Abstract
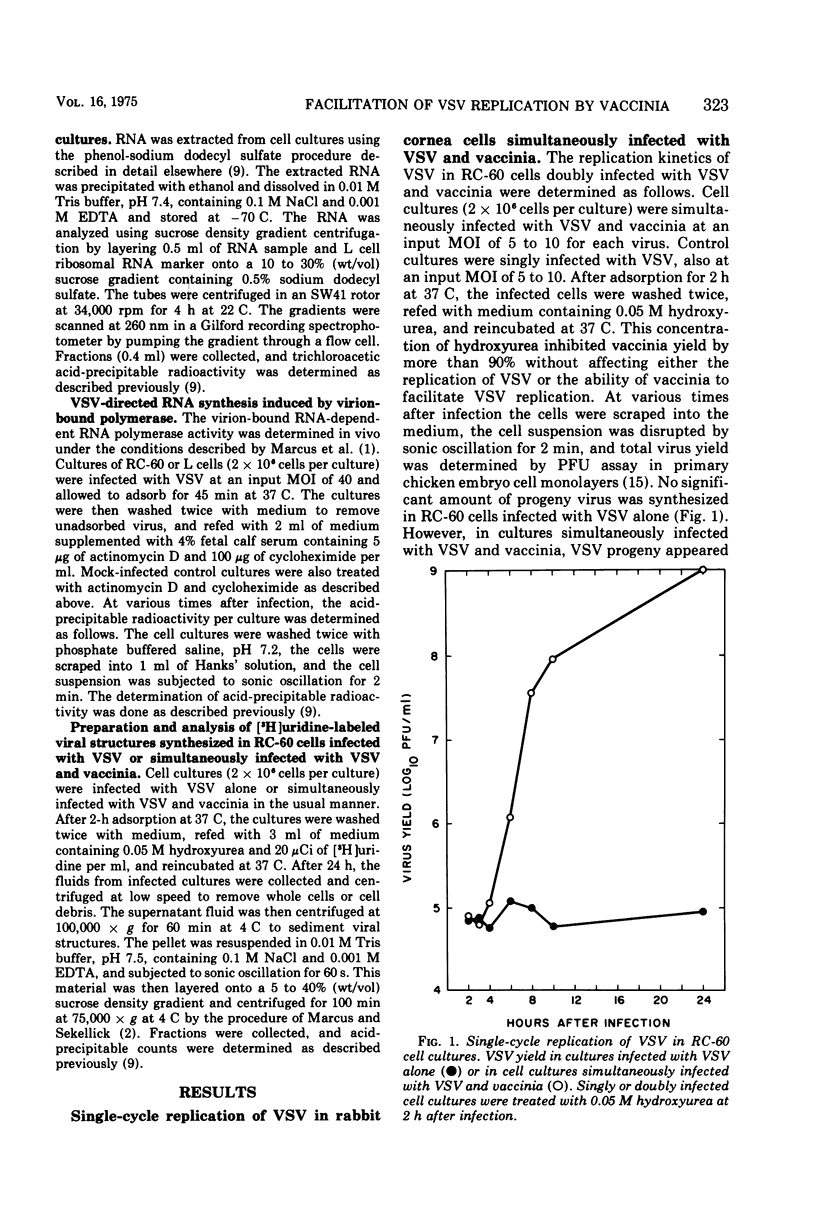
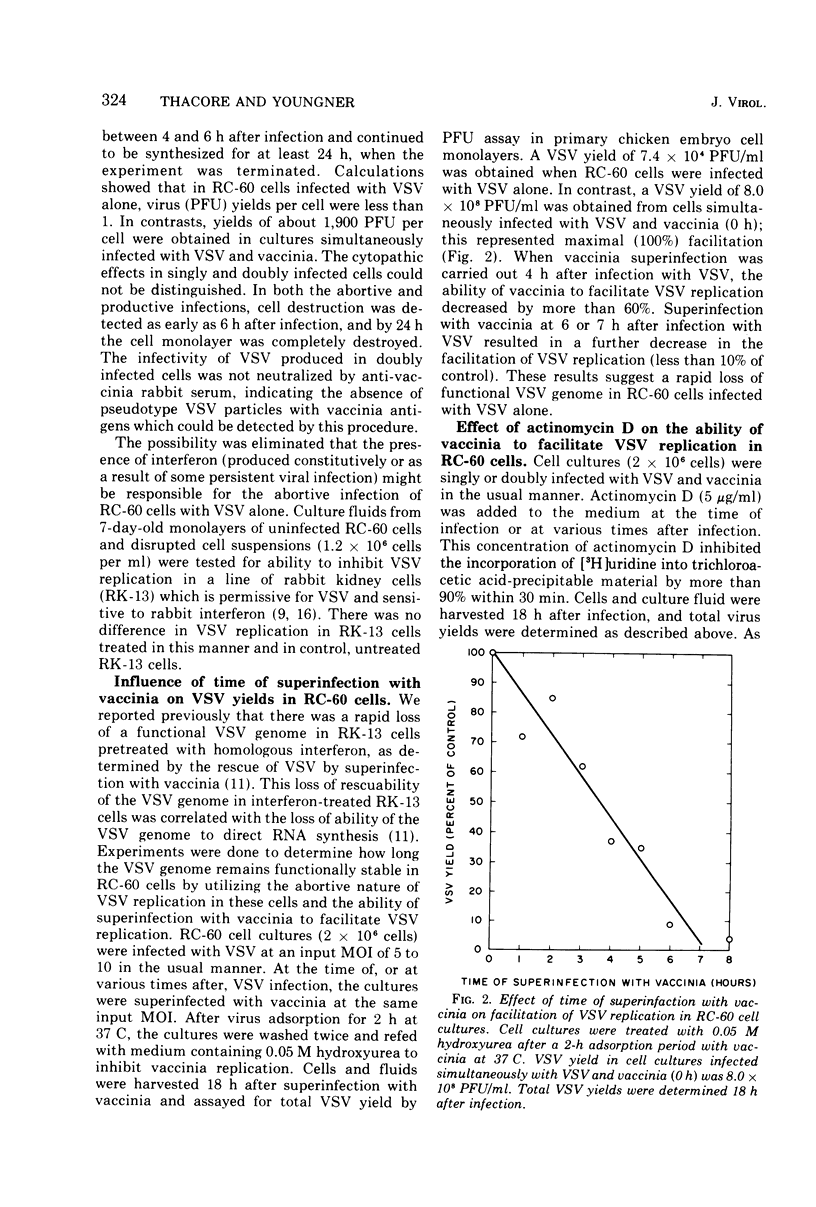
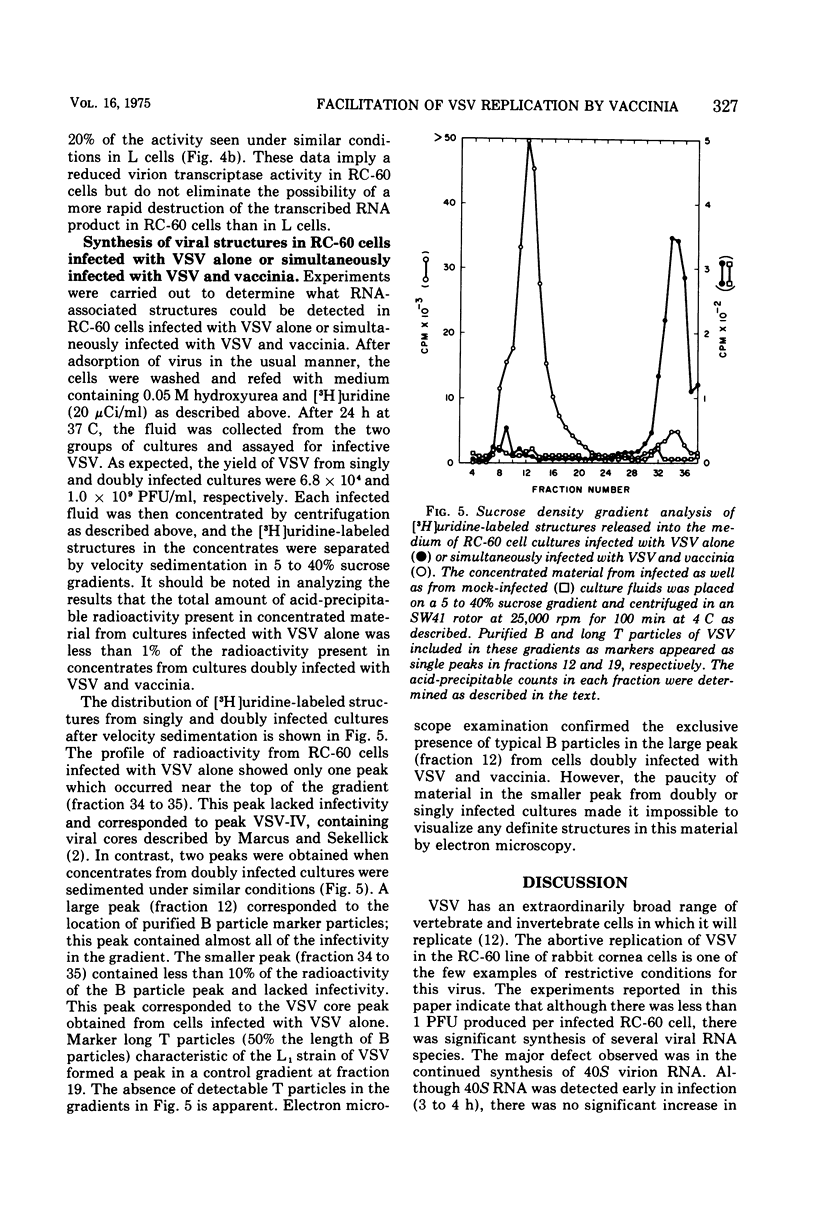
An abortive infection of a rabbit cornea cell line (RC-60) by vesicular stomatitis virus (VSV), yielding less than 1 PFU/cell, was converted to a productive infection, yielding 1,900 PFU/cell, when cells were superinfected with vaccinia. Studies on the synthesis of VSV-directed RNA in RC-60 cells suggest that the abortive infection by VSV alone may be due in part to (i) a limited production of 40S virion RNA and (ii) a markedly reduced activity of virion-bound transcriptase activity in RC-60 cells compared to the activity in mouse L cells, a permissive host for VSV. No recognizable VSV structures, except a small amount of viral core structures, were produced by the abortive infection. In contrast, double infection of RC-60 cells with VSV and vaccinia in the presence of hydroxyurea resulted in the production of infective B particles of VSV. Although the function supplied by vaccinia responsible for the productive replication of VSV in double infected RC-60 cells has not been identified, metabolic inhibitor studies indicate that continuous vaccinia-dependent RNA synthesis is required for maximal production of infective VSV. The possibility is considered that vaccinia may supply a product or function required for VSV replication which is ordinarily supplied by