Abstract
Hyperosmotic stress initiates several adaptive responses, including the remodeling of the cytoskeleton. Besides maintaining structural integrity, the cytoskeleton has emerged as an important regulator of gene transcription. Myocardin-related transcription factor (MRTF), an actin-regulated coactivator of serum response factor, is a major link between the actin skeleton and transcriptional control. We therefore investigated whether MRTF is regulated by hyperosmotic stress. Here we show that hypertonicity induces robust, rapid, and transient translocation of MRTF from the cytosol to the nucleus in kidney tubular cells. We found that the hyperosmolarity-triggered MRTF translocation is mediated by the RhoA/Rho kinase (ROK) pathway. Moreover, the Rho guanine nucleotide exchange factor GEF-H1 is activated by hyperosmotic stress, and it is a key contributor to the ensuing RhoA activation and MRTF translocation, since siRNA-mediated GEF-H1 downregulation suppresses these responses. While the osmotically induced RhoA activation promotes nuclear MRTF accumulation, the concomitant activation of p38 MAP kinase mitigates this effect. Moderate hyperosmotic stress (600 mosM) drives MRTF-dependent transcription through the cis-element CArG box. Silencing or pharmacological inhibition of MRTF prevents the osmotic stimulation of CArG-dependent transcription and renders the cells susceptible to osmotic shock-induced structural damage. Interestingly, strong hyperosmolarity promotes proteasomal degradation of MRTF, concomitant with apoptosis. Thus, MRTF is an osmosensitive and osmoprotective transcription factor, whose intracellular distribution is regulated by the GEF-H1/RhoA/ROK and p38 pathways. However, strong osmotic stress destabilizes MRTF, concomitant with apoptosis, implying that hyperosmotically induced cell death takes precedence over epithelial-myofibroblast transition, a potential consequence of MRTF-mediated phenotypic reprogramming.
Keywords: RhoA, GEF-H1, cell volume, osmoprotection, hypertonicity
deviation from the physiologic hydration state (i.e., “osmotic stress”) represents a major threat for normal cell functions and survival. Accordingly, osmotically challenged cells mobilize a set of adaptive responses, which include the activation of volume-correcting transport systems (29), the expression of osmoprotective genes (8), and the remodeling of the cytoskeleton (17). Hyperosmotic stress-induced cytoskeletal restructuring manifests in enhanced peripheral actin polymerization (18, 26, 52, 57), the formation of a polygonal actin-myosin lattice (38), and enhanced cell contractility (15, 16, 51). These responses are thought to reinforce the cell enabling it to withstand the shrinkage-provoked mechanical trauma. Regarding the underlying mechanisms, we and others have shown that hyperosmolarity activates several Rho family GTPases, which in turn are central mediators of the ensuing cytoskeletal effects (16, 37, 56, 68, 75). For example, RhoA/Rho kinase-mediated cofilin phosphorylation is a key contributor to the shrinkage-induced increase in F-actin (66).
While crucial for structural reinforcement, the hyperosmotic activation of Rho family GTPases and the consequent cytoskeleton remodeling itself may fulfill other roles too. Namely, the Rho family and the cytoskeleton have emerged as important regulators of gene expression (49). One key link between the state of the actin skeleton and transcriptional control is myocardin-related transcription factor (MRTF), the two isoforms of which, MRTF-A (also known a MAL or MKL1) and MRTF-B (MKL2), are ubiquitously expressed. MRTF is an actin-regulated transcriptional coactivator, which, when stimulated, forms a complex with serum response factor (SRF) thereby driving the expression of a variety of cytoskeletal genes through the CC(AT)6GG cis-element, called the CArG box (50, 53, 72). Under resting conditions, MRTF is bound to monomeric (G) actin and resides in the cytosol. According to the current view, upon enhanced actin polymerization, G-actin may be “stolen away” from MRTF, which consequently unmasks its nuclear localization sequence thereby promoting its nuclear accumulation (43, 45, 69). This scenario raises the intriguing possibility that MRTF might be an osmosensitive molecule whose nuclear transport is affected by cell shrinkage. This is of particular interest, since so far the only well-characterized mechanism linking osmotic alterations to transcriptional control is the one mediated by the tonicity-responsive