Abstract
Green synthesis of noble metal nanoparticles is a vastly developing area of research. Metallic nanoparticles have received great attention from chemists, physicists, biologists, and engineers who wish to use them for the development of a new-generation of nanodevices. In this study, silver nanoparticles were biosynthesized from aqueous silver nitrate through a simple and eco-friendly route using Curcuma longa tuber-powder extracts, which acted as a reductant and stabilizer simultaneously. Characterizations of nanoparticles were done using different methods, which included ultraviolet-visible spectroscopy, powder X-ray diffraction, transmission electron microscopy, scanning electron microscopy, energy-dispersive X-ray fluorescence spectrometry, and Fourier-transform infrared spectroscopy. The ultraviolet-visible spectrum of the aqueous medium containing silver nanoparticles showed an absorption peak at around 415 nm. Transmission electron microscopy showed that mean diameter and standard deviation for the formation of silver nanoparticles was 6.30 ± 2.64 nm. Powder X-ray diffraction showed that the particles are crystalline in nature, with a face-centered cubic structure. The most needed outcome of this work will be the development of value-added products from C. longa for biomedical and nanotechnology-based industries.
Keywords: silver nanoparticles, Curcuma longa, biosynthesis, green synthesis, transmission electron microscopy
Introduction
Green nanotechnology is an area with significant focus at present on the important objective of facilitating the manufacture of nanotechnology-based products that are eco-friendly and safer for all beings, with sustainable commercial viability. The “green synthesis” of metal nanoparticles receives great attention due to their unusual optical, chemical, photochemical, and electronic properties.1 Metal nanoparticles, especially the noble metals, have mainly been studied because of their strong optical absorption in the visible region caused by the collective excitation of free-electron gas.2
Among noble metal nanoparticles, silver nanoparticles (Ag-NPs) have a wide area of interest, as they have a large number of applications, such as in nonlinear optics, spectrally selective coating for solar energy absorption, biolabeling, intercalation materials for electrical batteries as optical receptors, catalyst in chemical reactions, and as antibacterial capacities.
Ag-NPs have particular properties that may perhaps have numerous applications in the fields of dentistry, clothing, catalysis, mirrors, optics, photography, electronics, and the food industry.3 Because of such a broad variety of applications, many different preparation methods have been developed. However, the methods developed for Ag-NP preparation must give preference to controlled size of Ag-NPs. Therefore, nanosilver with small particle size and devoid of aggregation between particles is favorable.
There are several ways to reduce Ag+, eg, use of γ-rays,4 ultraviolet (UV) irradiation,5 heating and electrochemical reduction,6 and application of reducing chemicals, such as hydrazine,7 sodium borohydride,8–10 polyethylene glycerol,11 N,N-dimethylformamide,12 glucose,13 ethylene glycol,14 formaldehyde,15 and sodium in liquid ammonia.16 However, there is still need for a more economic, commercially viable, and environmentally green synthesis route to synthesize Ag-NPs. The green synthesis of Ag-NPs involves three main steps, which must be evaluated based on green chemistry perspectives, including selection of solvent medium, reducing agent, and nontoxic stabilizers for Ag-NPs.17
The biosynthesis of nanoparticles, which represents a connection between biotechnology and nanotechnology, has received increasing consideration due to the growing need to develop environmentally friendly technologies for material syntheses. The search for appropriate biomaterials for the biosynthesis of nanoparticles continues through many different synthetic methods.18
The biosynthetic method using plant extracts has received more attention than chemical and physical methods and even the use of microbes. The method is suitable for nanoscale metal synthesis due to the absence of any requirement to maintain an aseptic environment.19 The possibility of using plant materials for the synthesis of nanoscale metals was reported initially by Gardea-Torresdey et al.20,21 Later, the bioreduction of various metals to nanosize materials of various shapes, capable of meeting the requirements of diverse industrial applications, was extensively studied.22 In continuation, we have demonstrated the prospect of using Vitex negundo L leaf and Callicarpa manigayi stem-bark methanolic extracts for the synthesis of Ag-NPs in ambient conditions, without any additive protecting nanoparticles from aggregating, template shaping nanoparticles or accelerants.23,24
In this study, the synthesis and characterization of Ag/Curcuma longa by a green method is reported. The Ag-NPs were prepared using silver nitrate as silver precursor and C. longa tuber-powder water extract as reducing agent and stabilizer.