the host but which is lacking in RC-60 cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Nowakowski M., Bloom B. R., Ehrenfeld E., Summers D. F. Restricted replication of vesicular stomatitis virus in human lymphoblastoid cells. J Virol. 1973 Dec;12(6):1272–1278. doi: 10.1128/jvi.12.6.1272-1278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA's from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968 Jun 10;31(5):685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. I. Viral specific RNA and nucleoprotein in infected L cells. Virology. 1970 Nov;42(3):732–743. doi: 10.1016/0042-6822(70)90319-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Obijeski J. F. Conditional lethal mutants of vesicular stomatitis virus. I. Phenotypic characterization of single and double mutants exhibiting host restriction and temperature sensitivity. Virology. 1974 Feb;57(2):357–368. doi: 10.1016/0042-6822(74)90175-5. [DOI] [PubMed] [Google Scholar]
- Soria M., Huang A. S. Association of polyadenylic acid with messenger RNA of vesicular stomatitis virus. J Mol Biol. 1973 Jul 5;77(3):449–455. doi: 10.1016/0022-2836(73)90450-6. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Persistence of vesicular stomatitis virus in interferon-treated cell cultures. Virology. 1975 Feb;63(2):345–351. doi: 10.1016/0042-6822(75)90308-6. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Rescue of vesicular stomatitis virus from interferon-induced resistance by superinfection with vaccinia virus. I. Rescue in cell cultures from different species. Virology. 1973 Dec;56(2):505–511. doi: 10.1016/0042-6822(73)90053-6. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Rescue of vesicular stomatitis virus from interferon-induced resistance by superinfection with vaccinia virus. II. Effect of UV-inactivated vaccinia and metabolic inhibitors. Virology. 1973 Dec;56(2):512–522. doi: 10.1016/0042-6822(73)90054-8. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Viral ribonuclei acid synthesis by Newcastle disease virus mutants isolated from persistently infected L cells: effect of interferon. J Virol. 1972 Mar;9(3):503–509. doi: 10.1128/jvi.9.3.503-509.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatitis virus. J Virol. 1970 Oct;6(4):476–484. doi: 10.1128/jvi.6.4.476-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild T. F. Replication of vesicular stomatitis virus: characterization of the virus-induced RNA. J Gen Virol. 1971 Nov;13(2):295–310. doi: 10.1099/0022-1317-13-2-295. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Thacore H. R., Kelly M. E. Sensitivity of ribonucleic acid and deoxyribonucleic acid viruses to different species of interferon in cell cultures. J Virol. 1972 Aug;10(2):171–178. doi: 10.1128/jvi.10.2.171-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