enhancer-binding protein (TonEBP, also termed as OREBP or NFAT5), which is regulated by osmolarity at multiple levels (transport, activity, expression) and drives the expression of osmosensitive solute transporters and osmolyte-generating enzymes through the osmotic response element (8, 30). Thus, while TonEBP is the master regulator of the synthesis and transport of nonperturbing osmolytes (and thus cell survival), it is conceivable that MRTF, a cytoskeleton-regulated and cytoskeleton-regulating transcription factor, might be involved in the structural adaptation to hyperosmolarity. However, regulation by MRTF is a potentially dangerous mechanism, especially in epithelial cells, since it can induce major transcriptional reprogramming of the cytoskeleton, facilitating the expression of mesenchymal or smooth muscle-specific proteins. Indeed, recent studies from our laboratory (14, 21, 40) and from others (31, 42, 44) have shown that MRTF is a key mediator of epithelial-mesenchymal transition (EMT), a phenotypic change that is thought to play an important role in the pathogenesis of organ fibrosis (13, 33, 48, 55). Accordingly, sustained translocation of MRTF induces epithelial-myofibroblast transition (EMyT), the most robust form of EMT characterized by the expression of α-smooth actin (SMA) (21, 40). Importantly, the tissue accumulation of SMA shows close correlation with the severity of organ (e.g., kidney) fibrosis (3, 54). Indeed, MRTF has recently been shown to be a direct mediator of myofibroblast generation during experimental fibrosis (63).
With this scenario in mind, we sought to determine the effect of osmotic stress on the intracellular trafficking of MRTF and to discern the underlying signaling and the potential consequences. We also investigated the hitherto largely unexplored upstream mechanism for RhoA activation under hyperosmotic stress. Our results suggest that osmolarity regulates MRTF at multiple levels in tubular cells. Modest hyperosmolarity induces nuclear MRTF accumulation through the GEF-H1/RhoA/Rho kinase pathway and facilitates nuclear efflux of MRTF through p38 MAP kinase. MRTF is an osmoprotective factor under these conditions. Strong hyperosmotic stress leads to rapid degradation of MRTF, ensuring that the “preferred” cell fate is death rather than epithelial-myofibroblast transition.1
MATERIALS AND METHODS
Chemicals and antibodies.
SB203580, Y-27632, CCG-1432, and zVAD-fmk were from EMD Biosciences (Mississauga, ON, Canada). MG132 was obtained from Sigma (Mississauga, ON, Canada). Bovine serum albumin (BSA) was from BioShop Canada (Burlington, ON, Canada). The Complete Mini Protease inhibitor tablets were from Roche Diagnostics (Laval, QC, Canada). Antibodies against the following proteins were used: RhoA, GEF-H1 (55B6), and SRF from Cell Signaling Technology (Danvers, MA); histones from Millipore (Temecula, CA); GAPDH, lamin A/C (N-18), p38 MAP kinase (designated as p38) and phospho-Thr180 p38 (pp38) from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal MRTF antibody (BSAC) has been described previously (59). Peroxidase and Cy3-labeled secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). DAPI nucleic acid stain was from Invitrogen.
Cells and cell treatment.
LLC-PK1, a kidney proximal tubule epithelial cell line (AT1), was used as in our earlier studies (16, 66). Cells were maintained in DMEM medium (Gibco Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum and 1% antibiotic suspension (penicillin and streptomycin, Invitrogen) in an atmosphere containing 5% CO2. The isotonic Na+ medium (290 ± 5 mosM) contained 130 mM NaCl, 3 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM glucose, and 20 mM Na-HEPES pH 7.4. Confluent cells were serum depleted for 3 h in DMEM before the experiments, followed by 15 min of incubation in Na+-medium before the indicated treatments. If not otherwise stated, hyperosmolarity was induced by adding 150 mM NaCl to the isotonic Na+ medium. When osmolarity was varied, the desired osmotic concentration was obtained by adding the appropriate concentration of NaCl. In a subset of experiments, instead of NaCl, equiosmotic sucrose was added with identical results. In long-term experiments (6 h or more), NaCl was added to the serum-free DMEM medium. To induce cell contact disassembly, cells were thoroughly washed with phosphate-buffered saline (PBS, Invitrogen) and cultured in nominally calcium chloride-free DMEM (low-calcium medium: LCM) as in our earlier studies (39, 40).
Gene silencing using siRNA.
