Abstract
Whereas adult sex differences in brain morphology and behavior result from developmental exposure to steroid hormones, the mechanism by which steroids differentiate the brain is unknown. Studies to date have described subtle sex differences in levels of proteins and neurotransmitters during brain development, but these have lacked explanatory power for the profound sex differences induced by steroids. We report here a major divergence in the response to injection of the γ-aminobutyric acid type A (GABAA) agonist, muscimol, in newborn male and female rats. In females, muscimol treatment primarily decreased the phosphorylation of cAMP response element binding protein (CREB) within the hypothalamus and the CA1 region of the hippocampus. In contrast, muscimol increased the phosphorylation of CREB in males within these same brain regions. Within the arcuate nucleus, muscimol treatment increased the phosphorylation of CREB in both females and males. Thus, the response to GABA can be excitatory or inhibitory on signal-transduction pathways that alter CREB phosphorylation depending on the sex and the region in developing brain. This divergence in response to GABA allows for a previously unknown form of steroid-mediated neuronal plasticity and may be an initial step in establishing sexually dimorphic signal-transduction pathways in developing brain.
Sex differences in adult brain morphology and behavior are determined by the release of testicular testosterone during a discrete developmental period. Testosterone is secreted at high levels by the testis around embryonic day 18 and again on the day of birth. Exposure to these two surges of testosterone in males leads to masculinization of the brain, whereas the relative absence of testosterone in females results in feminization of the brain. The conversion of testosterone to estradiol by neuronal tissue is a critical step in the process of sexual differentiation of the brain (1–3). Although the prenatal testosterone surge is important, altering postnatal hormone levels can profoundly affect sexual differentiation of brain and behavior. Castration of newborn male rats prevents behavioral masculinization (4, 5), and testosterone administration to newborn females induces behavioral masculinization (6, 7). Postnatal changes in steroid hormone levels also influence neuronal migration, neuronal survival, and the plasticity of both neurons and glia (8–10).
It is well established that many of the actions of steroid hormones occur by means of the activation of intracellular steroid receptors (11, 12); however, the cellular and molecular consequences that follow steroid receptor activation during sexual differentiation of the brain are largely unknown. Previous research has focused on quantitative differences in the levels of neurotransmitters (13) or other cellular proteins (14, 15). For instance, we have observed significantly higher levels of γ-aminobutyric acid (GABA; ref. 16) and calcium-binding proteins (17) in newborn males, as contrasted to females. Although these quantitative differences are clearly important, it is difficult to envision how relatively minor and transient changes in the amount of a particular transmitter or cellular protein could induce the profound differences in male versus female physiology and behavior. We report here a major point of divergence in a signal-transduction pathway mediating cellular responses to GABA in male and female brain during the critical period, and we propose this divergence as an essential step in establishing sex differences in adult brain.
The GABAA receptor is a chloride ionophore that is selectively permeable to chloride and bicarbonate ions. Chloride will flow through the channel in either direction, depending on its concentration gradient. In adulthood, activation of the GABAA receptor is associated with a hyperpolarizing (inhibitory) effect, because the resting anion gradient favors net chloride influx at resting membrane potential. Hence, opening the anion-selective GABAA ionophore results in accumulated negative charge intracellularly, hyperpolarization of the membrane, and synaptic inhibition. However, in recent years, it has become apparent that variation in the transmembrane anionic gradient can alter the properties of the cell such that activation of the GABAA receptor can result in a depolarizing or excitatory effect. More importantly, during development, it seems that most, if not all, neurons first respond to GABA with depolarization and gradually develop a hyperpolarizing response as they mature (18). The magnitude of the depolarization induced by GABAA receptor activation in immature neurons is sufficient to open L-type voltage-gated calcium channels leading to significant increases in intracellular free Ca2+. As a result, GABA action during development is important in influencing neuronal survival (19, 20), neurite outgrowth (21), and synapse formation (22).