Materials and methods
Materials
The C. longa tubers were purchased from a local market in Malaysia. AgNO3 (99.98%) was used as a silver precursor, and was provided by Merck (Darmstadt, Germany). HNO3 (70%) and HCl (37%) were obtained from Sigma-Aldrich (St Louis, MO). All reagents in this effort were analytical grade and were used as received without further purification. All solutions were freshly prepared using double-distilled water and kept in the dark to avoid any photochemical reactions. All glassware used in experimental procedures was cleaned in a fresh solution of HNO3/HCl (3:1, v/v), washed thoroughly with double-distilled water, and dried before use.
Extraction preparation
The C. longa plant and tubers are shown in Figure 1A and B. The C. longa tubers were washed to remove the adhering mud particles and possible impurities. Later they were dried under sunlight for a week to completely remove the moisture. The tubers were cut into small pieces, powdered in a mixer, and then sieved using a 20-mesh sieve to get uniform size range. The final sieved powder was used for all further studies (Figure 1C). For the production of extract, 0.1 g of C. longa tuber powder was added to a 100 mL Erlenmeyer flask with 20 mL sterile distilled water and then mixed for 4 hours at room temperature.
Figure 1.
Curcuma longa plant (A), C. longa tubers (B), and C. longa powder (C).
Synthesis of Ag/C. longa emulsion
Briefly, water extract of C. longa tubers (0.1 g) was added to distilled deionized water (20 mL) with vigorous stirring for 4 hours. Forty milliliters of AgNO3 (1 × 10−3 M) was then added and mixed at room temperature (25°C) for 24 hours. Ag-NPs were gradually obtained during the incubation period. Throughout the reduction process, the solution was kept at a room temperature in the dark to avoid any photochemical reactions. The solution component was purged with nitrogen gas prior to use. Subsequently, reduction proceeded in the presence of nitrogen to eliminate oxygen. The obtained colloidal suspensions of Ag/C. longa were then centrifuged at 15,000 rpm for 20 minutes and washed four times to remove silver ion residue. The precipitate nanoparticles were then dried overnight at 30°C under vacuum to obtain the Ag/C. longa.
Characterization methods and instruments
The prepared Ag/C. longa were characterized by UV-visible spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy dispersive X-ray fluorescence spectrometry (EDXRF) and Fourier-transform infrared (FT-IR) spectroscopy. The UV-visible spectra were recorded over the 300–800 nm range with a UV-1650 PC UV-visible spectrophotometer (Shimadzu, Osaka, Japan). The structures of the Ag-NPs produced were examined by XRD (XRD-6000; Shimadzu). The XRD patterns were recorded at a scan speed of 4°/minute. TEM observations were carried out on a H-7100 electron microscope (Hitachi, Tokyo, Japan), and the particle-size distributions were determined using the UTHSCSA Image Tool version 3.00 program (freeware). SEM was performed using a Philips XL-30 instrument (Philips, Eindhoven, Netherlands) to study the morphology of Ag/C. longa. The EDXRF was carried out on a DX-700HS spectrometer (Shimadzu). Meanwhile, the FT-IR spectra were recorded over the range of 400–4000 cm−1 using an FT-IR Series 100, 1650 PerkinElmer spectrophotometer (Los Angeles, CA).
Results and discussion
The reduction of Ag+ into Ag-NPs during exposure to water extract of C. longa tuber powder was able to be followed by the color change. The fresh suspension of C. longa was yellow. However, after the addition of AgNO3 and stirring for 24 hours at room temperature, the emulsion turned brown.
The color changes in aqueous solutions are due to the surface-plasmon resonance (SPR) phenomenon (Figure 2A and B). The result obtained in this investigation is interesting because it can serve as a foundation in terms of identification of potential forest plants for synthesizing Ag-NPs.
Figure 2.
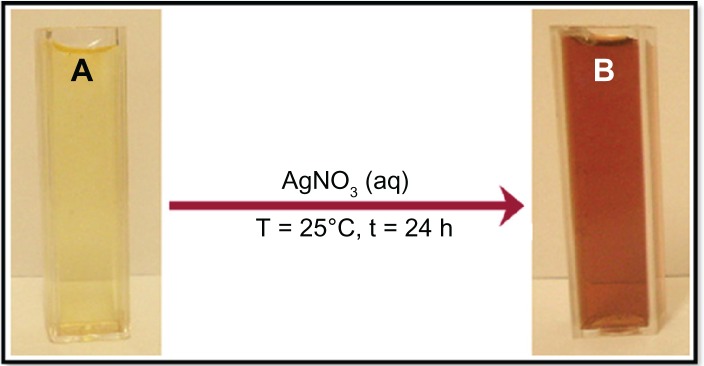
Photograph of Curcuma longa (A) and silver/C. longa (B) emulsions after 24 hours of stirring time.
