined participation (n = 174, 24%) primarily reported that it was due to feeling overwhelmed by the clinic visit (McCarthy et al., 2010).
Explanatory Variables
Behavioral Response
Child characteristics explaining child behavioral distress (OSBD-R) were child’s age, child’s typical coping with pain (e.g., from “silent with little emotion” to “loudly and highly emotional” on a 1 to 7 scale), and parent expectation of child’s behavior during intravenous insertion, a question from the PPQ (all p < .0001). This regression model explained 35.3% of the variation in OSBD-R. Parent characteristics were considered for addition into the model, including previous use of distraction to help the child cope with a painful event, how actively involved they would like to be during intravenous placement, parent trait anxiety, and parent level of distraction coaching measured using the DCI (<10, 10–19, and ≥20). With the three selected child variables already in the model, only DCI showed a significant incremental effect on OSBD-R (p = .002), with the model R-square increased to 36.9%. The final explanatory factors for increased behavioral distress were younger age, more emotional child’s typical coping, parent expectation of more distress, and lower score on the DCI indicating low quality and frequency of parent distraction coaching (Table 1).
TABLE 1.
Variable Explaining Behavioral Distress Response (observed scale of Behaviorals Distress)
| Explanatory variable | Model coefficient (SE)a | % Change (95% CI)b | p |
|---|---|---|---|
| Child age | −0.122 (0.017) | −11.5% (−8.5% to −14.3%) | <.0001 |
| Child typical coping | 0.124 (0.020) | 13.3% (8.8% to 17.9%) | <.0001 |
| Parent expectation of child distress with intravenous insertion | 0.162 (0.023) | 17.6% (12.4% to 23.0%) | <.0001 |
| Distraction Coaching Index | |||
| <10 (vs. >20) | 0.229 (0.074) | 25.7% (8.7% to 45.4%) | .002 |
| 10–20 (vs. >20) | 0.299 (0.094) | 34.9% (12.2% to 62.1%) | .002 |
Note. Adjusted R2 = 36.9%. CI = confidence interval; OSBD-R = Observation Scale of Behavioral Distress–Revised.
The model coefficients are for natural log transform of OSBD-R as the dependent variable.
For the continuous explanatory variables, this is the % change in OSBD-R for every one unit increase in the explanatory variable.
Biological Response
Using cortisol responsivity (percent change from baseline to after the intravenous insertion) as the outcome, the following variables were identified as explaining cortisol responsivity using a stepwise regression analysis: perception of how distressed the child becomes the morning of the intravenous insertion, child diagnosis of attention deficit hyperactivity disorder (ADHD), child diagnosis of anxiety disorder, and the timing of telling the child about the intravenous procedure (finding out within 24 hours of the procedure vs. at the clinic visit or more than 24 hours before the visit). This initial regression model explained 17.7% of the total variation in child cortisol responsivity after the intravenous insertion. The addition of DCI into the model increased the R-square to 19.7%, but explanatory factors were otherwise unchanged (Table 2).
TABLE 2.
Variables Explaining Biological Response (Salivary Cortisol Responsivity)
| Explanatory variable | Model coefficient (SE)a | % Change (95% CI)b | p |
|---|---|---|---|
| Parent’s perception of how distressed child becomes in the morning of the intravenous insertion | 0.145 (0.037) | 15.6% (7.4% to 24.5%) | .0001 |
| Diagnosis of ADHD | −1.181 (0.294) | −69.3% (−45.2% to −82.8%) | <.0001 |
| Diagnosis of anxiety disorder | 1.161 (0.417) | 219% (40% to 527%) | .006 |
| Time child finds out about the intravenous procedure (vs. < 24 hours) | |||
| At visit | 0.619 (0.223) | 85.7% (19.7% to 188.0%) | .006 |
| Greater than 24 hours | 0.418 (0.192) | 50.3% (3.0% to 119.5%) | .035 |
| Distraction Coaching Index | |||
| <10 (vs. >20) | 0.270 (0.165) | 30.9% (−5.4% to 81.3%) | .104 |
| 10–20 (vs. >20) | 0.394 (0.213) | 48.4% (−2.6% to 125.9%) | .066 |
Note. Cortisol Responsivity = (% change from baseline after the intravenous insertion); R2 = 19.7%. CI = confidence interval; ADHD = attention deficit hyperactivity disorder; IV = intravenous.