The developmental switch from excitatory to inhibitory GABA occurs gradually, and the precise timing is likely specific for each brain region. Recently, we have found that gonadal steroids extend the developmental duration of excitatory GABA in cultured hypothalamic neurons (23). Extrapolating from in vitro findings, we have formulated the working model that in the newborn male hypothalamus, GABA action is predominantly excitatory, whereas in the newborn female, GABA action is inhibitory (23, 24). To test this model, we hypothesized that GABA would increase the activity of signal-transduction pathways associated with the phosphorylation of cAMP response element binding protein (CREB) in male but not in female rat brain. CREB is a member of a large family of transcription factors that bind to DNA and increase transcription of target genes. CREB alters gene expression after phosphorylation of Ser-133 by protein kinase A, Ca2+-activated calmodulin kinase, ribosomal S6 kinase 2, or mitogen-activated protein kinase-activated protein kinase 2 (25). The phosphorylation of CREB has been implicated in many processes that are known to be critical for sexual differentiation of the brain, such as neuronal survival, activity, and synaptic plasticity (25, 26).
Methods
Animals.
Adult female Sprague–Dawley rats (Charles River Breeding Laboratories) were mated in our animal facility; mating was confirmed by the presence of sperm in vaginal smears. Pregnant females were allowed to deliver normally, and cages were checked regularly to determine the day of birth.
Injections.
The selective GABAA receptor agonist, muscimol (25 μg in 0.05 cc), or saline vehicle were administered s.c. to male (muscimol, n = 6; vehicle, n = 5) and female (muscimol, n = 9; vehicle, n = 9) rat pups on the day of birth. Treatments were done by two investigators so that injections of muscimol or saline occurred simultaneously in two different pups at a time. The brains were removed simultaneously from muscimol- or saline-treated pups 30 min after injection, immersed in 4% (vol/vol) paraformaldehyde for 72 h, and then placed into 0.1 M PBS containing 20% (wt/vol) sucrose overnight. Brains were sectioned at 50 μm with a cyrostat, and alternating sections were placed into two separate wells containing cryoprotectant. This procedure allowed the use of half the brain for immunocytochemical detection of phosphorylated CREB (pCREB) and the other half for total CREB (phosphorylated plus unphosphorylated).
Immunocytochemistry.
Female and male brain sections were processed separately to maximize the immunocytochemical detection of pCREB and CREB protein for each sex. This procedure was necessary because the linear range of immunocytochemical detection is limited by saturation on the upper end and lack of sensitivity at the lower end. Conditions for the immunocytochemical detection (ICC) of pCREB were optimized to avoid saturation in males and increased sensitivity at the low end in females. We performed four separate immunocytochemical runs: pCREB ICC for females, pCREB ICC for males, CREB ICC for females, and CREB ICC for males.
Sections were rinsed three times for 5 min each in 0.1 M PBS (pH 7.6), and placed into 1% H2O2, 20% (vol/vol) normal goat serum (NGS) and 1% BSA for 30 min to reduce nonspecific staining and endogenous peroxidase activity. Sections were then incubated with pCREB or CREB antibody (rabbit polyclonal; Upstate Biotechnology, Lake Placid, NY; 1:1,000 dilution) overnight at room temperature in PBS containing 0.3% Triton X-100 and 1% NGS. The pCREB antibody is specific for Ser-133 phosphorylated CREB. The CREB antibody recognizes both the Ser-133 phosphorylated and unphosphorylated forms of CREB. After primary incubation, sections were rinsed three times in PBS for 5 min each time and then incubated with biotinylated goat anti-rabbit IgG (7.5 μg/ml) diluted in PBS for 90 min at room temperature. After three additional rinses in PBS, sections were incubated with the DH:biotinylated horseradish peroxidase H complex (1:200 in PBS) for 90 min. Sections were rinsed three more times in PBS for 5 min each, and reacted with 0.05% diaminobenzidine and 0.05% H2O2 in PBS. The reaction was stopped for all sections simultaneously in any one immunocytochemical run as soon as positive staining of CREB or pCREB was observed. The ICC procedure was carried out by two investigators working simultaneously to ensure that all sections were treated and reacted equally. Developed sections were mounted onto gelatin-coated slides and coverslipped with Permount mounting medium. The antibodies for CREB and pCREB have been extensively characterized (27), and the omission of primary antibodies in the immunocytochemical procedure eliminated all staining. In addition, preadsorption of primary antibodies with control peptides for pCREB and CREB blocked all immunoreactivity.
Computer-Aided Image Analysis.