The siRNAs targeting the sequence in porcine MRTF-A (CCAAGGAGCTGAAGCCAAA), MRTF-B (CGACAAACACCGTAGCAAA), GEF-H1 [AACAAGAGCATCACAGCCAAG (no. 1) and AACGGGCATCTCTTCACCACC (no. 2)] (32, 71), and RhoA (AAAGCAGGTAGAGTTGGCTTT) were obtained from Applied Biosystems/Ambion (Austin, TX). Cells were transfected with either 100 nM siRNA (GEF-H1 or RhoA siRNA) or (50 nM MRTF-A+50 nM MRTF-B siRNAs) oligonucleotide using the Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) according to the manufacturer's instructions. Control cells were transfected with 100 nM Silencer siRNA negative control no. 2 (nonrelated siRNA) (Applied Biosytems/Ambion). Experiments were performed 24–48 h after transfection. The levels of the silenced proteins were routinely checked by Western blotting.
Constructs and transient transfection.
GST-RBD (RhoA-binding domain of Rhotekin, amino acids 7–89) and GST-RhoA(G17A) (2, 23) were kind gifts from Dr. K. Burridge (Univ. of North Carolina, Chapel Hill). The hemagglutinin (HA)-tagged MRTF construct was generated as described previously (14). LLC-PK1 cells were transfected using the transfection reagent FuGENE 6 (Roche Molecular Biochemicals) according to the manufacturer's instructions using 1 μg DNA/well for six-well plates or as specified in the figures.
Western blot analysis.
Following treatment, cells were lysed on ice with cold lysis buffer [100 mM NaCl, 30 mM HEPES (pH 7.5), 20 mM NaF, 1 mM EGTA, 1% Triton X-100, supplemented with 1 mM Na3VO4, 1 mM PMSF, and Complete Mini Protease Inhibitor Cocktail Tablet (Roche)]. Protein concentration was determined by the bicinchoninic acid assay (BCA) (Pierce Biotechnology) with BSA used as standard. SDS-PAGE and Western blotting were performed as described previously (40). Blots were blocked in Tris-buffered saline containing 3% BSA and incubated with the primary antibody overnight. Antibody binding was visualized with the corresponding peroxidase-conjugated secondary antibodies and the enhanced chemiluminescence method (kit from GE Healthcare Lifesciences). Where indicated, blots were stripped and reprobed to demonstrate equal loading.
Preparation of nuclear extracts.
Nuclear extracts were prepared from confluent layers of LLC-PK1 cells grown on 6-cm dishes, as described previously (40), using the NE-PER Nuclear Extraction Kit from Pierce Biotechnology (Rockford, IL) according to the manufacturer's recommendation. The nuclear extracts were collected, and their protein concentration was determined. Samples containing 10 μg protein were analyzed by Western blotting. Antibodies against histones or lamin were used as markers of nuclear fraction.
GEF-H1 and RhoA activation assays.
GST-RBD and GST-RhoA(G17A) were used to capture active RhoA and GEFs, respectively, as described (32, 70, 71). Briefly, confluent LLC-PK1 cells were grown on 6- or 10-cm dishes and treated as indicated in the respective figures. Cells were lysed with ice-cold buffer containing 100 mM NaCl, 50 mM Tris base (pH 7.6), 20 mM NaF, 10 mM MgCl2, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, 1 mM PMSF, 1 mM Na3VO4 and protease inhibitors (RhoA activation assay) or 20 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 1 mM DTT, 1 mM PMSF, and protease inhibitors (GEF activation assay). After centrifugation, aliquots for determination of total RhoA or GEF-H1 were removed. The remaining supernatants were incubated at 4°C for 45 min with 20–25 μg of GST-RBD or GST-RhoA(G17A)-covered beads, followed by extensive washing. Total cell lysates and the captured proteins were analyzed by Western blotting using RhoA or GEF-H1 antibody. Results were quantified by densitometry (see below).
Densitometry.
To quantify results, films with nonsaturated exposures were scanned and densitometry analysis was performed using a GS-800 calibrated densitometer and the “band analysis” option of the Quantity One software (Bio-Rad). In each assay the amount of the investigated protein species was normalized to the appropriate control (e.g., active RhoA to total RhoA, active GEF-H1 to total GEF-H1, nuclear MRTF to nuclear marker protein). Data are expressed as fold changes. Since the basal levels of active RhoA and nuclear MRTF were often either undetectable or just slightly above the background, to achieve accurate and stringent comparison, signals were expressed relative to the response detected in stimulated cells at a selected time point (taken as unity) as described in the figures.
