Abstract
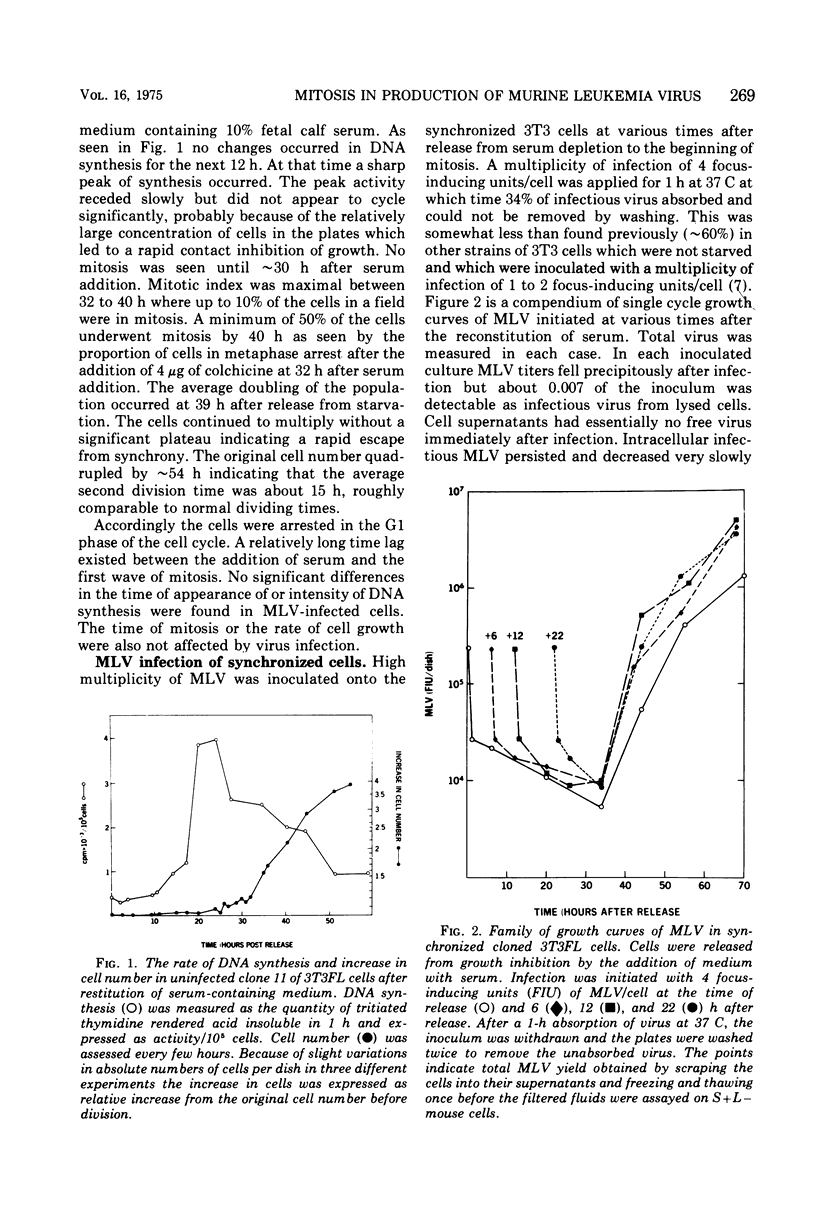
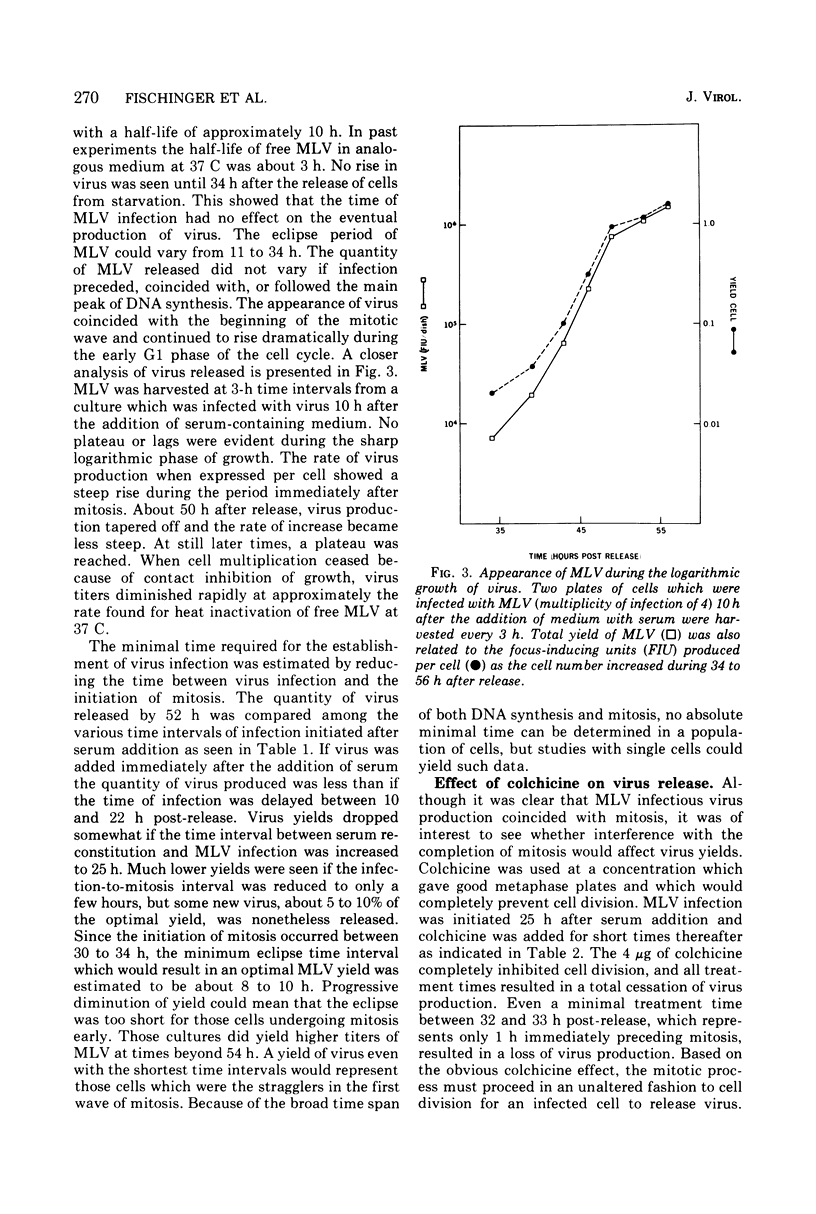
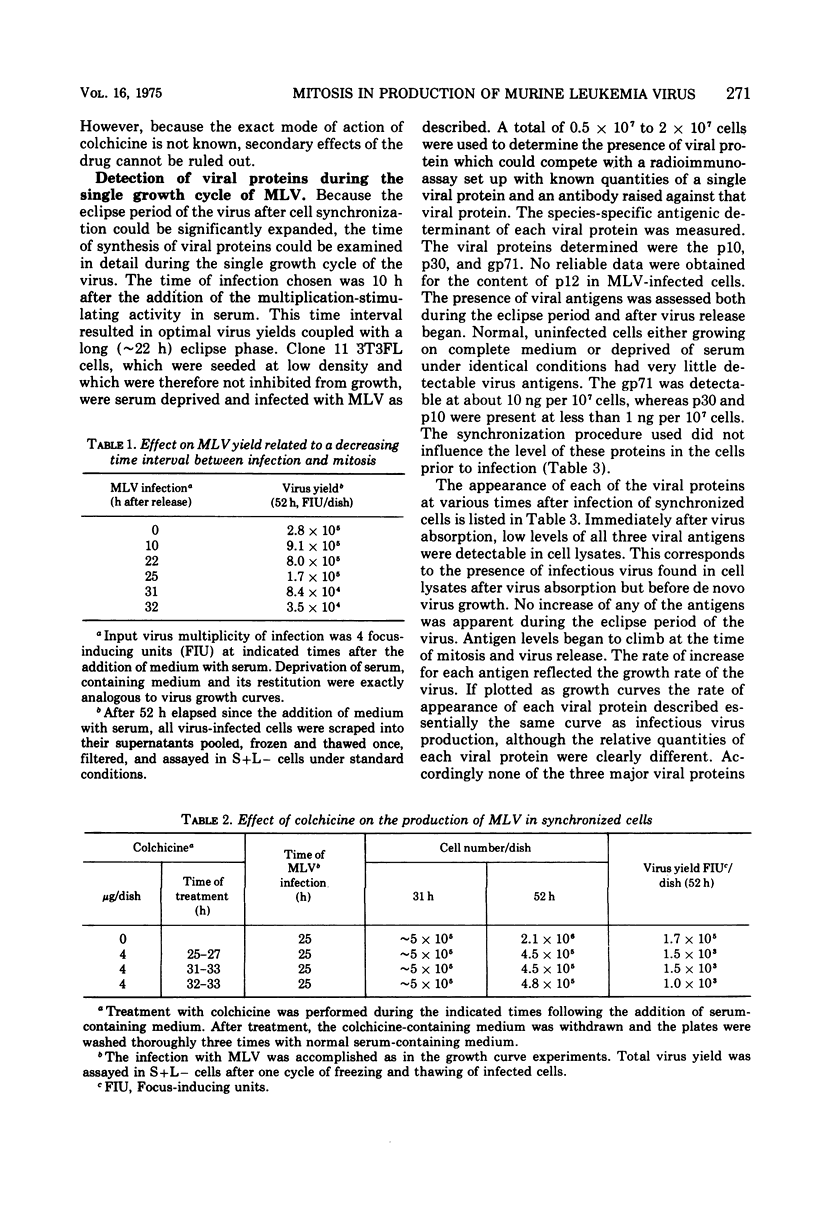
Cloned 3T3FL cells were synchronized in G1 phase of the cell cycle by deprivation of multiplication stimulatory activity of serum and were then infected with Moloney leukemia virus. Eclipse period of virus could be made to vary from less than 10 to 34 h. All virus release was completely dependent and occurred immediately after the first mitosis following serum reconstitution. Virus yield was not affected by the time of virus inoculation as related to the cell DNA synthetic phase. Colchicine arrested the cells in mitosis and prevented the formation of infectious virus. Viral proteins p10, p30, and gp71 were assayed in cell lysates during the growth curve of virus in synchronized cells. The group-specific determinants of each protein were measured in a competition radioimmunoassay. None of the virus proteins appeared during the eclipse period of the virus. All three proteins appeared simultaneously, coincident with mitosis, and continued to rise during the G1 phase. The absolute quantities of each protein were proportional to the amount of Moloney leukemia virus produced. The relative amounts of some of the viral proteins in the cell did not correspond to their content in purified virions suggesting several possible mechanisms of control.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Luftig R., Shaper J. H. Localization of RNA tumor virus polypeptides. I. Isolation of further virus substructures. Virology. 1973 Dec;56(2):549–564. doi: 10.1016/0042-6822(73)90057-3. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Haapala D. K. Quantitative interactions of feline leukaemia virus and its pseudotype of murine sarcoma virus in cat cells: requirement for DNA synthesis. J Gen Virol. 1971 Nov;13(2):203–214. doi: 10.1099/0022-1317-13-2-203. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Moore C. O., O'Connor T. E. Isolation and identification of a helper virus found in the Moloney sarcoma-leukemia virus complex. J Natl Cancer Inst. 1969 Apr;42(4):605–622. [PubMed] [Google Scholar]
- Fischinger P. J., O'Connor T. E. Replication of defective and competent forms of murine sarcoma virus in mouse cell cultures. Virology. 1970 Jun;41(2):233–243. doi: 10.1016/0042-6822(70)90075-9. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. P., Bigner D. D., Fischinger P. J., Bolognesi D. P. Expression of murine leukemia virus structural antigens on the surface of chemically induced murine sarcomas. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5037–5041. doi: 10.1073/pnas.71.12.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P., Schäfer W., Pister L., Hunsmann G., De Noronha F. Polypeptides of mammalian oncornaviruses. I. Isolation and serological analysis polypeptides from murine and feline C-type viruses. Virology. 1973 Dec;56(2):565–579. doi: 10.1016/0042-6822(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Cell cycle-dependent activation of rous sarcoma virus-infected stationary chicken cells: avian leukosis virus group-specific antigens and ribonucleic acid. J Virol. 1972 Jul;10(1):82–87. doi: 10.1128/jvi.10.1.82-87.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J Virol. 