CREB-immunoreactive cell counts were carried out with a Zeiss microscope fitted with an MTI CCD72 camera (Dage–MTI, Michigan City, IN) connected to a Macintosh computer and the National Institutes of Health nih image program (http://rsb.info.nih.gov/nih-image/) for image analysis. Before tissue examination, the microscope was adjusted for Kohler illumination at 10 × 10 magnification. Optical densities were read by the nih image analysis program as pixels, which ranged in value from 0 (white) to 255 (black). At this magnification, a single pixel represented a 2-μm area, and the average particle size measured was ≈10 pixels (one particle represents one cell). To maximize the detection of darkly stained nuclei over background, the video camera gain and black levels were adjusted so that no more than 10% of the immunoreactive cells were above a mean pixel value of 200. The mean pixel value of an area on the slide adjacent to the tissue also was measured and then set to a value of ≈5 by adjusting the black level and gain level on the video camera. This method allowed us to exploit the full dynamic range of the nih image system. Once the camera gain and black levels were adjusted, they remained calibrated for all sections analyzed.
A critical component of this analysis is the establishment of stringent criteria for the counting of individual particles (cells). The threshold was established as follows: (i) the mean pixel value for the entire area and its SD was determined; (ii) the contribution of darkly immunostained cells was removed, and a new mean and SD were calculated; (iii) a threshold was set that was four SDs above this mean; and (iv) particles that exceeded this threshold were counted. Thus, only cells that were darker than four SDs above background were included in the analysis. The threshold remained constant for all sections. All sections had a variation in background that was within 2–3 SDs of the average background for the immunocytochemical run. Individual cells within the CA1 region of the hippocampus could not be resolved with nih image; therefore, we drew a 200 by 100 pixel box within the CA1 region and measured the area covered by immunoreactivity.
Neuroanatomical Areas Analyzed.
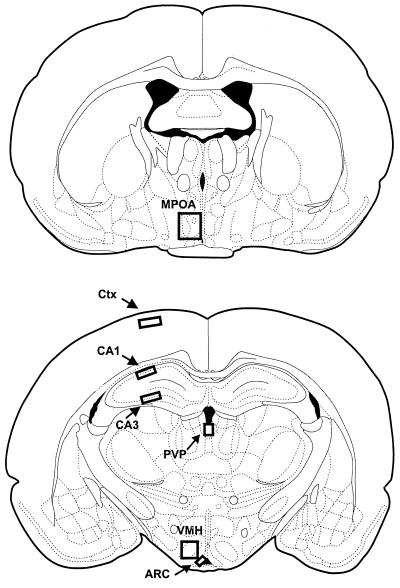
One brain section per area was anatomically matched according to the rat brain atlas of Paxinos and Watson (28). Areas examined expressed high levels of gonadal steroid receptors, such as the medial preoptic area (MPOA), arcuate nucleus (Arc), ventromedial hypothalamus (VMH), and the CA1 and CA3 regions of the hippocampus (Fig. 1). A portion of the Ctx was also examined as well as the PVP, an area that exhibits few to no gonadal steroid receptors. A box of preset size was optimized for each brain region and used to analyze the same overall area for each section. The relative size of these boxes and areas analyzed are depicted in Fig. 1.
Figure 1.
Schematic drawing of areas in which the pCREB-immunoreactive cell number was quantified. Boxes indicate the area used for image analysis within a given region. PVP, paraventricular thalamic nucleus, posterior part; and Ctx, frontal cortex.
Statistical Analysis.
Bilateral measurements of each area were added and analyzed with statview statistical software (SAS Institute, Cary, NC). Results were analyzed by using a two-factor ANOVA. Posthoc comparisons were made by a Fisher's least significance difference test. Means were considered significantly different at P < 0.05.
Results
Influence of Muscimol on pCREB-ir in Females.
