Abstract
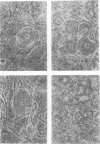
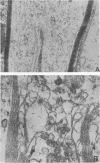
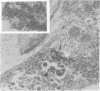
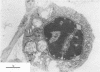
Replication of encephalomyocarditis virus and its cytopathic effects were studied in myelinated cultures of dorsal root ganglia obtained from newborn mice. Six hours after infection virus progeny was detected in the culture. At 24 h the virus titer reached 2 times 10(6) PFU per culture and remained at this level until 48 h. The first cytopathic alterations began at 24 h and consisted of rounding of Schwann and satellites cells and their detachment from neurons. Later, bead-like swellings of the myelin appeared along the axons followed by splitting and degeneration of lamellae. The cytopathic effect in the neurons started 29 h after infection, reaching complete neuronolysis at 48 h. Virus particles, scattered or arranged in crystal-like aggregates, were first seen in the cytoplasm of glial cells and then in neurons and axons.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Peterson E. R., Murray M. R. A light and electron microscope study of long-term organized cultures of rat dorsal root ganglia. J Cell Biol. 1967 Feb;32(2):439–466. doi: 10.1083/jcb.32.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge R. P., Harter D. H. Cytopathic effects of Visna Virus in cultured mammalian nervous tissue. J Neuropathol Exp Neurol. 1969 Apr;28(2):185–194. [PubMed] [Google Scholar]
- Burch G. E., Harb J. M. Encephalomyocarditis (EMC) virus infection of the femoral artery of newborn mice. Proc Soc Exp Biol Med. 1974 Oct;147(1):11–15. doi: 10.3181/00379727-147-38271. [DOI] [PubMed] [Google Scholar]
- Campbell J. B., Colter J. S. Studies of three variants of Mengo encephalomyelitis virus. IV. Affinities for mouse tissues in vitro and in vivo. Virology. 1967 May;32(1):69–73. doi: 10.1016/0042-6822(67)90253-x. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Pathogenicity of the M and E variants of the encephalomyocarditis (EMC) virus. I. Myocardiotropic and neurotropic properties. Am J Pathol. 1966 Feb;48(2):333–345. [PMC free article] [PubMed] [Google Scholar]
- DALES S., FRANKLIN R. M. A comparison of the changes in fine structure of L cells during single cycles of viral multiplication, following their infection with the viruses of Mengo and encephalomyocarditis. J Cell Biol. 1962 Aug;14:281–302. doi: 10.1083/jcb.14.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson L., Griffiths A. J. The pathogenesis of experimental infections with encephalomyocarditis (EMC) virus. Br J Exp Pathol. 1966 Feb;47(1):35–44. [PMC free article] [PubMed] [Google Scholar]
- Feldman L. A., Sheppard R. D., Bornstein M. B. Herpes simplex virus-host cell relationships in organized cultures of mammalian nerve tissues. J Virol. 1968 Jun;2(6):621–628. doi: 10.1128/jvi.2.6.621-628.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak N. R., Zimmerman H. M. Spinal ganglion in herpes zoster. A light and electron microscopic study. Arch Pathol. 1973 Jun;95(6):411–415. [PubMed] [Google Scholar]
- HINZ R. W., BARSKI G., BERNHARD W. An electron microscopic study of the development of the encephalomyocarditis (EMC) virus propagated in vitro. Exp Cell Res. 1962 Mar;26:571–586. doi: 10.1016/0014-4827(62)90163-5. [DOI] [PubMed] [Google Scholar]
- Leestma J. E., Bornstein M. B., Sheppard R. D., Feldman L. A. Ultrastructural aspects of herpes simplex virus infection in organized cultures of mammalian nervous tissue. Lab Invest. 1969 Jan;20(1):70–78. [PubMed] [Google Scholar]
- Matsumoto S., Schneider L. G., Kawai A., Yonezawa T. Further studies on the replication of rabies and rabies-like viruses in organized cultures of mammalian neural tissues. J Virol. 1974 Oct;14(4):981–996. doi: 10.1128/jvi.14.4.981-996.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON E. R., MURRAY M. R. PATTERNS OF PERIPHERAL DEMYELIMINATION IN VITRO. Ann N Y Acad Sci. 1965 Mar 31;122:39–50. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Barbosa L. H., Bornstein M. B. Subacute sclerosing panencephalitis virus. Observations on a neuroadapted and non-neuroadapted strain in organotypic central nervous system cultures. Lab Invest. 1974 Jul;31(1):42–53. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Subacute sclerosing panencephalitis virus in cultures of organized central nervous tissue. Lab Invest. 1973 May;28(5):627–640. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructural study of long-term measles infection in cultures of hamster dorsal-root ganglion. J Virol. 1971 Sep;8(3):318–329. doi: 10.1128/jvi.8.3.318-329.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar A., Grunfeld Y., Spiegelstein M. Y., Monzain R. Myelinization in long-term cultures of dissociated mammalian neurons. Brain Res. 1975 Apr 25;88(1):44–51. doi: 10.1016/0006-8993(75)90945-2. [DOI] [PubMed] [Google Scholar]
- Shahar A., Monzain R., Straussman Y. Silicone rubber membrane as a support for long-term cultivation and electron microscopic processing of nervous tissue. Tissue Cell. 1973;5(4):691–696. doi: 10.1016/s0040-8166(73)80054-0. [DOI] [PubMed] [Google Scholar]