Microscopy.
Confluent cells grown on coverslips were treated as indicated in the corresponding figures and fixed with 4% paraformaldehyde. Immunofluorescence staining was carried out as described (39). Briefly, following permeabilization with 0.1% Triton X-100, the coverslips were blocked with 3% BSA in PBS. Next, cells were incubated with anti-MRTF (1:100). Bound antibody was detected using the corresponding fluorescent secondary antibody (1:1,000), which also contained DAPI to counterstain nuclei. All samples were viewed using an Olympus IX81 microscope (Melville, NY) coupled to an Evolution QEi Monochrome camera (Media Cybernetics, Bethesda, MD). Nuclear translocation was quantified as described in our earlier studies (21, 40). Briefly, MRTF localization was denoted as cytosolic, if clear nuclear exclusion was seen, and nuclear if MRTF was accumulated in the nucleus to the extent that the demarcation of nucleus was obvious. These conditions correspond to a nuclear/cytoplasmic ratio of <0.6 and >1.5, respectively, as determined by quantitative image analysis (40). At least 10 randomly selected fields (>200 cells) were quantified for each condition in three experiments.
Phase contrast images were obtained by a Nikon eclipse TS100 microscope (×40 objective), using the Nikon digital sight system and SPOT software.
Caspase-3 activity assay.
For total caspase-3 activity assay, cells were grown in 12-well plates and treated as indicated in the corresponding figures. Following treatment, cells were permeabilized with caspase-3 lysis buffer (10 mM Tris·HCl, 10 mM NaH2PO4/NaHPO4 pH 7.5, 130 mM NaCl, 10 mM sodium pyrophosphate, 1% Triton X-100). The collected lysates were then added to the protease assay buffer (20 mM HEPES pH 7.5, 10% glycerol, 2 mM DTT) in conjunction with 20 μM fluorogenic caspase-3 substrate (Ac-DEVD-AMC) (BD Biosciences). The samples were incubated at 37°C for 1 h. The generated fluorescence of the cleaved reporter subunit was measured in a cuvette fluorimeter (Photon Technologies International) using excitation and emission wavelengths of 380 nm and 440 nm, respectively. The obtained readings were normalized to total protein levels as determined by BCA assay.
Luciferase reporter assays.
These were performed as described previously (40). Briefly, cells were plated onto 12-well plates and at ∼60% confluence were transfected with 0.5 μg/well reporter plasmid pGL3–3DA-Luc, which contains the firefly luciferase gene under the control of a CArG box triplet (64), or pSMA-Luc, which harbors the proximal 765-base pair segment of the rat SMA promoter in the pGL3 vector (40). Normalization was carried out by cotransfection of the enhancer-less Renilla luciferase control plasmid pHRG-B (Promega) (0.125 μg/well), which is optimal for this purpose, as it is not responsive to hypertonicity, as verified in our previous studies (24). At 16–20 h posttransfection, cells were serum deprived for 3 h, and treatments were initiated by adding fresh, serum-free medium supplemented with NaCl, in the presence or absence of inhibitors, as indicated. Finally, cells were lysed and luciferase activity was determined using the Dual-Luciferase Reporter Assay System kit (Promega) and a Berthold Lumat 9507 luminometer according to the manufacturers' instructions. For each condition, treatments were done in duplicate and experiments were repeated at least three times. From each sample the firefly luciferase activity corresponding to a specific promoter construct was normalized to the Renilla luciferase activity of the same sample. Results are expressed as fold changes compared with the mean firefly/Renilla ratio of the untreated controls taken as a unity.
Statistical analysis.
Data are presented as representative blots or images from at least three similar experiments or as the means ± SE for the number of experiments indicated. Statistical significance was determined by one-way analysis of variance (Tukey or Dunn post hoc testing for parametric and nonparametric ANOVA, as appropriate), or with Student's t-test. P < 0.05 was accepted as significant.
RESULTS
Hyperosmotic stress induces nuclear translocation of MRTF.