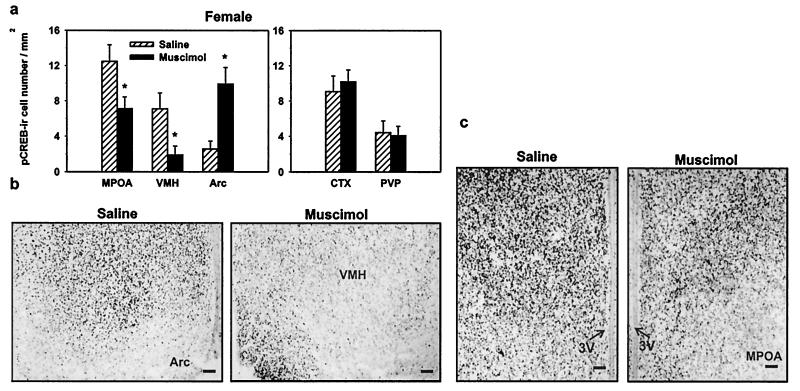
In neonatal females, injection of muscimol significantly decreased pCREB immunoreactivity within the VMH by 73% and the MPOA by 43%; however, muscimol increased pCREB immunoreactivity within the Arc by 287% (Fig. 2; P < 0.05, two-way ANOVA). Muscimol treatment also decreased pCREB immunoreactivity within the CA1 region of the hippocampus by 18% (P < 0.05). Muscimol treatment did not alter pCREB immunoreactivity in any other brain region examined in females, such as the Ctx, PVP, or the CA3 region of the hippocampus (P > 0.05). In adjacent sections, there were no differences in the total number of CREB-immunoreactive cells between the treatment groups (data not shown; P > 0.05), indicating that changes in pCREB immunoreactivity were specifically caused by alterations in the phosphorylation state of CREB. Because of the semiquantitative nature of the immunocytochemical procedure, cell counts may be underestimates of the total number of cells that actually express pCREB or CREB.
Figure 2.
In females, GABAA-receptor activation either increases or decreases the phosphorylation of CREB in sexually dimorphic brain regions. (a) Activation of GABAA receptors by muscimol leads to a decrease in the number of cells expressing pCREB within the MPOA and the VMH of female rat brain. In contrast, GABAA-receptor activation leads to an increase in the number of cells expressing pCREB within the Arc of female rat brain (*, P < 0.05). GABAA-receptor activation by muscimol does not alter pCREB-immunoreactive cell number within the CTX or the PVP in female rat brain. (b) Representative photomicrographs of pCREB immunoreactivity within the VMH and the Arc in saline- or muscimol-treated rats. Note the opposite effects on the expression of pCREB immunoreactivity in the VMH and the Arc after GABAA-receptor activation by muscimol. (c) Photomicrographs of pCREB immunoreactivity within the MPOA of saline- or muscimol-treated female rats. (Bar, 50 μm.)
Influence of Muscimol on pCREB-ir in Males.
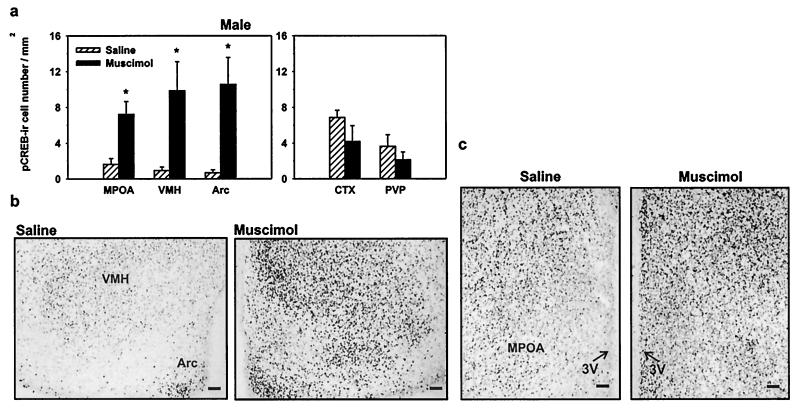
In contrast to females, injection of muscimol significantly increased pCREB immunoreactivity in male brain within the VMH by 962% (P < 0.05), the MPOA by 343%, and the Arc by 1,463% (Fig. 3; P < 0.05). Muscimol treatment in males also increased pCREB immunoreactivity within the CA1 region of the hippocampus by 76% (Fig. 4; P < 0.05). Muscimol had no effect on pCREB immunoreactivity in any other brain region examined in males, such as the Ctx, PVP, or the CA3 region of the hippocampus (P > 0.05). In adjacent sections, there were no differences found in the total number of CREB-immunoreactive cells between the two groups (data not shown; P > 0.05), indicating that changes in the pCREB-immunoreactive number were caused by alterations in the phosphorylation of CREB.