The model coefficients are for natural log transform of cortisol as the dependent variable.
For the continuous explanatory variables, this is the % change for every one-unit increase in the explanatory variable.
Child Self-report of Pain
The final model explaining child report of pain (Oucher Pain Scale) is presented in Table 3. The child variables that were identified by stepwise logistic regression analysis that explained high pain (Oucher Pain Scale > 3) were the child’s age, the Pediatric Behavior Scale impulsivity subscale, the parent expectation of child’s behavior during intravenous insertion, the special assistance at school, the Child Behavior Style Scale monitoring total score, and the child’s typical coping with pain. Younger age was associated with high pain; all other variables in the final model had a positive association with high pain. There was no statistically significant incremental effect of parent’s level of distraction coaching (DCI p = .896) on high pain after accounting for the significant child variables.
TABLE 3.
Variables Explaining Child self-Report of pain Response (Oucher pain Scale)
| Explanatory variable | Model coefficient (SE)a | Odds ratio (95% CI)b | p |
|---|---|---|---|
| Child’s age | −0.312 (0.061) | 0.73 (0.65–0.83) | <.0001 |
| Pediatric Behavior Scale, Impulsivity subscale | 0.162 (0.049) | 1.18 (1.07–1.30) | .0009 |
| Parent expectation of child’s distress during intravenous insertion | 0.228 (0.082) | 1.26 (1.07–1.47) | .005 |
| Special assistance at school | 0.874 (0.431) | 2.40 (1.03–5.57) | .042 |
| Child Behavior Style Scale monitor scale score | 0.048 (0.033) | .138 | |
| Child typical coping | 0.107 (0.073) | .141 |
Coefficients from logistic regression model.
Odds ratio of moderate/high pain: Oucher Pain Scale >3 versus low pain 0 to 3.
Parent Report of Child Distress
Variables explaining high (5–7) versus low (1 and 2) PRCD were identified by stepwise generalized logistic regression analysis; the final model is shown in Table 4. Child variables included child’s typical coping with pain, child’s age, parent expectation of child’s behavior during intravenous insertion, parent’s perception of how distressed the child becomes the morning of the intravenous insertion, and parent’s perception of how distressed the child becomes during routine clinic visits when no medical procedures are scheduled. All of these variables have a positive association with parent report of high distress, except for child age, where younger age was associated with higher distress. There was also a significant effect of DCI on level of PRCD (p = .022).
TABLE 4.
Variables Explaining parent Report of Child Distress Response (PRCD)
| High/low |
Medium/low |
|||||
|---|---|---|---|---|---|---|
| Explanatory variable | Model coefficient (SE)* |
Odds ratio (95% CI) |
p | Model coefficient (SE)* |
Odds ratio (95% CI) |
p |
| Child’s typical coping | 0.333 (0.081) | 1.40 (1.19, 1.64) | <.0001 | −0.014 (0.086) | 0.99 (0.83, 1.17) | .869 |
| Child’s age | −0.258 (0.069) | 0.77 (0.67, 0.89) | .0002 | −0.061 (0.074) | 0.94 (0.81, 1.09) | .410 |
| Parent expectation of child’s distress during intravenous insertion | 0.333 (0.099) | 1.40 (1.15, 1.70) | .0008 | 0.071 (0.109) | 1.07 (0.87, 1.33) | .515 |
| Parent’s perception of how distressed child becomes during routine clinic visit | 0.183 (0.098) | 1.20 (0.99, 1.46) | .061 | 0.193 (0.116) | 1.21 (0.97, 1.52) | .096 |
| Parent’s perception of how distressed child becomes in the morning on a day of intravenous insertion | 0.259 (0.080) | 1.30 (1.11, 1.52) | .001 | 0.028 (0.093) | 1.03 (0.86, 1.23) | .759 |
| Child’s preferred coping style (watch) | −0.175 (0.247) | 0.84 (0.52, 1.36) | .480 | −0.716 (0.266) | 0.49 (0.29, 0.82) | 0.007 |
| Distraction Coaching Index | ||||||
| <10 (vs. ≥20) | 0.525 (0.296) | 1.69 (0.95, 3.02) | .076 | −0.398 (0.305) | 0.67 (0.37, 1.22) | .192 |
| 10–<20 (vs. ≥20) | 0.856 (0.375) | 2.35 (1.13, 4.90) | .022 | 0.372 (0.380) | 1.45 (0.69, 3.05) | .327 |
Note. Parent Report of Child Distress categorized data: high = 5–7, medium = 3 and 4, low = 1 and 2.