C. longa as an aldehyde can reduce silver ions to Ag-NPs. The possible chemical equations for preparing the Ag-NPs are:
| (1) |
| (2) |
After dispersion of silver ions in the C. longa aqueous solution matrix (Equation 1), the extract was reacted with the Ag+(aq) to form [Ag (C. longa)]+ complex, which reacted with aldehyde groups in the molecular structure of the methanolic extract to form [Ag (C. longa)], due to the reduction of silver ions through the oxidation of aldehyde to carboxylic acid groups (Equation 2).
UV-visible spectroscopy analysis
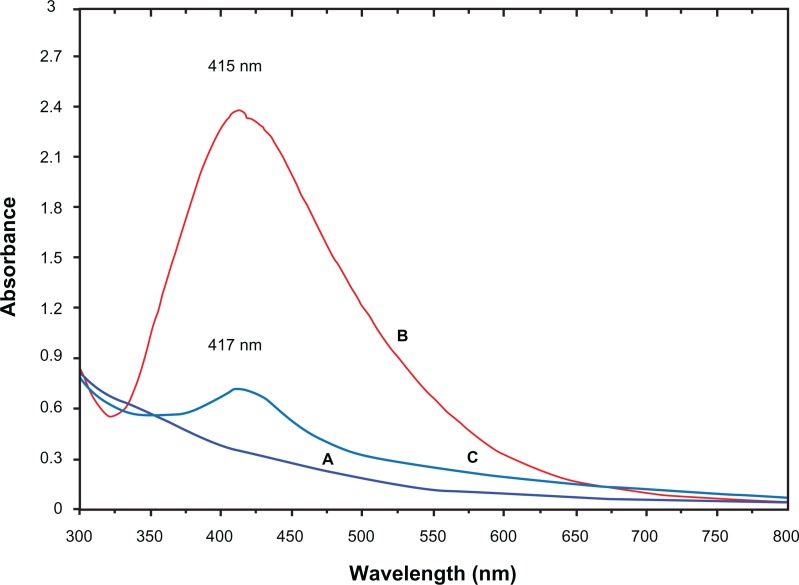
The formation of Ag-NPs was followed by measuring the SPR of the C. longa and Ag/C. longa emulsions over the wavelength range of 300–800 nm (Figure 3B). The SPR bands are influenced by the size, shape, morphology, composition, and dielectric environment of the prepared nanoparticles.25,26 Previous studies have shown that the spherical Ag-NPs contribute to the absorption bands at around 400–420 nm in the UV-visible spectra.26,27 These absorption bands were assumed to correspond to the Ag-NPs’ extra-fine nature, with relatively small size. UV-visible absorption spectra (Figure 3B) showed that the broad SPR band contained one peak at 415 nm. This peak illustrates the presence of a homogeneous distribution of hydrosol Ag-NPs after 24 hours stirring.11,23 For the stability test of the Ag-NP emulsion, the absorption spectrum of the sample was measured after storage for 3 months (Figure 3C). The absorption peak of the Ag-NPs shifted slightly from 415 to 417 nm, but the spectra for these two samples showed significant changes in either peak intensity or spectral shape.27 Thus, with the comparison of Figure 3B and C, it can be concluded that for emulsion stability testing, due to the decreases in absorbance intensity and deposits of Ag-NPs, at first the stability of the Ag/C. longa emulsion decreases and then gradually the size of the Ag-NPs increases.
Figure 3.
UV-visible absorption spectra of Curcuma longa (A) and silver (Ag)/C. longa emulsion (B) after 24 hours of stirring; Ag/C. longa emulsion (C) after 3 months.