Figure 3.
In males, GABAA-receptor activation increases the phosphorylation of CREB in sexually dimorphic brain regions. (a) Activation of GABAA receptors by muscimol leads to an increase in the number of cells expressing pCREB within the MPOA, VMH, and the Arc of male rat brain (*, P < 0.05). GABAA-receptor activation by muscimol does not change the pCREB-immunoreactive cell number within the CTX or the PVP of male brain. (b) Representative photomicrographs of pCREB immunoreactivity within the VMH and the Arc in saline- or muscimol-treated rats. Note that GABAA-receptor activation by muscimol increases pCREB-immunoreactive cell number within the VMH and the Arc. (c) Photomicrographs of pCREB immunoreactivity within the MPOA of saline- or muscimol-treated male rats. (Bar, 50 μm.)
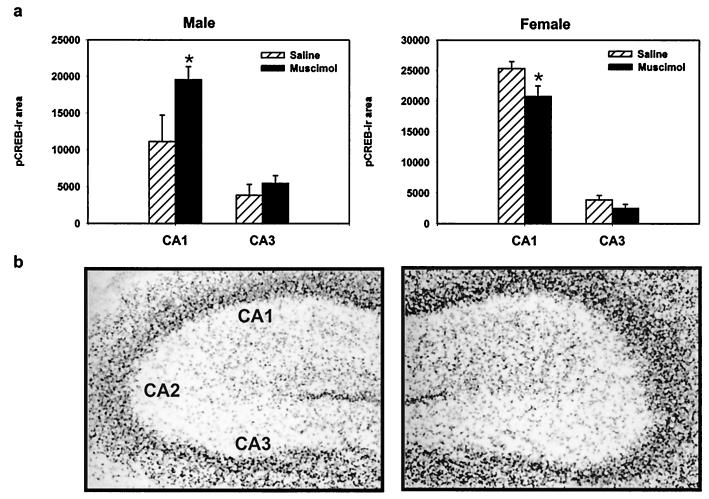
Figure 4.
GABAA-receptor activation alters immunoreactivity for phosphorylated CREB within the CA1 but not in the CA3. (a) In males (Left), muscimol treatment increases the pCREB-immunoreactive area within the CA1 region but not in the CA3 region (*, P < 0.05). (b) In females (Right), muscimol treatment decreases the pCREB-immunoreactive area within the CA1 region of the hippocampus but not in the CA3 region of the hippocampus. (c) Photomicrographs of pCREB immunoreactivity within the hippocampus of saline- (Left) or muscimol-treated males (Right).
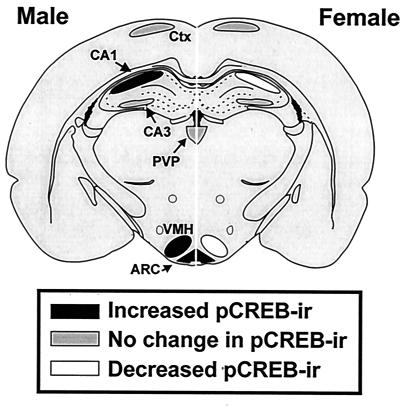
Results of the muscimol-induced changes in pCREB immunoreactivity in neonatal male and female rat brain are summarized in Fig. 5.
Figure 5.
Summary of the effects of GABAA-receptor activation by muscimol on the phosphorylation of CREB. (Left) Effects of muscimol relative to saline on pCREB immunoreactivity in male rat brain. (Right) Effects of muscimol on pCREB immunoreactivity in female rat brain. Black, increased pCREB-immunoreactive cell number; gray, unchanged pCREB-immunoreactive cell number; white, decreased pCREB-immunoreactive cell number.
