Abstract
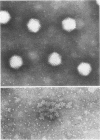
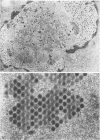
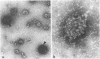
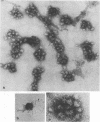
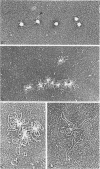
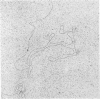
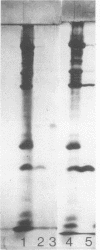
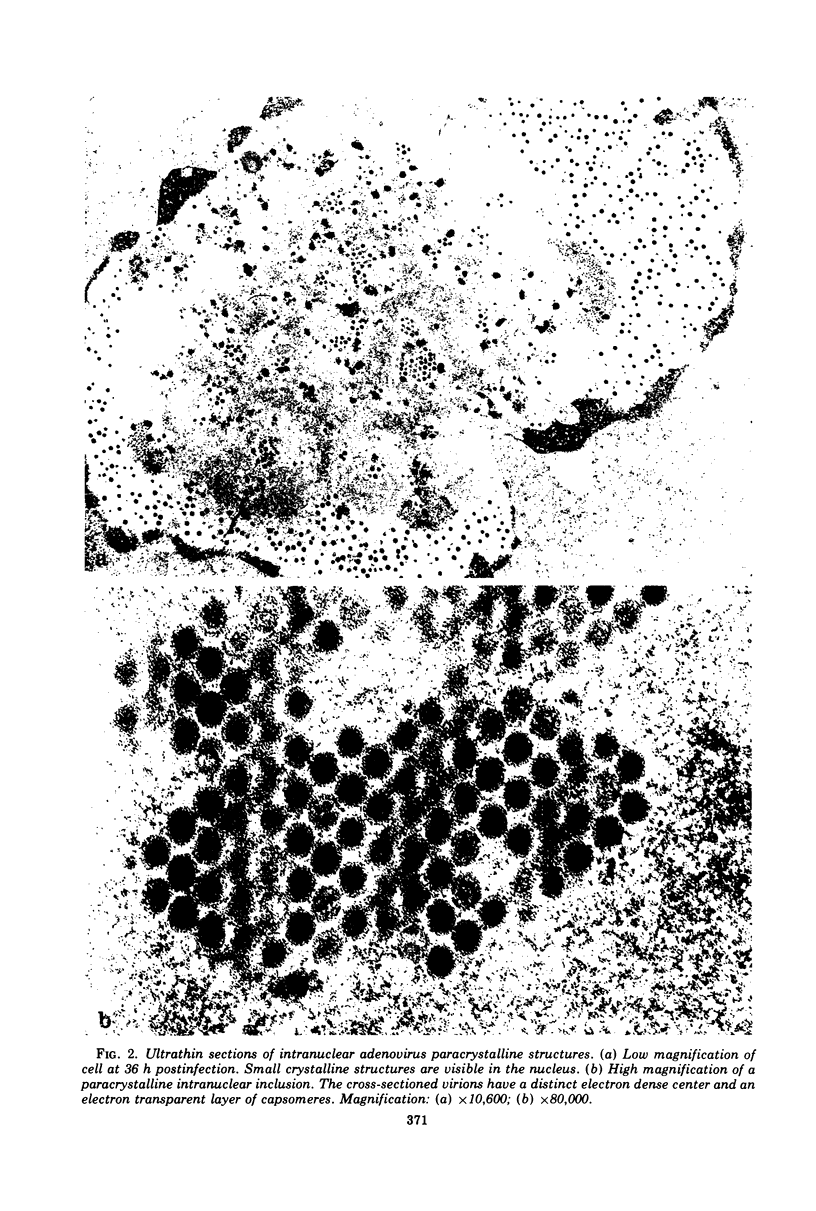
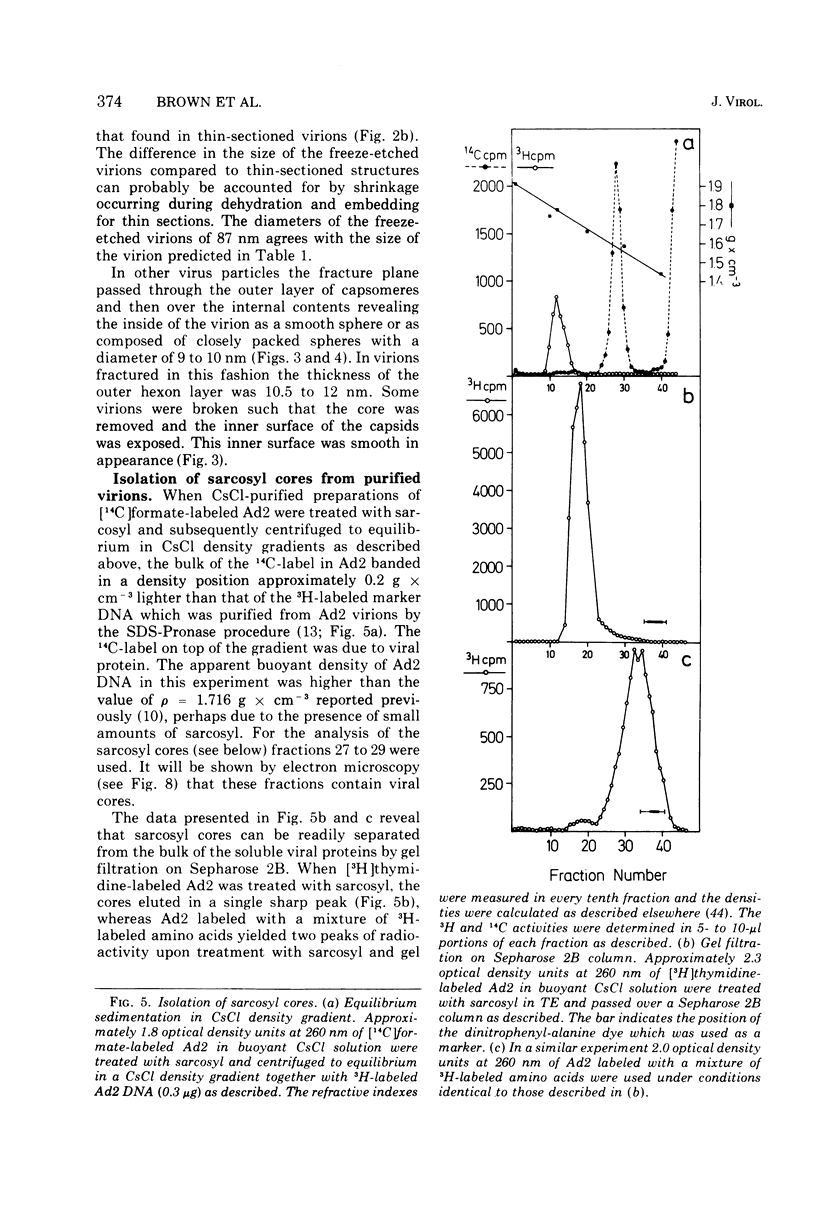
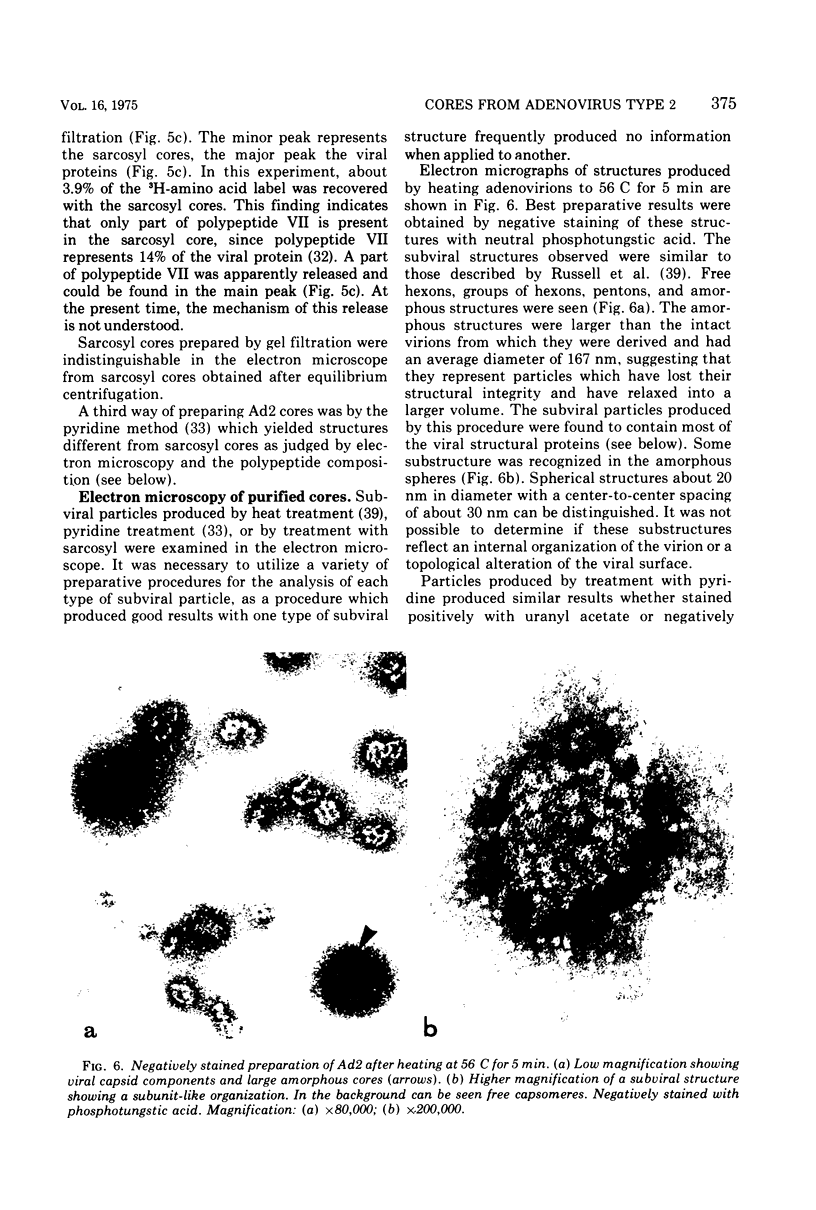
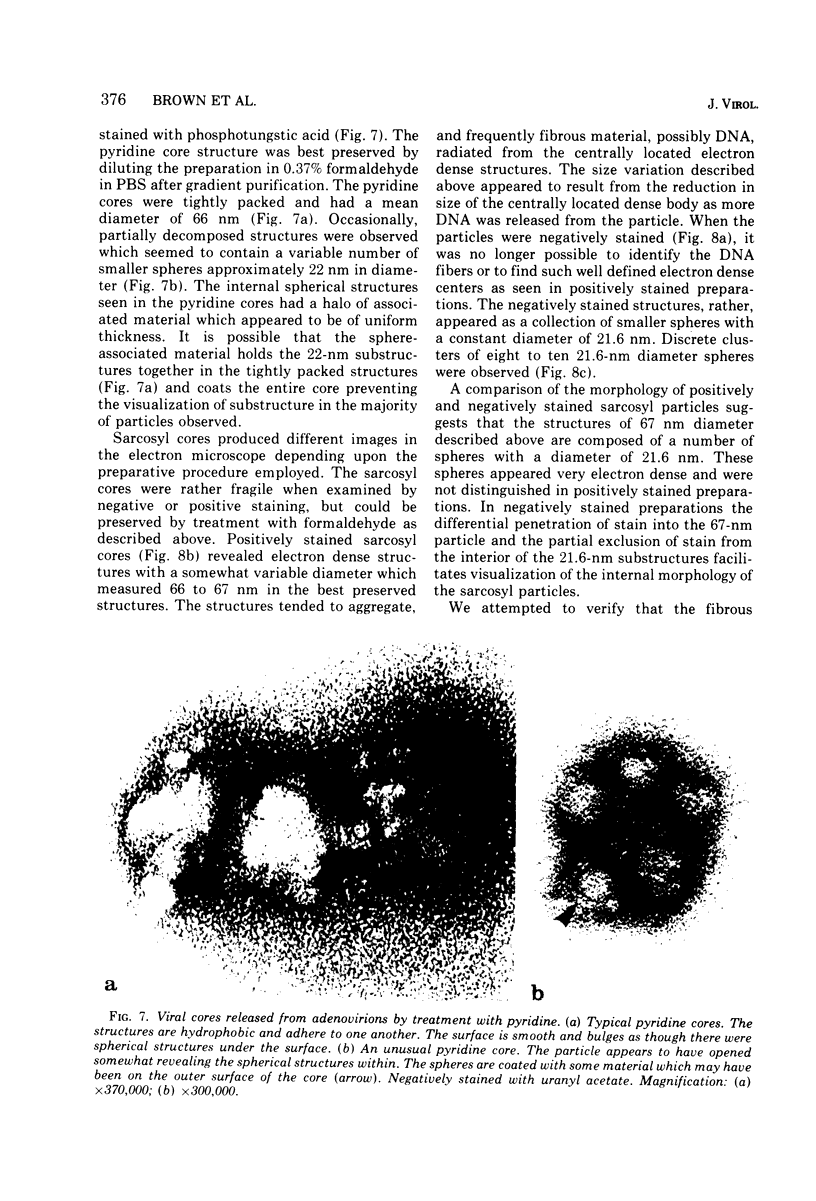
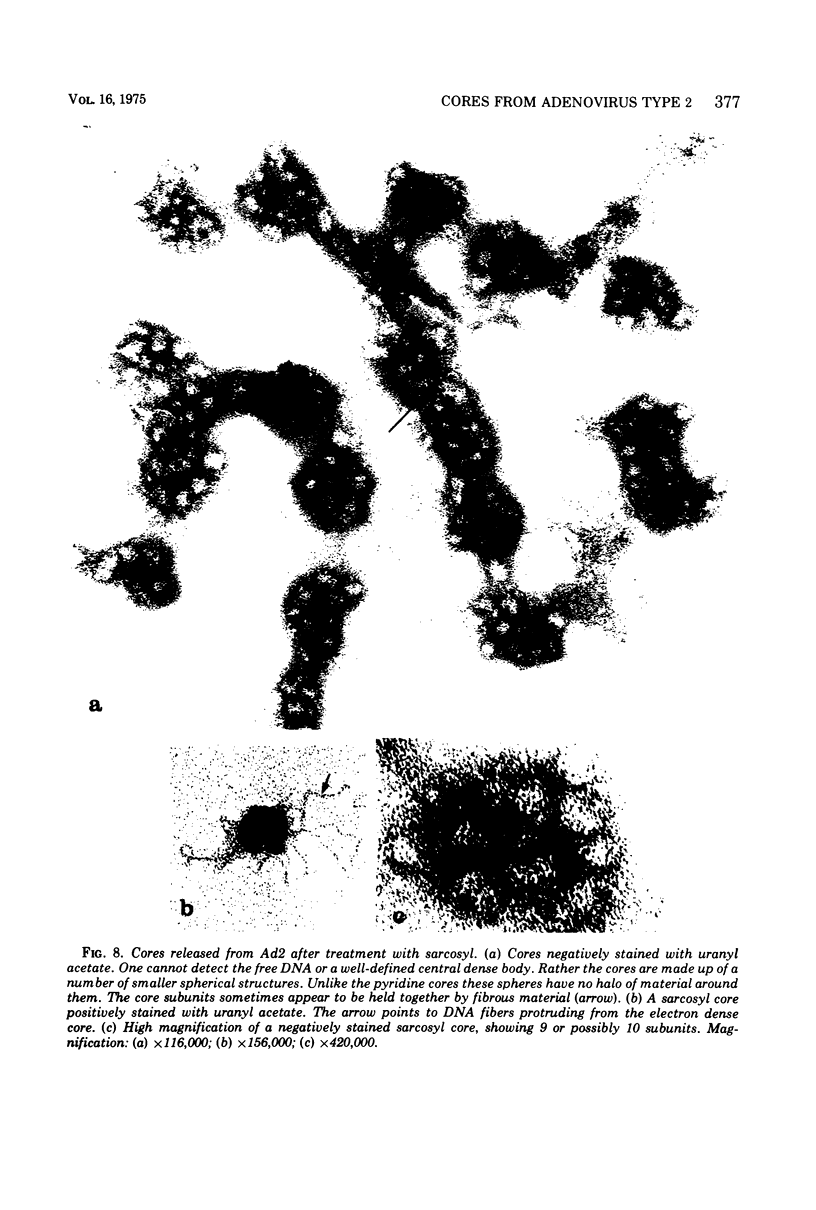
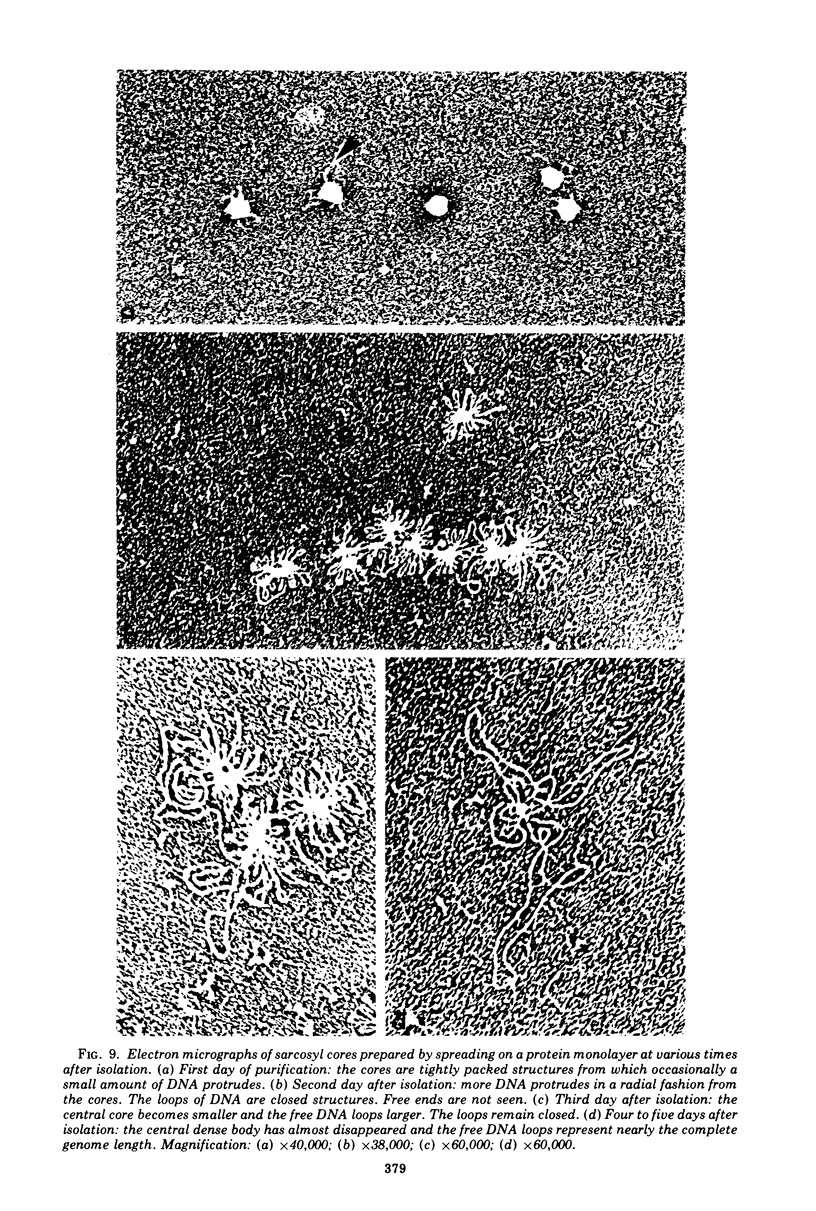
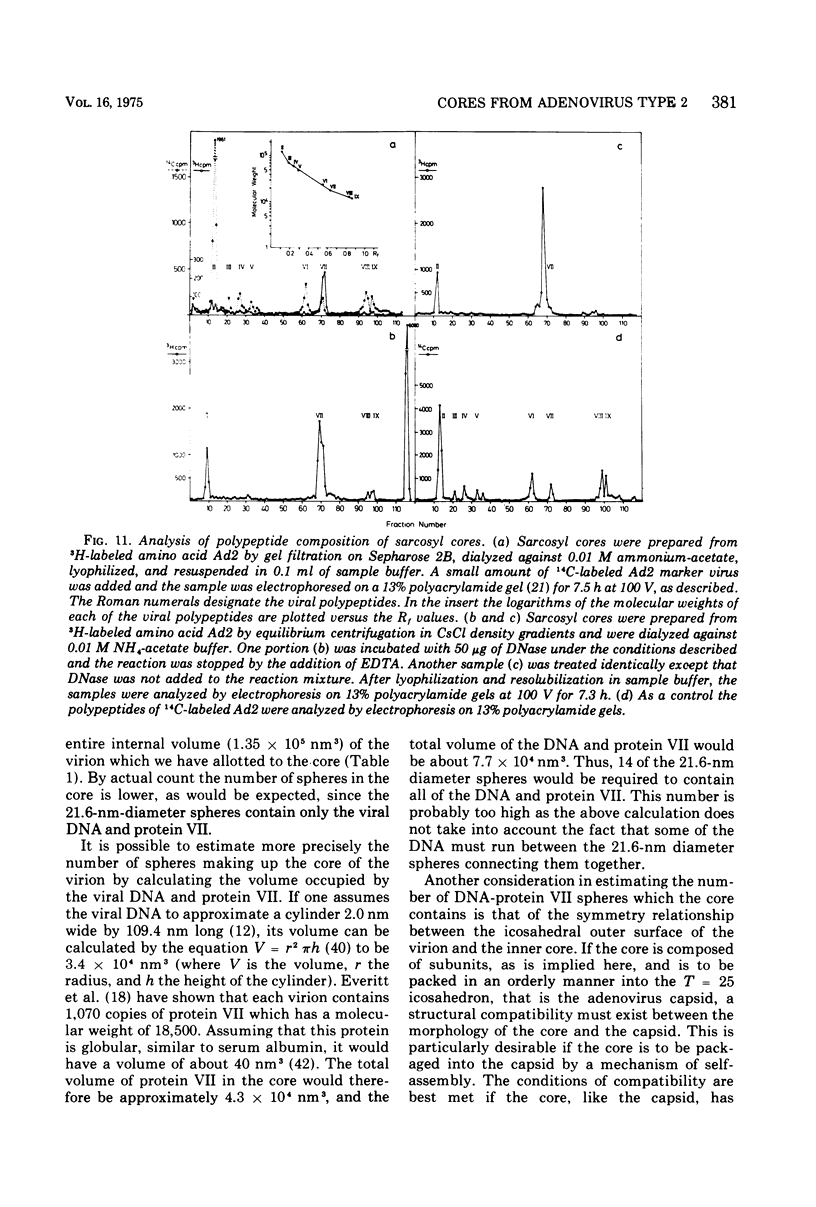
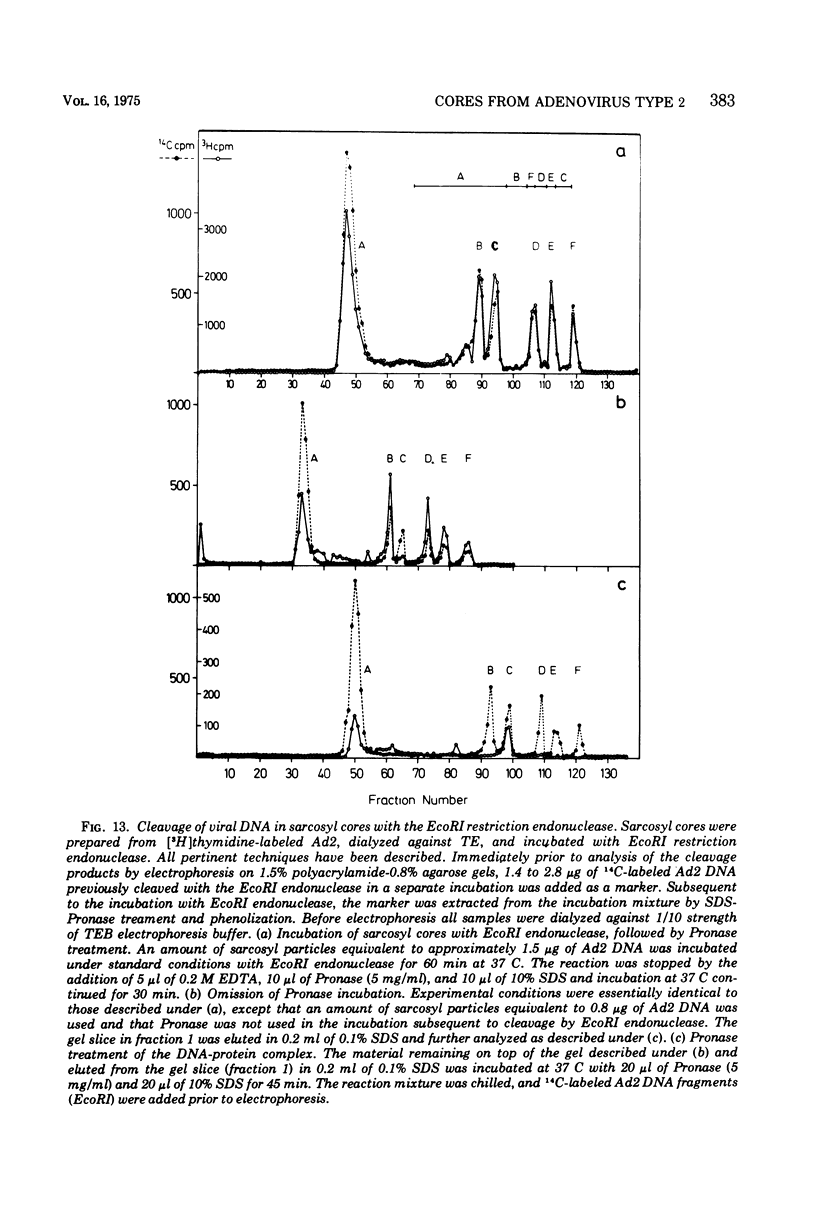
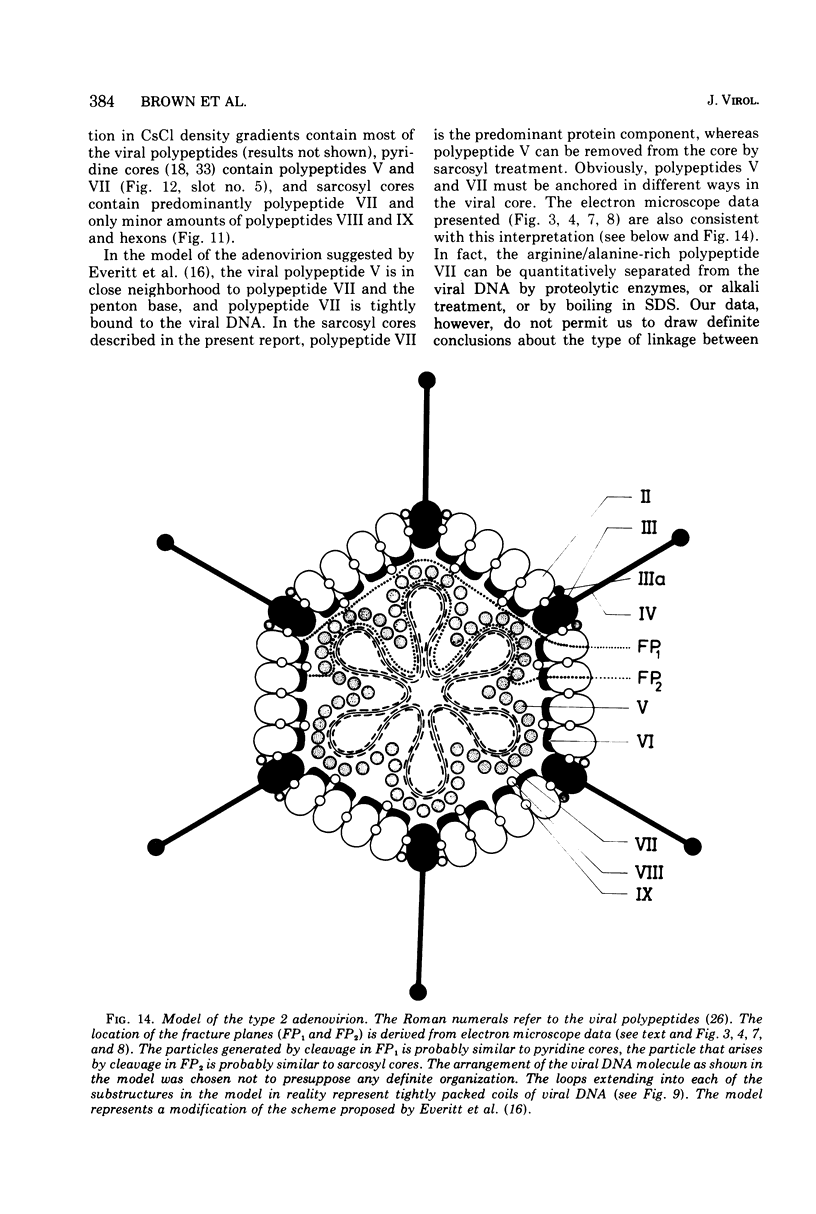
The structure and composition of the core of adenovirus type 2 were analyzed by electron microscopy and biochemical techniques after differential degradation