Powder X-ray diffraction
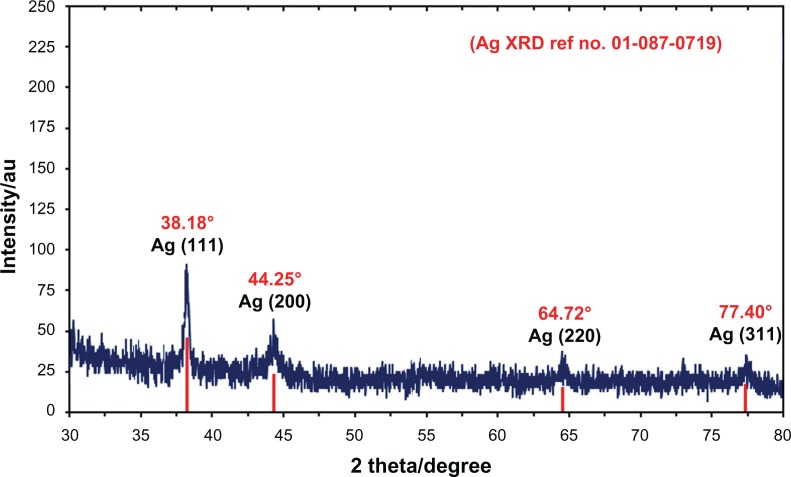
Figure 4 shows the XRD patterns of vacuum-dried Ag-NPs synthesized using C. longa. The XRD patterns of Ag/C. longa indicated that the structure of Ag-NPs is face-centered cubic.27 In addition, all the Ag-NPs had a similar diffraction profile, and XRD peaks at 2θ of 38.18°, 44.25°, 64.72°, and 77.40° could be attributed to the 111, 200, 220, and 311 crystallographic planes of the face-centered cubic silver crystals, respectively.28 The XRD pattern thus clearly illustrated that the Ag-NPs formed in this study were crystalline in nature. The main crystalline phase was silver, and there were no obvious other phases as impurities were found in the XRD patterns (Figure 4).
Figure 4.
X-ray diffraction patterns of silver nanoparticles (Ag-NPs) synthesized in Curcuma longa for determination of Ag-NPs after 24 hours of stirring.
Abbreviation: XRD, X-ray diffration.
The average particle size of Ag-NPs can be calculated using the Debye–Scherrer equation (3):
| (3) |
where K is the Scherrer constant with value from 0.9 to 1 (shape factor), where λ is the X-ray wavelength (1.5418 Å), β½ is the width of the XRD peak at half-height and θ is the Bragg angle. From the Scherrer equation, the average crystallite size of Ag-NPs for the sample at 24 hours of stirring are found to be lower than 10 nm in size, which is also in line with the TEM results discussed later.
Morphology study
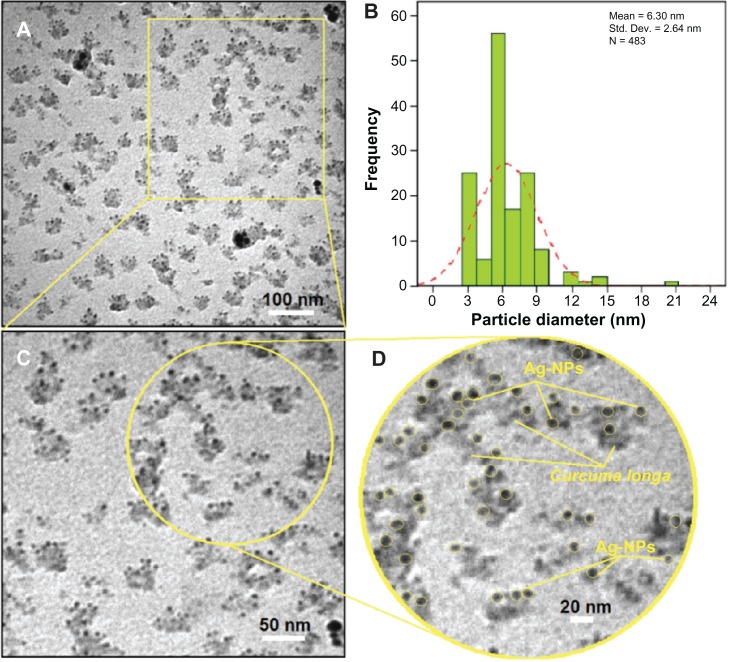
For TEM, a drop of the Ag-NP solution synthesized by treating silver nitrate solution with C. longa was deposited onto a TEM copper grid. After drying, the grid was imaged using TEM. The TEM images and their size distribution are shown in Figure 5A and B; the result showed narrow particle-size distributions, with diameters in the range of 3.66–8.94 nm. Moreover, the mean diameter and standard deviation of Ag-NPs was 6.30 ± 2.64 nm.
Figure 5.
