Abstract
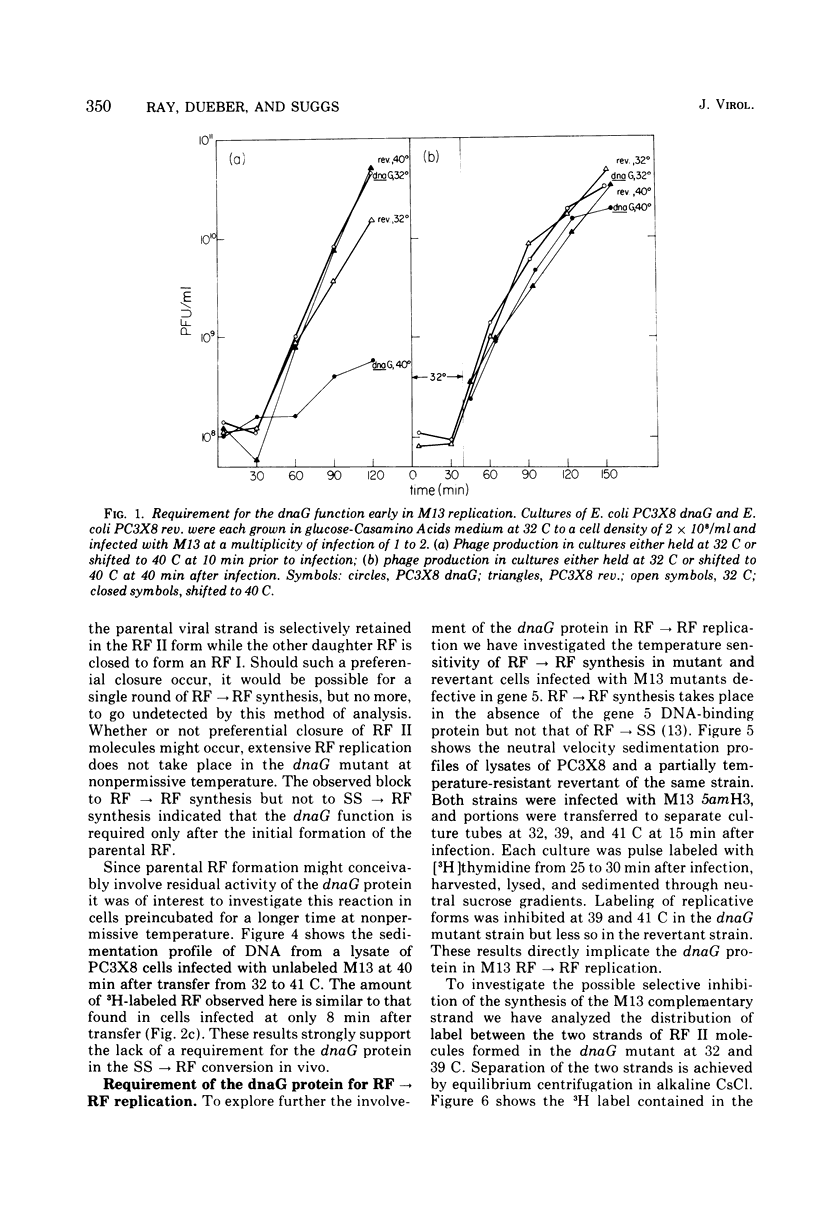
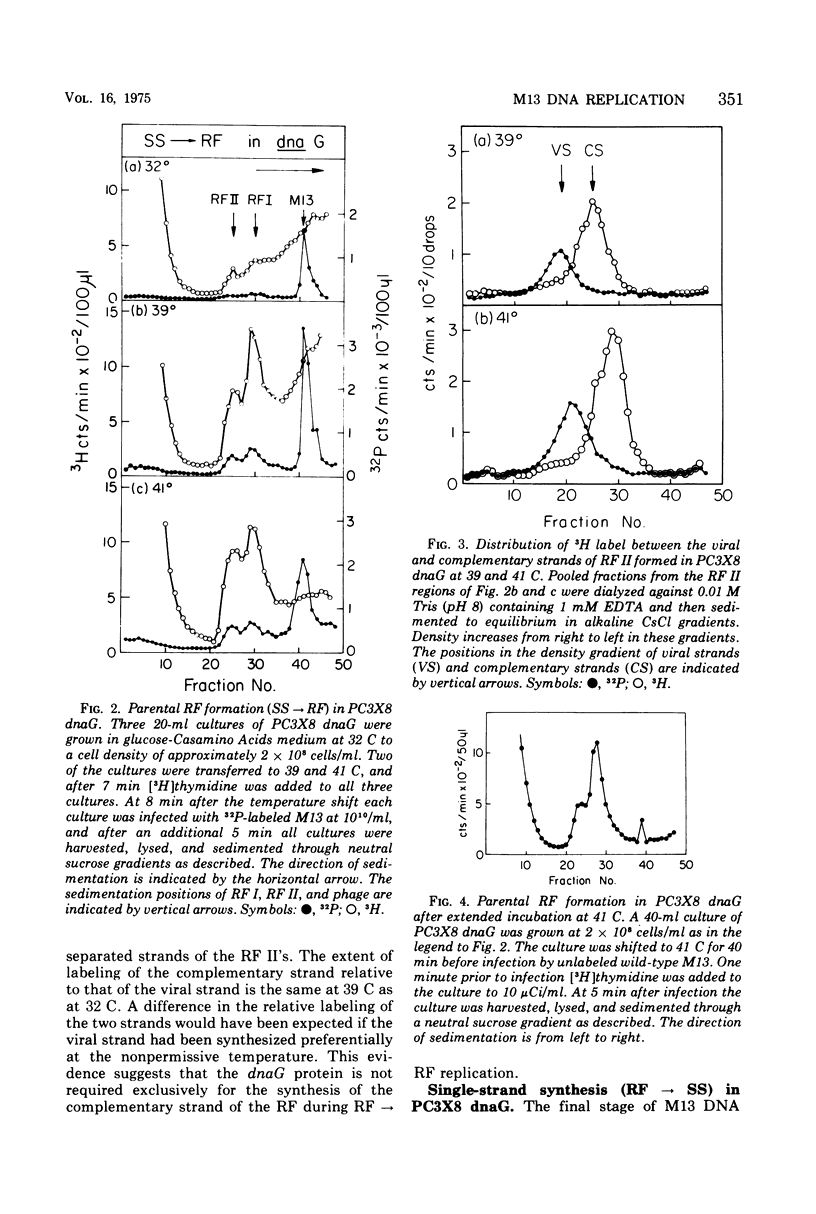
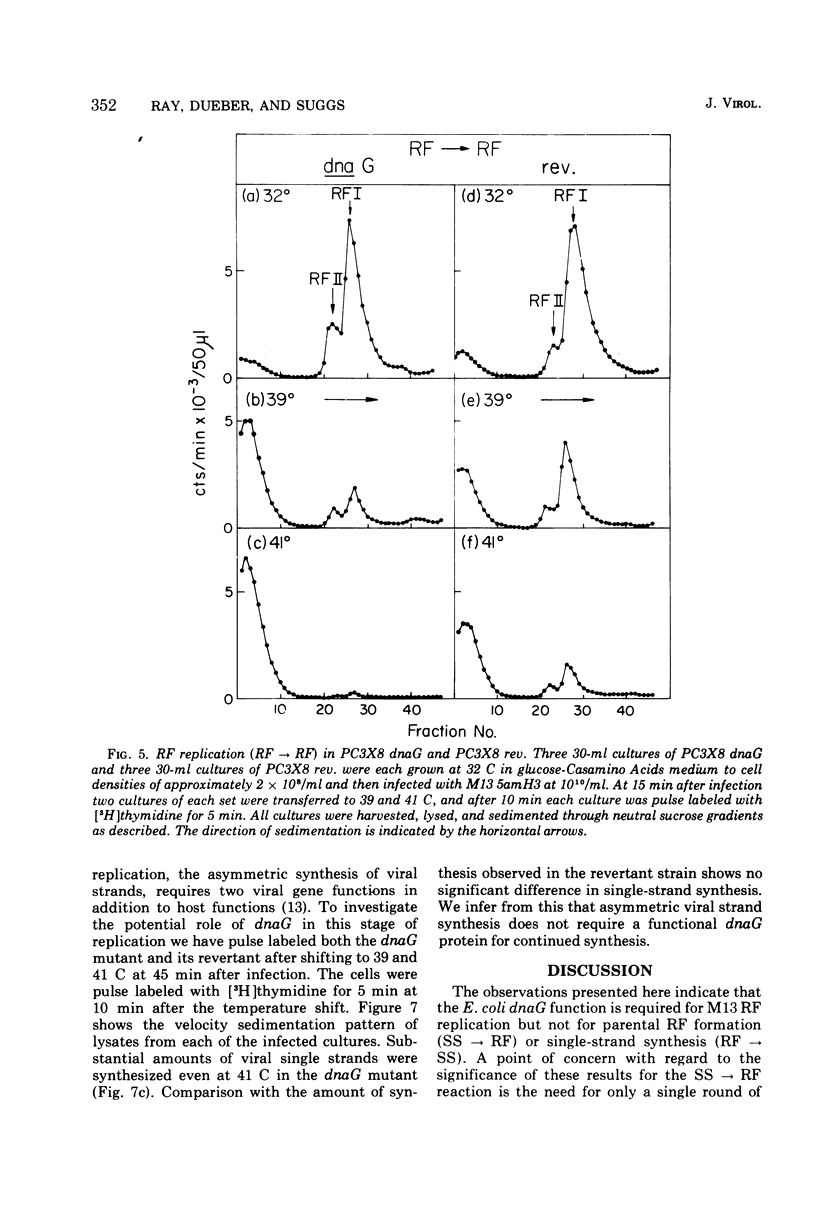
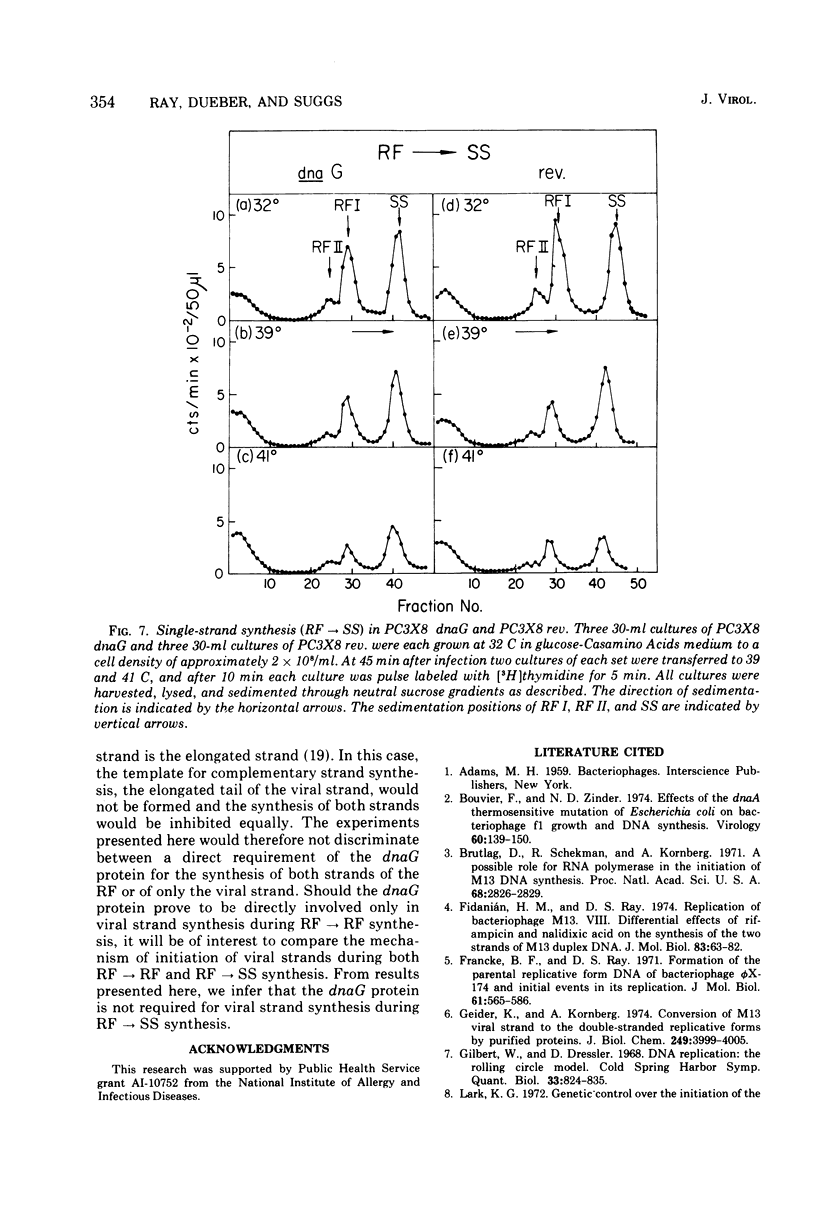
Temperature-shift experiments with an Escherichia coli dnaG strain indicate a requirement for the dnaG function for M13 phage production only at an early stage of infection. Mutant cells infected at nonpermissive temperature form the parental RF (SS leads to RF) but do not replicate further. A shift to nonpermissive temperature after infection inhibits RF leads to RF replication but not RF leads to SS synthesis. The synthesis of both strands of the duplex RF was inhibited equally after a temperature shift during RF leads to RF replication. We infer that the dnaG protein is required for M13 production only during RF replication and that it is required for the synthesis of both strands of the RF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvier F., Zinder N. D. Effects of the dnaA thermosensitive mutation of Escherichia coli on bacteriophage fl growth and DNA synthesis. Virology. 