of the virion by heat, by pyridine, or by sarcosyl treatment. In negatively stained preparations purified sarcosyl cores reveal spherical subunits of 21.6-nm diameter in the electron microscope. It is suggested that these subunits are organized as an icosahedron which has its axes of symmetry coincident with those of the viral capsid. The subunits are connected by the viral DNA molecule. The sarcosyl cores contain the viral DNA and predominantly the arginine/alanine-rich core polypeptide VII. When sarcosyl cores are spread on a protein film, tightly coiled particles are observed which gradually unfold giving rise to a rosette-like pattern due to the uncoiling DNA molecule. Completely unfolded DNA molecules are circular. Pyridine cores consist of the viral DNA and polypeptides V and VII. In negatively stained preparations of pyridine cores the subunit arrangement apparent in the sarcosyl cores is masked by an additional shell which is probably formed by polypeptide V. In freeze-cleaved preparations of the adenovirion two fracture planes can be recognized. One fracture plane probably passes between the outer capsid of the virion and polypeptide V exposing a subviral particle which corresponds to the pyridine core. The second fracture plane observed could be located between polypeptide V and the polypeptide VII-DNA complex, thus uncovering a subviral structure which corresponds to the sarcosyl core. In the sarcosyl core polypeptide VII is tightly bound to the viral DNA which is susceptible to digestion with DNase. The restriction endonuclease EcoRI cleaves the viral DNA in the sarcosyl cores into the six specific fragments. These