Discussion
Our data indicate that in developing brain, GABA can be excitatory or inhibitory on signal-transduction pathways associated with the phosphorylation of CREB, depending on sex and region. Administration of muscimol to newborn females decreased the phosphorylation of CREB in the VMH, POA, and the CA1 region of the hippocampus; however, muscimol treatment increased the phosphorylation of CREB within the Arc. Therefore, muscimol treatment in females can be inhibitory or excitatory on signal-transduction pathways that lead to the phosphorylation of CREB in a region-specific manner. In contrast to females, the administration of muscimol to newborn males rapidly increased the phosphorylation of CREB in brain regions where it decreased phosphorylation in females. Muscimol treatment also increased the phosphorylation of CREB in the Arc of males, suggesting that the effects of GABA may be selectively retained in this brain region for both sexes during this developmental period.
A potential source of the diversity in response to GABA is the differential expression of ion cotransporters regulating the transmembrane chloride gradient (29). In cells where GABA is inhibitory, intracellular chloride concentration is low, and GABA action results in a chloride influx which leads to membrane hyperpolarization. In cells where GABA is excitatory, intracellular chloride concentration is high, and GABA action results in a chloride efflux which leads to membrane depolarization. The K-Cl cotransporter (KCC2), which keeps intracellular chloride concentrations low, is expressed at lower levels in neonate contrasted to adult; furthermore, reducing the expression of KCC2 in mature neurons with antisense oligodeoxynucleotides results in a positive shift in the reversal potential of GABA (30). Another cotransporter, bumetanide-sensitive cotransporter 2 (BSC2), which transports chloride into cells, is expressed at higher levels in the neonatal brain contrasted to adult brain (31). The voltage-gated chloride channel (ClC2) allows chloride to flow out of the cell maintaining low intracellular chloride and is also differentially expressed over development. Low levels of expression are found embryonically and during the first few days of life, and higher levels are found with increasing age (32). More importantly, GABA-mediated depolarization of dorsal root ganglion cells can be attenuated by de novo expression of ClC2 (33). Therefore, the differential expression of chloride cotransporters and channels, such as KCC2, BSC2, and ClC2, may be a dominant mechanism for the heterogeneous response to GABA in males and females.
An alternative explanation for the divergence in response to GABA on the day of birth is that there are sex differences in the expression of phosphatases or phosphatase inhibitors. For example, the perinatal surges in testosterone could up-regulate the expression or phosphorylation of phosphatase inhibitors, such as the dopamine- and cAMP-regulated phosphoprotein, DARPP-32. Phosphorylation of DARPP-32 leads to the inhibition of phosphatase-1 which dephosphorylates proteins (34). Therefore, increased phosphorylation of DARPP-32 can lead to increased phosphorylation within a cell by decreasing the activity of phosphatase-1. Recent data indicate estradiol can increase the phosphorylation of DARPP-32 within the hypothalamus (35). Because DARPP-32 is a potent phosphatase inhibitor, males may experience greater phosphorylation in response to GABA because of the inhibition of phosphatases by DARPP-32. In contrast, the lack of the perinatal surges in testosterone in females may prevent increased activity of DARPP-32, resulting in increased activity of phosphatases which could lead to decreased phosphorylation of proteins. Support for this alternative hypothesis comes from data showing that phosphatase-1 can be activated by calcium influx after activation of the GABAA receptor (34).
Regardless of the mechanism, the response to GABA seems to be a major point of divergence in the process of steroid-mediated sexual differentiation of the brain. That is, the influence of GABA can be excitatory or inhibitory on signal-transduction pathways that influence the phosphorylation state of CREB between newborn males and females. Central to this divergence is the pattern of calcium influx experienced by the cell in response to GABA. In hypothalamic neurons, the magnitude of calcium transients induced by excitatory GABA in the presence of gonadal hormones is similar to that induced by glutamate (23), whereas in the absence of gonadal hormones, GABA results in low calcium influx (36). Changes in the amplitude as well as the location of calcium influx within a cell can selectively activate or inhibit particular kinases and phosphatases (26), and may be the basis for the ability of muscimol to induce phosphorylation or dephosphorylation of CREB depending on brain region and sex. The broader significance of this polarity is that developing neurons have the capacity to diametrically respond to the same stimulus, leading to different functional outcomes.
Research from our lab has indicated that GABA-mediated outcomes can be sex specific in developing brain. Reducing GABA levels immediately after birth interferes with behavioral masculinization in males and behavioral feminization in females (37). Therefore, GABA action is critical in the development of normal male sex behavior as well as to the development of normal female sex behavior. The sexually dimorphic pattern of CREB phosphorylation in response to muscimol may be involved in mediating the effects of GABA during development.