1974 Jul;60(1):139–150. doi: 10.1016/0042-6822(74)90371-7. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidanián H. M., Ray D. S. Replication of bacteriophage M13. 8. Differential effects of rifampicin and nalidixic acid on the synthesis of the two strands of M13 duplex DNA. J Mol Biol. 1974 Feb 15;83(1):63–82. doi: 10.1016/0022-2836(74)90424-0. [DOI] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Lark K. G. Genetic control over the initiation of the synthesis of the short deoxynucleotide chains in E. coli. Nat New Biol. 1972 Dec 20;240(103):237–240. doi: 10.1038/newbio240237a0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden G., Denhardt D. T. Mechanism of replication of phi x174 single-stranded DNA. IX. Requirement for the Escherichia coli dnaG protein. J Virol. 1974 Nov;14(5):1070–1075. doi: 10.1128/jvi.14.5.1070-1075.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Stallions D. R. Role of dna genes of Escherichia coli in M13 phage replication. Virology. 1973 Apr;52(2):417–424. doi: 10.1016/0042-6822(73)90336-x. [DOI] [PubMed] [Google Scholar]
- Olsen W. L., Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M13: specificity of the Escherichia coli dnaB function for replication of double-stranded M13 DNA. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2570–2573. doi: 10.1073/pnas.69.9.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. I. Sedimentation analysis of crude lysates of M13-infected bacteria. Biochim Biophys Acta. 1969 Apr 22;179(2):398–407. [PubMed] [Google Scholar]
- Staudenbauer W. L., Olsen W. L., Hofschneider P. H. Analysis of bacteriophage-M 13-DNA replication in an Escherichia coli mutant thermosensitive in DNA polymerase 3. Eur J Biochem. 1973 Jan 15;32(2):247–253. doi: 10.1111/j.1432-1033.1973.tb02604.x. [DOI] [PubMed] [Google Scholar]
- Truitt C. L., Walker J. R. Growth of phages lambda, phiX174, and Ml3 requires the dnaZ (previously dnaH) gene product of Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1036–1042. doi: 10.1016/0006-291x(74)90259-9. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Marvin D. A. Filamentous bacterial viruses. V. Asymmetric replication of fd duplex deoxyribonucleic acid. J Virol. 1972 Sep;10(3):371–383. doi: 10.1128/jvi.10.3.371-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Wright M., Wickner S., Hurwitz J. Conversion of phiX174 and fd single-stranded DNA to replicative forms in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3233–3237. doi: 10.1073/pnas.69.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Kornberg A. A novel form of RNA polymerase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4425–4428. doi: 10.1073/pnas.71.11.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