fragments can be resolved on polyacrylamide-agarose gels provided the sarcosyl cores are treated with pronase after incubation with the restriction endonuclease. When pronase digestion is omitted, a complex of the terminal EcoRI fragments adenovirus DNA and protein can be isolated. From this complex the terminal DNA fragments can be liberated after pronase treatment. The complex described is presumably responsible for the circularization of the viral DNA inside the virion. The nature of the protein(s) involved in circle formation has not yet been elucidated.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. T., Burlingham B. T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973 Aug;12(2):386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. An endonuclease in cells infected with adenovirus and associated with adenovirions. Virology. 1972 Apr;48(1):1–13. doi: 10.1016/0042-6822(72)90108-0. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Burger H., Ortin J., Fanning E., Brown D. T., Mestphal M., Winterhoff U., Weiser B., Schick J. Integration of adenovirus DNA into the cellular genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):505–521. doi: 10.1101/sqb.1974.039.01.063. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Kleinschmidt A. K. Denaturation pattern of the DNA of adenovirus type 2 as determined by electron microscopy. J Mol Biol. 1970 Jun 28;50(3):579–593. doi: 10.1016/0022-2836(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Rensing U., Philipson L. Intracellular forms of Adenovirus DNA. II. Isolation in dye-buoyant density gradients of a DNA-RNA complex from KB cells infected with Adenovirus type 2. J Virol. 