Coefficients from the generalized logit model.
No variables were associated significantly with high versus medium (3 and 4) PRCD scores. For the medium versus low analysis, two variables were statistically significant: parent perception of how distressed the child becomes during a routine clinic visit and child’s preferred coping style (preference to watch the procedure). Parents of children in the medium group reported their children were more likely to be distressed during a routine clinic visit and that the child was more likely to look away.
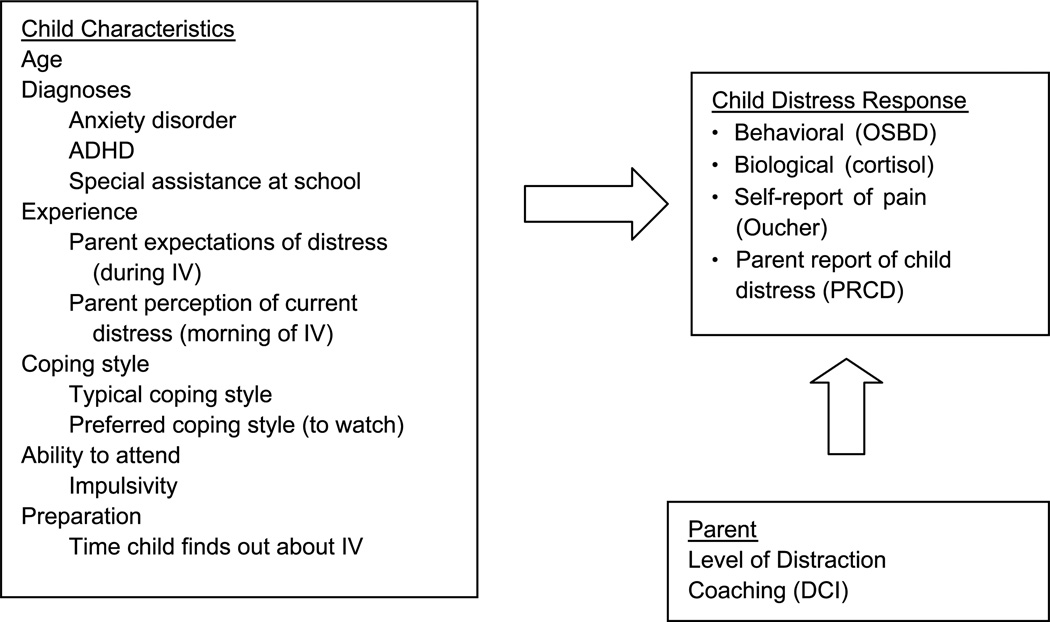
Combined Variables
Figure 2 includes the significant explanatory variables for the combination of distress outcomes. Although Tables 1 through 4 include variables significant at p < .15, this figure only includes those significant at p < .05.
FIGURE 2.
Model for child distress with intravenous insertions when parent distraction is provided; variables with p > .05. Measures: OSBD-R, salivary cortisol, Oucher Pain Scale, PRCD, DCI, and IV. OSBD-R = Observation Scale of Behavioral Distress–Revised; PRCD = parent report of child distress; DCI = Distraction Coaching Index; IV = intravenous. From “A conceptual model of factors influencing children’s responses to a painful procedure when parents are distraction coaches,” by A. M. McCarthy and C. Kleiber, 2006, Journal of Pediatric Nursing, 21(2), p. 89. Copyright 2006 by the Journal of Pediatric Nursing. Adapted with permission of the author.
Discussion
Child, parent, and procedural variables were examined concurrently in this study with the goal of explaining children’s distress responses to an intravenous insertion. Identifying specific variables to explain distress allowed for refining and updating the original model and list of variables (Figure 1) to develop a more concise model (Figure 2). A number of child characteristics from the original model remained in the new model: age, diagnoses, experience with procedures, ability to attend, anxiety (state), and coping. None of the original parent characteristics significantly explained child distress, although parent performance of distraction, originally a parent response, does explain child distress response. Neither of the two procedural variables originally proposed, difficulty of the procedure and use of topical anesthetic, explained child distress.