(A–D) Transmission electron microscopy images and corresponding size distribution of silver/Circuma longa after 24 hours of stirring.
Abbreviations: Std Dev, standard deviation; Ag-NPs, silver nanoparticles.
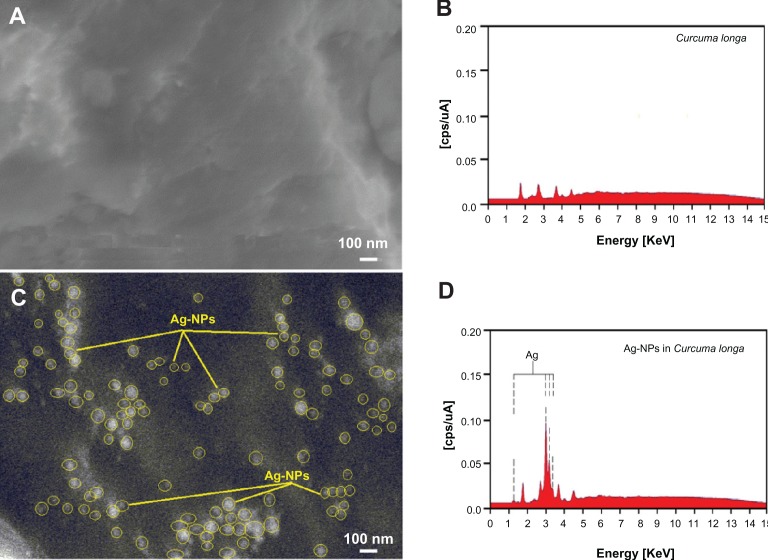
The presence of one narrow distribution of Ag-NPs in the TEM images is in accordance with the UV-visible spectral study. Figure 5C and D show the Ag-NPs surrounded by the extract of C. longa. The dark points in this figure represent the large-scale distribution of Ag-NPs. The Ag-NPs surrounded by C. longa extract is shown by TEM in Figure 5 and confirmed by FT-IR spectroscopy. The number of Ag-NPs counted for TEM imaging was around 483 at 24 hours stirring. Figure 6A–D shows the SEM images and EDXRF spectra for the C. longa and Ag/C. longa emulsion after 24 hours stirring. These results confirm that extract of C. longa can effectively control the shape and size of the Ag-NPs.
Figure 6.
Scanning electron microscopy image and energy-dispersive X-ray fluorescence spectrometry spectra of Curcuma longa (A and B) and silver/C. longa (C and D) formation after 24 hours of stirring.
Abbreviation: Ag-NPs, silver nanoparticles.
The exterior surfaces of Ag/C. longa due to the presence of small Ag-NPs become shiny in the spots’ spherical shapes (Figure 6C). Figure 6B shows the EDXRF spectra for the C. longa; the peaks around 1.7, 2.8, 3.8, and 4.5 keV are related to the binding energies of C. longa. In Figure 6D, the peaks around 1.3, 3.1, 3.3, and 3.4 keV are related to the silver elements in the C. longa.29
Additionally, the EDXRF spectra for the Ag/C. longa confirmed the presence of Ag-NPs in the tuber-powder extraction without any impurity peaks. From EDXRF spectra, it is clear that C. longa has a yield of 45.53% of Ag-NPs. The results indicate that the synthesized nanoparticles are composed of high-purity Ag-NPs.