1973 Oct;12(4):793–807. doi: 10.1128/jvi.12.4.793-807.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Everitt E., Philipson L. Structural proteins of adenoviruses. XI. Purification of three low molecular weight virion proteins of adenovirus type 2 and their synthesis during productive infection. Virology. 1974 Nov;62(1):253–269. doi: 10.1016/0042-6822(74)90320-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Pereira H. G., Russell W. C., Valentine R. C. Isolation of an internal component from adenovirus type 5. J Mol Biol. 1968 Nov 14;37(3):379–386. doi: 10.1016/0022-2836(68)90109-5. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. I. Structure of the contracted sheath and polysheath. J Mol Biol. 1967 Apr 28;25(2):167–200. doi: 10.1016/0022-2836(67)90136-2. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Höglund S. Sructural proteins of adenoviruses. 3. Purification and characterization of the adenovirus type 2 penton antigen. Virology. 1969 Sep;39(1):90–106. doi: 10.1016/0042-6822(69)90351-1. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Philipson L. Internal basic proteins in adenovirus. Virology. 1968 Nov;36(3):508–511. doi: 10.1016/0042-6822(68)90178-5. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Price P. A., Stein W. H., Moore S. Effect of divalent cations on the reduction and re-formation of the disulfide bonds of deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):929–932. [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Rosenwirth B., Tjia S., Westphal M., Doerfler W. Incomplete particles of adenovirus. II. Kinetics of formation and polypeptide composition of adenovirus type 2. Virology. 1974 Aug;60(2):431–437. doi: 10.1016/0042-6822(74)90337-7. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Laver W. G., Sanderson P. J. Internal components of adenovirus. Nature. 1968 Sep 14;219(5159):1127–1130. doi: 10.1038/2191127a0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Valentine R. C., Pereira H. G. The effect of heat on the anatomy of the adenovirus. J Gen Virol. 1967 Oct;1(4):509–522. doi: 10.1099/0022-1317-1-4-509. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S., ANDERSON T. F. STRUCTURE OF TYPE 5 ADENOVIRUS. II. FINE STRUCTURE OF VIRUS SUBUNITIS. MORPHOLOGIC RELATIONSHIP OF STRUCTURAL SUBUNITS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:307–314. doi: 10.1084/jem.118.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]