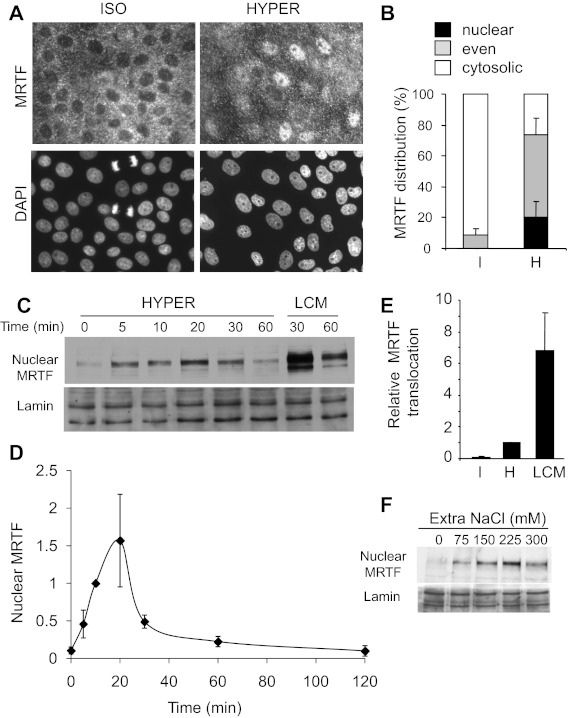
To test whether hyperosmolarity impacts on MRTF traffic, confluent monolayers of LLC-PK1 tubular cells were left untreated or exposed to hyperosmolarity (600 mosM total, 20 min), and MRTF distribution was visualized by immunofluorescence staining. Under isotonic conditions, MRTF was cytosolic, as verified by the nuclear exclusion of the label, visible in nearly every cell (Fig. 1A left, see DAPI-positive, MRTF-negative nuclear areas). Upon hypertonic exposure, some cells showed strong nuclear MRTF accumulation (over the cytosolic level), while in many others the nuclear exclusion disappeared, indicating that the nuclear MRTF concentration rose to the cytosolic level (Fig. 1A, right). We quantified MRTF localization by determining the percentages of cells that showed cytosolic, even, and nuclear distribution (Fig. 1B), as defined in our previous studies (Refs. 21 and 40 and see materials and methods). While under isotonic conditions no cells exhibited nuclear MRTF accumulation, and <10% had even distribution, hyperosmolarity provoked MRTF redistribution in ∼80% of the cells, with 20% and 60% showing highly nuclear and even localization, respectively. Essentially similar results were obtained irrespective of whether hyperosmolarity was induced by NaCl (Fig. 1, A and B) or osmotically equivalent sucrose (not shown). To quantify MRTF protein accumulation and to investigate the time dependence of the effect, we prepared nuclear extracts from cells that had been exposed to hyperosmolarity for 0–120 min. An increase in nuclear MRTF was clearly detectable after 5 min, peaked at 10–20 min, and gradually decayed thereafter (Fig. 1, C and D). At the peak, the nuclear MRTF content of the monolayer was approximately 20-fold compared with the resting (isotonic) level. To assess the relative magnitude of this effect, we also used low-calcium medium as a stimulus (LCM, 30 min), which, as our previous studies have shown (39, 40), uncouples intercellular contacts and thereby induces robust nuclear accumulation of MRTF (Fig. 1C). The maximal translocation elicited by LCM was approximately sixfold higher than the peak response induced by hyperosmolarity (normalized to 1 in each experiment) (Fig. 1E). Next we tested the osmotic dependence of the effect: increasing the NaCl concentration added over the isotonic level from 75 to 225 mM was accompanied by a gradual rise in the nuclear MRTF content, while stronger osmotic stress (300 mM extra) consistently resulted in less translocation, indicating that at the selected time point (20 min), the maximal effect was obtained at an overall osmolarity of 600–750 mosM (Fig. 1F). Taken together, these data show that hyperosmolarity induces a rapid, robust (20-fold) but submaximal (compared with LCM), transient and osmolarity-dependent nuclear accumulation of MRTF.
Fig. 1.