Factors explaining child distress varied by outcome, although some commonality exists. Age, child’s typical coping with pain, and parent expectations of child distress behavior during the intravenous insertion explained behavioral, parent-report, and child-report outcomes. The inverse relationship between child age and behavioral and self-report of distress has been reported in previous research (Bournaki, 1997; Gagliese & Katz, 2003; Goodenough et al., 1999) and is corroborated by clinical observations. Younger children may not have developed the self-regulation skills to remain outwardly calm in stressful situations. Not surprisingly, children who had a history of highly emotional responses to pain and whose parents expected a high level of distress were more distressed during the intravenous procedure.
One variable, parent performance of distraction coaching, explained behavioral, biological, and parent-report outcomes. This effect remained significant even after accounting for effects of child age, previous experience, and parent expectations. Previous studies evaluating distraction have not measured the quantity and quality of distraction coaching. This is the first study to demonstrate that child distress outcomes varied with the level of parent distraction coaching. The results suggest that when high-quantity and high-quality distraction (>20 DCI) is provided, children have less distress with intravenous procedures compared with children provided with low (<10 DCI) or moderate (10 to <20 DCI) levels of distraction. A smaller effect was noted in children provided with low versus moderate levels of distraction. Although parents in the low group provided less distraction, it may be that their children were less distressed and did not require distraction. Provision of little or no distraction may be appropriate for children with little distress in response to a procedure.
A strength of this study was the inclusion of multiple outcome measures of child distress, which provided a broad view of distress and captured a range of factors that contribute to a child’s distress response. Studies that include only one measure of distress may miss factors that uniquely explain another aspect of a child’s distress response. For example, cortisol responsivity was explained by five variables. One (the level of parent-provided distraction) was associated with two other outcomes; however, the other four variables were not associated with any other outcome. Children with higher cortisol responsivity were more upset before arriving at the clinic for the procedure, suggesting that some children are more biologically reactive to anticipated pain. The timing of telling the child about the procedure was also an explanatory factor for cortisol response. Children who were told either just before the procedure or more than 24 hours before the procedure had an increased biological response to the stress of the intravenous insertion. This supports the finding that information given too close to an event may not allow the child time to process the information (Jaaniste et al., 2007). Two child diagnoses also emerged as explanatory factors for cortisol response: ADHD and anxiety disorder. Compared with other children, children with ADHD displayed a low level of cortisol responsivity in response to the intravenous insertion. Others have reported similar findings between behavioral disorders and low cortisol responsivity, suggesting an atypical response in these children (Kaneko, Hoshino, Hashimoto, Okano, & Kumashiro, 1993; McBurnett, Lahey, Rathouz, & Loeber, 2000). State anxiety has been associated with increased distress during a painful procedure (Claar et al., 2002; Kleiber et al., 2002). In this study, a diagnosis of anxiety disorder, which can be viewed as a proxy for trait anxiety, explained cortisol responsivity. Unlike earlier research, child self-report of state or trait anxiety did not explain any of the outcomes.
Some limitations of this study should be noted. The focus was on child distress during intravenous insertions; findings may not be applicable to other medical procedures that children experience. Although a concerted effort was made to recruit an ethnically diverse sample, the largely White Midwestern participants limit these findings. Individuals from other backgrounds may have unique factors explaining child distress responses. The parents in the sample were primarily mothers, and the results may not be generalizable to fathers or other support persons. A number of families declined to participate (n = 174, 24%), primarily because of feeling overwhelmed by the clinic visit. Thus, the participants in this study may be less distressed by the procedure than the entire population of children undergoing a potentially painful procedure such as an intravenous insertion.
Clinical and Research Implications
The explanatory factors identified in this study can be used clinically to assess a child’s risk for distress with an intravenous insertion. These results identify the need for parental education and support in providing their children with an appropriate level of distraction to decrease child distress during stressful medical procedures. Clinicians should assess children on the explanatory factors identified in this study and identify those who may be at particular risk for increased distress. When possible, parents of children who appear to be at increased risk for distress should be provided support from professional staff, either to teach the parent to perform distraction coaching or to provide the distraction to the child in partnership with the parent. Clinical settings where children receive potentially painful medical procedures need to develop procedural guidelines for the routine screening of children before a medical procedure to identify the child’s risk for distress and to provide appropriate cognitive behavioral interventions such as distraction. For some families, simply providing distraction materials, encouraging families to use them before, during, and after the procedure, and minimizing environmental obstacles to the use of distraction may be sufficient. For other families, professional support or intervention may be needed. This research team is developing a computer application on the basis of these data to identify child risk for distress and test appropriate interventions, which include parent distraction coaching. After testing in clinical setting, this computerized assessment program will facilitate tailoring of interventions to the individual child’s profile and incorporating educational interventions to support parents in providing distraction to their children.