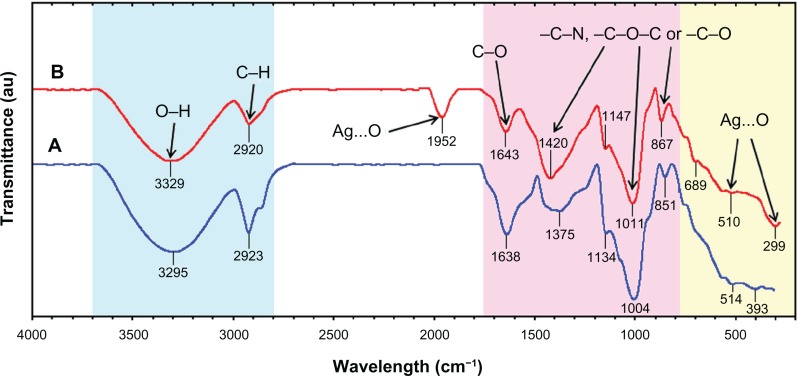
FT-IR chemical analysis
The FT-IR spectra were recorded to identify the possible biomolecules responsible for the reduction of the Ag+ ions and capping of the bioreduced Ag-NPs synthesized by the C. longa extract. After complete bioreduction of Ag+, the C. longa tuber-powder extract was centrifuged at 15,000 rpm for 20 minutes to isolate the Ag-NPs from proteins and other compounds present in the solution. Figure 7A shows the FT-IR spectrum of C. longa tuber powder that did not contain AgNO3, whereas Figure 7B shows the spectrum containing Ag-NPs after extract bioreduction with AgNO3. The spectrum in Figure 7A shows transmission peaks at 3295, 2923, 1638, 1375, 1134, 1004, 851, 514, and 393 cm−1. Similarly, transmission peaks for the tuber-powder extract containing Ag-NPs were at 3329, 2920, 1952, 1643, 1420, 1147, 1011, 867, 689, 510, and 299 cm−1. Three absorption peaks located around 867, 1011 and 1147 cm−1 can be assigned as the absorption peaks of –C–N stretching vibrations of the amine, –C–O–C or –C–O groups, respectively.24,30 The bonds or functional groups such as –C–O–C–, –C–O, and –C═C– derived from heterocyclic compounds, eg, alkaloid or flavones, and the amide (I) bond derived from the proteins that are present in the tuber-powder extract are the capping ligands of the nanoparticles.31 The broad and strong bands at 3329–2920 cm−1 were due to bonded hydroxyl (–OH) or amine groups (–NH) and aliphatic C–H of the C. longa tuber-powder extract, respectively. The peak at 1643 cm−1 is attributed to the carboxyl group (–C═O) stretching vibration. The adsorption at around 1375–1420 cm−1 notably showed that –NO3 existed in residual amounts.24 The broad peaks at 510 and 299 cm−1 and also the peak in 1952 cm−1 are related to Ag-NP banding with oxygen from hydroxyl groups of C. longa compounds (Figure 7B).27
Figure 7.
Fourier-transform infrared spectra for the Curcuma longa tuber-powder extract (A) and Ag/C. longa (B) after 24 hours from biosynthesis reaction.
Conclusion
Ag-NPs with an average size of 6.30 ± 2.64 nm and spherical shapes were synthesized using aqueous tuber-powder extract of C. longa. The Ag-NPs were characterized by UV-visible, XRD, TEM, SEM, EDXRF, and FT-IR spectra. Biosynthesis of Ag-NPs using green resources like C. longa is a better alternative to chemical synthesis, since this green synthesis is pollutant-free and eco-friendly. From the results obtained in this research, one can affirm that C. longa tuber powder can play an important role in the bioreduction and stabilization of silver ions to Ag-NPs.
Acknowledgments
The authors thank the University Putra Malaysia (UPM) for its financial support (RUGS, Project no 9199840). The authors are also grateful to the staff of the Department of Chemistry, UPM, for their help in this research, and the Institute of Bioscience (IBS/UPM) for technical assistance.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10:507–517. [Google Scholar]
- 2.Mohamed MB, Volkov V, Link S, Sayed MAE. The ‘lightning’ gold nanorods: fluorescence enhancement of over a million compared to the gold metal. Chem Phys Lett. 2000;317:517–523. [Google Scholar]
- 3.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Shameli K, Ahmad MB, Wan Yunus WMZ, Ibrahim NA, Gharayebi Y, Sedaghat S. Synthesis of silver/montmorillonite nanocomposites using γ-irradiation. Int J Nanomedicine. 2010;5:1067–1077. doi: 10.2147/IJN.S15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shameli K, Ahmad MB, Wan Yunus WMZ, et al. Green synthesis of silver/montmorillonite/chitosan bionanocomposites using the UV-irradiation method and evaluation of antibacterial activity. Int J Nanomedicine. 2010;5:875–887. doi: 10.2147/IJN.S13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Chen F, Zhuang J, et al. Synthesis of silver nanoparticles via electrochemical reduction on compact zeolite film modified electrodes. Chem Commun (Camb) 2002:2814–2815. doi: 10.1039/b208222e. [DOI] [PubMed] [Google Scholar]
- 7.Szczepanowicz K, Stefańska J, Socha RP. Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity. Physicochem Probl Miner Process. 2010;45:85–98. [Google Scholar]