The sex difference in the phosphorylation of CREB may also be a mechanism for the sex difference in cell death within sexually dimorphic areas, such as the sexually dimorphic nucleus (SDN) of the preoptic area. The SDN is 3–4 times larger in males than in females (38). Castration of males on the day of birth reduces the size of the SDN, and testosterone treatment of females on the day of birth increases the size of the SDN. The sex difference in the size of the SDN is known to be caused by cell death, which occurs at a higher rate in females contrasted to males from postnatal days 6–10 (39, 40). Interestingly, phosphorylation of CREB protects against cell death induced by okadaic acid (41) and is correlated with increased neuronal survival after hypoxia-ischemia (42). As GABA levels on the day of birth are higher in males as contrasted to females (16), perhaps GABA-induced CREB phosphorylation protects cells against the naturally occurring cell death within the SDN. GABA-induced phosphorylation of CREB may also be involved in mediating the effects of steroid hormones on the later changes in neuronal and glial plasticity observed in sexually dimorphic regions.
The differential regulation of CREB phosphorylation may have profound effects on gene expression given that increased phosphorylation of CREB can increase gene expression, whereas decreased phosphorylation of CREB can decrease gene expression (25, 26). Alternatively, the phosphorylation of CREB may be actively recruiting corepressors to the transcriptional complex and thereby decreasing gene expression. Lastly, the possibility that unphosphorylated CREB influences gene expression cannot be ruled out. The potential for GABA to exert opposing effects on gene expression is demonstrated by the observation that excitatory GABA increases the expression of brain-derived neurotrophic factor, whereas inhibitory GABA decreases its expression (43).
Our data are consistent with those showing that GABA can be excitatory during development, and further demonstrate that GABA action can be excitatory or inhibitory on signal-transduction pathways associated with the phosphorylation of CREB in developing rat brain. The sexually dimorphic regulation of CREB phosphorylation observed here may have implications for the differential expression of genes that underlie brain development and sexual differentiation.
Abbreviations
- GABA
γ-aminobutyric acid
- CREB
cAMP response element binding protein
- pCREB
phosphorylated CREB
- ICC
immunocytochemical detection
- MPOA
medial preoptic area
- Arc
arcuate nucleus
- VMH
ventromedial hypothalamus
- Ctx
frontal cortex
- PVP
paraventricular thalamic nucleus, posterior part
- SDN
sexually dimorphic nucleus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Naftolin F, Ryan K J, Petro Z. J Clin Endocrinol Metab. 1971;33:368–370. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- 2.Ryan K J, Naftolin F, Reddy V, Flores F, Petro Z. Am J Obstet Gynecol. 1972;114:454–460. doi: 10.1016/0002-9378(72)90204-9. [DOI] [PubMed] [Google Scholar]
- 3.MacLusky N J, Naftolin F. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 4.Whalen R E, Edwards D A. Anat Rec. 1967;157:173–180. doi: 10.1002/ar.1091570208. [DOI] [PubMed] [Google Scholar]
- 5.Beach F, Holz A M. J Exp Zool. 1946;101:91–142. doi: 10.1002/jez.1401010107. [DOI] [PubMed] [Google Scholar]
- 6.Wilson J G, Hamilton J B, Young W C. Yale J Biol Med. 1940;13:189–202. [PMC free article] [PubMed] [Google Scholar]
- 7.Feder H H, Phoenix C H, Young W C. J Endocrinol. 1966;34:131–132. doi: 10.1677/joe.0.0340131. [DOI] [PubMed] [Google Scholar]
- 8.Cooke B, Hegstrom C D, Villeneuve L S, Breedlove S M. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 9.Mong J A, McCarthy M M. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Gorski R A. Can J Physiol Pharmacol. 1985;63:577–594. doi: 10.1139/y85-098. [DOI] [PubMed] [Google Scholar]