The results of this study suggest areas for additional study. The primary aim of this study was to identify factors that explained the child’s distress response to a medical procedure. Although many of the factors that were identified were supported by previous studies, there are some new areas emerging. No parental characteristics emerged as explanatory factors of child distress, which differs from some previous research. Since this study began, other factors, such as pain catastrophizing, that may contribute to a child’s level of distress have emerged and need to be studied in conjunction with the findings of this research. The genetic data obtained in this study are being analyzed to identify the role of genetics in children’s distress response.
Since the quality and the quantity of distraction coaching provided by parents explained a significant amount of the child’s distress response, further research is needed to understand parent’s roles as distraction coaches and to develop and test interventions that teach parents how to use high-quality distraction. The data from this study are being analyzed further to identify the factors that explain parents’ performance of distraction as measured by the DCI and satisfaction with being a distraction coach.
In conclusion, the findings from this study advance understanding of factors explaining a child’s distress response to a medical procedure and demonstrate the value of parent-provided distraction coaching during this stressful experience. The development of a clinically useful predictive model to identify children at risk for distress with medical procedures and to provide parents training in distraction coaching is an important next step.
Acknowledgments
This study was funded by R01 grant no. NR05269-01A2 from the National Institute for Nursing Research to the first author.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.nursingresearchonline.com).
Contributor Information
Ann Marie McCarthy, College of Nursing.
Charmaine Kleiber, College of Nursing.
Kirsten Hanrahan, University of Iowa Health Care.
M. Bridget Zimmerman, Department of Biostatistics, University of Iowa.
Nina Westhus, School of Nursing, Saint Louis University, Missouri.
Susan Allen, Department, PICU, Internal Resource Team, Blank Children’s Hospital, Des Moines, Iowa.
References
- Aradine CR, Beyer JE, Tompkins JM. Children’s pain perception before and after analgesia: A study of instrument construct validity and related issues. Journal of Pediatric Nursing. 1988;3(1):11–23. [PubMed] [Google Scholar]
- Beyer JE, Aradine CR. Content validity of an instrument to measure young children’s perceptions of the intensity of their pain. Journal of Pediatric Nursing. 1986;1(6):386–395. [PubMed] [Google Scholar]
- Bournaki MC. Correlates of pain-related responses to venipunctures in school-age children. Nursing Research. 1997;46(3):147–154. doi: 10.1097/00006199-199705000-00005. [DOI] [PubMed] [Google Scholar]
- Broome ME, Rehwaldt M, Fogg L. Relationships between cognitive behavioral techniques, temperament, observed distress, and pain reports in children and adolescents during lumbar puncture. Journal of Pediatric Nursing. 1998;13(1):48–54. doi: 10.1016/S0882-5963(98)80068-7. [DOI] [PubMed] [Google Scholar]
- Brown DA, Salmon K, Pipe ME, Rutter M, Craw S, Taylor B. Children’s recall of medical experiences: The impact of stress. Child Abuse & Neglect. 1999;23(3):209–216. doi: 10.1016/s0145-2134(98)00127-6. [DOI] [PubMed] [Google Scholar]
- Chen E, Craske MG, Katz ER, Schwartz E, Zeltzer LK. Pain-sensitive temperament: Does it predict procedural distress and response to psychological treatment among children with cancer? Journal of Pediatric Psychology. 2000;25(4):269–278. doi: 10.1093/jpepsy/25.4.269. [DOI] [PubMed] [Google Scholar]
- Claar RL, Simons LE, Logan DE. Parental response to children’s pain: The moderating impact of children’s emotional distress on symptoms and disability. Pain. 2008;138(1):172–179. doi: 10.1016/j.pain.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Claar RL, Walker LS, Smith CA. The influence of appraisals in understanding children’s experiences with medical procedures. Journal of Pediatric Psychology. 2002;27(7):553–563. doi: 10.1093/jpepsy/27.7.553. [DOI] [PubMed] [Google Scholar]