1974 Sep;14(3):531–546. doi: 10.1128/jvi.14.3.531-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moennig V., Frank H., Hunsmann G., Schneider I., Schafer W. Properties of mouse leukemia viruses. VII. The major viral glycoprotein of friend leukemia virus. Isolation and physicochemical properties. Virology. 1974 Sep;61(1):100–111. doi: 10.1016/0042-6822(74)90245-1. [DOI] [PubMed] [Google Scholar]
- Nomura S., Fischinger P. J., Mattern C. F., Peebles P. T., Bassin R. H., Friedman G. P. Revertants of mouse cells transformed by murine sarcoma virus. I. Characterization of flat and transformed sublines without a rescuable murine sarcoma virus. Virology. 1972 Oct;50(1):51–64. doi: 10.1016/0042-6822(72)90345-5. [DOI] [PubMed] [Google Scholar]
- Panem S., Schauf V. Cell-cycle dependent appearance of murine leukemia-sarcoma virus antigens. J Virol. 1974 Jun;13(6):1169–1175. doi: 10.1128/jvi.13.6.1169-1175.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Fischinger P. J., Bassin R. H., Papageorge A. G. Isolation of human amnion cells transformed by rescuable murine sarcoma virus. Nat New Biol. 1973 Mar 28;242(117):98–101. doi: 10.1038/newbio242098a0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Strand M., Lilly F., August J. T. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3682–3686. doi: 10.1073/pnas.71.9.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. II. Relationship of virus-stimulated DNA synthesis to other viral functions. Virology. 1969 Dec;39(4):653–665. doi: 10.1016/0042-6822(69)90004-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]