- 11.Tsai M J, O'Malley B W. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 12.Blaustein J D, Olster D H. In: Advances in Comparative and Environmental Physiology. Balthazart J, editor. Berlin: Springer; 1989. pp. 31–104. [Google Scholar]
- 13.DeVries G J. J Neuroendocrinol. 1990;2:1–13. [Google Scholar]
- 14.Wagner C K, Nakayama A Y, DeVries G J. Endocrinology. 1998;139:3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Yoshida S, Nishihara M, Takahashi M. Neurosci Lett. 1998;242:127–130. doi: 10.1016/s0304-3940(98)00008-1. [DOI] [PubMed] [Google Scholar]
- 16.Davis A M, Ward S C, Selmanoff M, Herbison A E, McCarthy M M. Neuroscience. 1999;90:1471–1482. doi: 10.1016/s0306-4522(98)00511-9. [DOI] [PubMed] [Google Scholar]
- 17.Brager D H, Sickel M J, McCarthy M M. J Neurobiol. 2000;42:315–322. [PubMed] [Google Scholar]
- 18.Obrietan K, van den Pol A N. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda Y, Nishiyama N, Saito H, Katsuki H. Brain Res Dev Brain Res. 1997;98:253–258. doi: 10.1016/s0165-3806(96)00183-6. [DOI] [PubMed] [Google Scholar]
- 20.Obata K. Dev Neurosci (Basel) 1997;19:117–119. doi: 10.1159/000111195. [DOI] [PubMed] [Google Scholar]
- 21.Barbin G, Pollard H, Gaiarsa J L, Ben Ari Y. Neurosci Lett. 1993;152:150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C K, Redburn D A. Brain Res Dev Brain Res. 1996;95:63–71. doi: 10.1016/0165-3806(96)00064-8. [DOI] [PubMed] [Google Scholar]
- 23.Perrot-Sinal T S, Davis A M, Gregerson K A, Kao J P Y, McCarthy M M. Endocrinology. 2001;142:2238–2243. doi: 10.1210/endo.142.6.8180. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy M M, Perrot-Sinal T S, Auger A P, Sickel M J. In: Neuroplasticity, Development, and Steroid Hormone Action. Handa R J, Terasawa E, Hayashi S, Kawata M, editors. Boca Raton, FL: CRC; 2000. pp. 323–345. [Google Scholar]
- 25.Shaywitz A J, Greenberg M E. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 26.Finkbeiner S, Greenberg M E. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- 27.Ginty D D, Kornhauser J M, Thompson M A, Bading H, Mayo K E, Takahashi J S, Greenberg M E. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic; 1986. [Google Scholar]
- 29.Smith R L, Clayton G H, Wilcox C L, Escudero K W, Staley K J. J Neurosci. 1995;15:4057–4067. doi: 10.1523/JNEUROSCI.15-05-04057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera C, Voipio J, Payne J A, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. Nature (London) 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin M D, Snyder E Y, Hebert S C, Delpire E. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Clayton G H, Staley K J, Wilcox C L, Owens G C, Smith R L. Brain Res Dev Brain Res. 1998;108:307–318. doi: 10.1016/s0165-3806(98)00045-5. [DOI] [PubMed] [Google Scholar]
- 33.Staley K, Smith R, Schaack J, Wilcox C, Jentsch T J. Neuron. 1996;17:543–551. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 34.Greengard P, Allen P B, Nairn A C. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 35.Mani S K, Fienberg A A, O'Callaghan J P, Snyder G L, Allen P B, Dash P K, Moore A N, Mitchell A J, Bibb J, Greengard P, et al. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- 36.Xu W, Cormier R, Fu T, Covey D F, Isenberg K E, Zorumski C F, Mennerick S. J Neurosci. 2000;20:3147–3156. doi: 10.1523/JNEUROSCI.20-09-03147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis A M, Grattan D R, McCarthy M M. Behav Neurosci. 2000;114:923–933. [PubMed] [Google Scholar]
- 38.Gorski R A, Gordon J H, Shryne J E, Southam A M. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 39.Chung W C J, Swaab D F, De Vries G J. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- 40.Davis E C, Popper P, Gorski R A. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 41.Walton M, Woodgate A M, Muravlev A, Xu R, During M J, Dragunow M. J Neurochem. 1999;73:1836–1842. [PubMed] [Google Scholar]
- 42.Walton M R, Dragunow I. Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- 43.Berninger B, Marty S, Zafra F, da Penha B M, Thoenen H, Lindholm D. Development (Cambridge, UK) 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]