- Cohen LL, Blount RL, Cohen RJ, Ball CM, McClellan CB, Bernard RS. Children’s expectations and memories of acute distress: Short- and long-term efficacy of pain management interventions. Journal of Pediatric Psychology. 2001;26(6):367–374. doi: 10.1093/jpepsy/26.6.367. [DOI] [PubMed] [Google Scholar]
- Corbo-Richert BH. Coping behaviors of young children during a chest tube procedure in the pediatric intensive care unit. Maternal-Child Nursing Journal. 1994;22(4):134–146. [PubMed] [Google Scholar]
- Dahlquist LM, Power TG, Cox CN, Fernbach DJ. Parenting and child distress during cancer procedures: A multidimensional assessment. Children’s Health Care. 1994;23(3):149–166. doi: 10.1207/s15326888chc2303_1. [DOI] [PubMed] [Google Scholar]
- Frank NC, Blount RL, Smith AJ, Manimala MR, Martin JK. Parent and staff behavior, previous child medical experience, and maternal anxiety as they relate to child procedural distress and coping. Journal of Pediatric Psychology. 1995;20(3):277–289. doi: 10.1093/jpepsy/20.3.277. [DOI] [PubMed] [Google Scholar]
- Gagliese L, Katz J. Age differences in postoperative pain are scale dependent: A comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103(1–2):11–20. doi: 10.1016/s0304-3959(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Goodenough B, Thomas W, Champion GD, Perrott D, Taplin JE, von Baeyer CL, et al. Unravelling age effects and sex differences in needle pain: Ratings of sensory intensity and unpleasantness of venipuncture pain by children and their parents. Pain. 1999;80(1–2):179–190. doi: 10.1016/s0304-3959(98)00201-2. [DOI] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research. 2006;19(2):95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jaaniste T, Hayes B, von Baeyer CL. Providing children with information about forthcoming medical procedures: A review and synthesis. Clinical Psychology: Science and Practice. 2007;14(2):124–143. [Google Scholar]
- Jacobsen PB, Manne SL, Gorfinkle K, Schorr O, Rapkin B, Redd WH. Analysis of child and parent behavior during painful medical procedures. Health Psychology. 1990;9(5):559–576. doi: 10.1037//0278-6133.9.5.559. [DOI] [PubMed] [Google Scholar]
- Jay SM, Elliott C. Observation Scale of Behavioral Distress–Revised. Los Angeles, California: Susan M. Jay, Psychosocial Program, Children’s Hospital of Los Angeles; 1986. Unpublished scoring manual, [Google Scholar]
- Kaneko M, Hoshino Y, Hashimoto S, Okano T, Kumashiro H. Hypothalamic-pituitary-adrenal axis function in children with attention-deficit hyperactivity disorder. Journal of Autism and Developmental Disorders. 1993;23(1):59–65. doi: 10.1007/BF01066418. [DOI] [PubMed] [Google Scholar]
- Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, Halonen PM. Parents’ perceptions of their 1–6-year-old children’s pain. European Journal of Pain. 2003;7(3):203–211. doi: 10.1016/S1090-3801(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Penati B, Waibel MK, Blackall GF. The Perception of Procedures Questionnaire: Psychometric properties of a brief parent report measure of procedural distress. Journal of Pediatric Psychology. 1996;21(2):195–207. doi: 10.1093/jpepsy/21.2.195. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kleiber C, Craft-Rosenberg M, Harper DC. Parents as distraction coaches during I.V. insertion: A randomized study. Journal of Pain and Symptom Management. 2001;22(4):851–861. doi: 10.1016/s0885-3924(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Kleiber C, Harper DC. Effects of distraction on children’s pain and distress during medical procedures: A metaanalysis. Nursing Research. 1999;48(1):44–49. doi: 10.1097/00006199-199901000-00007. [DOI] [PubMed] [Google Scholar]
- Kleiber C, McCarthy AM. Evaluating instruments for a study on children’s responses to a painful procedure when parents are distraction coaches. Journal of Pediatric Nursing. 2006;21(2):99–107. doi: 10.1016/j.pedn.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kleiber C, McCarthy AM, Hanrahan K, Myers L, Weathers N. Development of the Distraction Coaching Index. Children’s Health Care. 2007;36(3):219–235. [Google Scholar]
- Kleiber C, Sorenson M, Whiteside K, Gronstal BA, Tannous R. Topical anesthetics for IV insertion in children: A randomized equivalency study. Pediatrics. 2002;110(4):758–761. doi: 10.1542/peds.110.4.758. [DOI] [PubMed] [Google Scholar]