- 8.Shameli K, Ahmad MB, Zargar M, Wan Yunus WMZ, Rustaiyan A, Ibrahim NA. Synthesis of silver nanoparticles in mont-morillonite and their antibacterial behavior. Int J Nanomedicine. 2010;6:581–590. doi: 10.2147/IJN.S17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shameli K, Ahmad MB, Wan Yunus WMZ, Ibrahim NA. Synthesis and characterization of silver/talc nanocomposites using the wet chemical reduction method. Int J Nanomedicine. 2010;5:743–751. doi: 10.2147/IJN.S13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shameli K, Ahmad MB, Zargar M, Wan Yunus WMZ, Ibrahim NA. Fabrication of silver nanoparticles doped in the zeolite framework and antibacterial activity. Int J Nanomedicine. 2011;6:331–341. doi: 10.2147/IJN.S16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shameli K, Ahmad MB, Jazayeri SD, et al. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int J Mol Sci. 2012;13:6639–6650. doi: 10.3390/ijms13066639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastoriza-Santos I, Liz-Marzán LM. Formation and stabilization of silver nanoparticles through reduction by N,N-dimethylformamide. Langmuir. 1999;15:948–951. [Google Scholar]
- 13.Ahmad MB, Shameli K, Wan Yunus WMZ, Ibrahim NA. Synthesis and characterization of silver/clay/starch bionanocomposites by green method. Aust J Basic Appl Sci. 2010;4:2158–2165. [Google Scholar]
- 14.Setua P, Pramanik R, Sarkar S. Synthesis of silver nanoparticle inside the nonaqueous ethylene glycol reverse micelle and a comparative study to show the effect of the nanoparticle on the reverse micellar aggregates through solvation dynamics and rotational relaxation measurements. J Phys Chem B. 2010;114:7557–7564. doi: 10.1021/jp1008048. [DOI] [PubMed] [Google Scholar]
- 15.Praus P, Turicová M, Klementová M. Preparation of silver-montmorillonite nanocomposites by reduction with formaldehyde and borohydride. J Braz Chem Soc. 2009;20:1351–1357. [Google Scholar]
- 16.Sun L, Zhang Z, Dang H. A novel method for preparation of silver nanoparticles. Mater Lett. 2003;57:3874–3879. [Google Scholar]
- 17.Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125:13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- 18.Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloid Surface B. 2009;73:332–338. doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Rai A, Singh A, Ahmad A, Sastry M. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir. 2006;22:736–741. doi: 10.1021/la052055q. [DOI] [PubMed] [Google Scholar]
- 20.Gardea-Torresdey JL, Parsons JG, Dokken K, et al. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002;2:397–401. [Google Scholar]
- 21.Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357–1361. [Google Scholar]
- 22.Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M. Biological synthesis of triangular gold nanoprisms. Nat Mater. 2004;3:482–488. doi: 10.1038/nmat1152. [DOI] [PubMed] [Google Scholar]
- 23.Zargar M, Hamid AA, Bakar FA, et al. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules. 2011;16:6667–6676. doi: 10.3390/molecules16086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shameli K, Ahmad MB, Al-Mulla EAJ, et al. Green biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extraction. Molecules. 2012;17:8506–8517. doi: 10.3390/molecules17078506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape and dielectric environment. J Phys Chem B. 2003;107:668–677. [Google Scholar]
- 26.Stepanov AL. Optical properties of metal nanoparticles synthesized in a polymer by ion implantation: a review. Tech Phys. 1997;49:143–153. [Google Scholar]
- 27.Shameli K, Ahmad MB, Jazayeri SD, et al. Investigation of antibacterial properties silver nanoparticles prepared via green method. Chem Cent J. 2012;(6):73. doi: 10.1186/1752-153X-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad MB, Shameli K, Darroudi M, Wan Yunus WMZ, Ibrahim NA. Synthesis and characterization of silver/clay nanocomposites by chemical reduction method. Am J Appl Sci. 2009;6:1909–1914. [Google Scholar]
- 29.Shameli K, Ahmad MB, Zargar M, et al. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int J Nanomedicine. 2011;6:271–284. doi: 10.2147/IJN.S16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Li Q, Sun D, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18:1–11. [Google Scholar]
- 31.Luo L, Yu S, Qian S, Zhou T. Large-scale fabrication of flexible silver/cross-linked poly(vinyl alcohol) coaxial nanoscale by a facial solution approach. J Am Chem Soc. 2005;127:2822–2823. doi: 10.1021/ja0428154. [DOI] [PubMed] [Google Scholar]