- Kleiber C, Suwanraj M, Dolan LA, Berg M, Kleese A. Pain sensitive temperament and postoperative pain. Journal for Specialists in Pediatric Nursing. 2007;12(3):149–158. doi: 10.1111/j.1744-6155.2007.00108.x. [DOI] [PubMed] [Google Scholar]
- Lee LW, White-Traut RC. The role of temperament in pediatric pain response. Issues in Comprehensive Pediatric Nursing. 1996;19(1):49–63. doi: 10.3109/01460869609026854. [DOI] [PubMed] [Google Scholar]
- Liossi C, White P, Franck L, Hatira P. Parental pain expectancy as a mediator between child expected and experienced procedure-related pain intensity during painful medical procedures. Clinical Journal of Pain. 2007;23(5):392–399. doi: 10.1097/AJP.0b013e31804ac00c. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57(1):38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McCarthy AM, Hanrahan K, Kleiber C, Zimmerman MB, Lutgendorf S, Tsalikian E. Normative salivary cortisol values and responsivity in children. Applied Nursing Research. 2009;22(1):54–62. doi: 10.1016/j.apnr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, Kleiber C. A conceptual model of factors influencing children’s responses to a painful procedure when parents are distraction coaches. Journal of Pediatric Nursing. 2006;21(2):88–98. doi: 10.1016/j.pedn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- McCarthy AM, Kleiber C, Hanrahan K, Zimmerman MB, Westhus N, Allen S. Impact of parent provided distraction on child responses to an IV insertion. Children’s Health Care. 2010;39(2):125–141. doi: 10.1080/02739611003679915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt KA, Ornstein PA, Spicker B. Children’s memory for a salient medical procedure: Implications for testimony. Pediatrics. 1994;94(1):17–23. [PubMed] [Google Scholar]
- Mogil JS. Complex traits genetics of pain. In: Mogil JS, editor. The Genetics of Pain. Seattle, WA: IASP Press; 2004. pp. 123–149. [Google Scholar]
- Moon EC, Chambers CT, Larochette AC, Hayton K, Craig KD, McGrath PJ. Sex differences in parent and child pain ratings during an experimental child pain task. Pain Research & Management. 2008;13(3):225–230. doi: 10.1155/2008/457861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Dennig MD, Weisz JR. Determinants and consequences of children’s coping in the medical setting: Conceptualization, review, and critique. Psychological Bulletin. 1995;118(3):328–357. doi: 10.1037/0033-2909.118.3.328. [DOI] [PubMed] [Google Scholar]
- Salmon K, Pereira JK. Predicting children’s response to an invasive medical investigation: The influence of effortful control and parent behavior. Journal of Pediatric Psychology. 2002;27(3):227–233. doi: 10.1093/jpepsy/27.3.227. [DOI] [PubMed] [Google Scholar]
- Salmon K, Price M, Pereira JK. Factors associated with young children’s long-term recall of an invasive medical procedure: A preliminary investigation. Journal of Developmental and Behavioral Pediatrics. 2002;23(5):347–352. doi: 10.1097/00004703-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Taddio A, Soin HK, Schuh S, Koren G, Scolnik D. Liposomal lidocaine to improve procedural success rates and reduce procedural pain among children: A randomized controlled trial. CMAJ. 2005;172(13):1691–1695. doi: 10.1503/cmaj.045316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uman LS, Chambers CT, McGrath PJ, Kisely S. A systematic review of randomized controlled trials examining psychological interventions for needle-related procedural pain and distress in children and adolescents: An abbreviated Cochrane review. Journal of Pediatric Psychology. 2008;33(8):842–854. doi: 10.1093/jpepsy/jsn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: Overview and implications for practice. Journal of Pain. 2004;5(5):241–249. doi: 10.1016/j.jpain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Walker LK, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137(2):266–275. doi: 10.1016/j.pain.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman SJ, Bernstein B, Schechter NL. Consequences of inadequate analgesia during painful procedures in children. Archives of Pediatrics & Adolescent Medicine. 1998;152(2):147–149. doi: 10.1001/archpedi.152.2.147. [DOI] [PubMed] [Google Scholar]
- Zempsky WT, Schechter NL. What’s new in the management of pain in children? Pediatrics in Review. 2003;24(10):337–348. doi: 10.1542/pir.24-10-337. [DOI] [PubMed] [Google Scholar]