Hyperosmolarity induces rapid nuclear translocation of myocardin-related transcription factor (MRTF). A: LLC-PK1 cells were grown to confluence on coverslips, serum deprived for 3 h, and preincubated with isotonic Na+ medium (15 min). Subsequently, cells were exposed to the same isotonic (ISO) or hyperosmotic medium (HYPER, 600 mosM total) for 20 min. At the end of the treatment, the cells were fixed, and MRTF and the nuclei were visualized by immunofluorescence staining with an MRTF-specific antibody (top) and DAPI (bottom), respectively. B: quantification of MRTF nuclear translocation at 30 min of hypertonic exposure was performed as described in materials and methods. Data are from three separate experiments (n = 3), in each of which >250 cells (960 cells in total) were analyzed. I, isotonic; H, hyperosmotic. C: confluent LLC-PK1 cells were treated with hyperosmotic or low-calcium medium (HYPER and LCM, respectively) for the indicated times, followed by cell lysis and isolation of nuclear fractions. Samples of these fractions containing equal amounts of protein were subjected to Western blotting using antibodies against MRTF and lamin (nuclear marker). D: MRTF was quantified in each nuclear fraction using densitometry and normalized to the amount of lamin in the corresponding sample. The MRTF/lamin ratio obtained at 10 min of hypertonic exposure was set to 1, and data were obtained at the various time points and normalized to this value. The graph shows the results of n = 7 separate experiments (means ± SE). E: the maximal MRTF accumulation detected after hyperosmotic treatment (normalized to the nuclear marker) was taken as unity (column H), and the isotonic MRTF level (I) or the maximal LCM-induced MRTF accumulation (LCM, 30 min) is expressed as fold change compared with the hypertonic level in each experiment (n = 3). F: osmotic concentration dependence of nuclear MRTF accumulation. Cells were exposed to various osmolarities for 20 min by supplementing the isotonic medium with the indicated concentration of extra NaCl. Nuclear fractions were prepared and tested as in C.
The GEF-H1/RhoA/Rho kinase pathway mediates hyperosmolarity-induced nuclear translocation of MRTF.
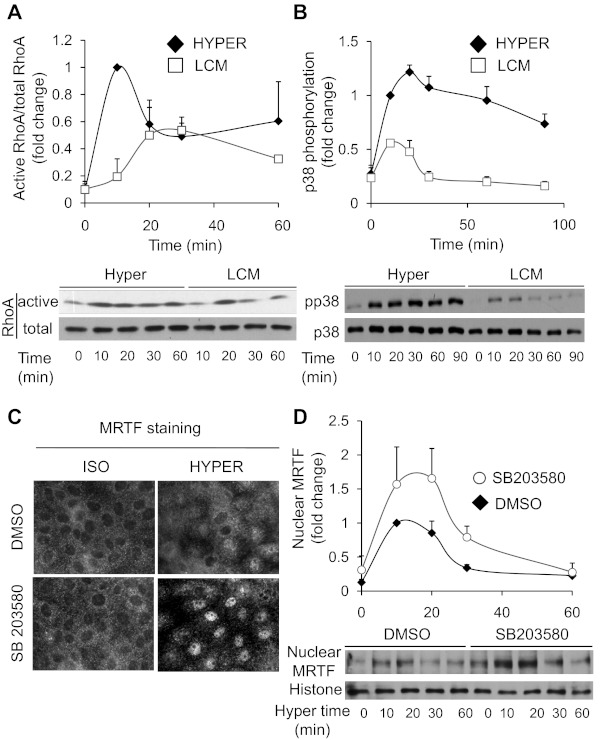
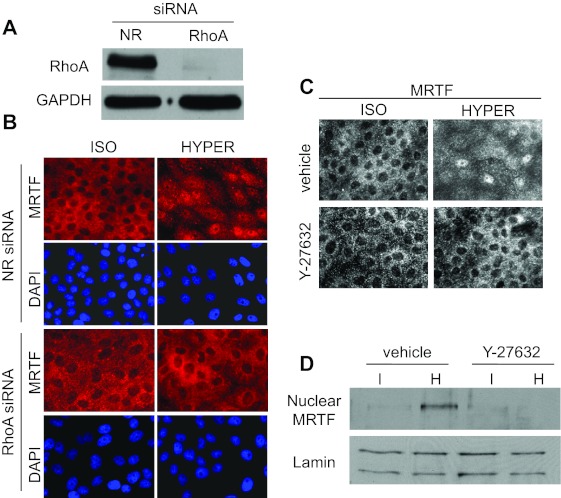
Next we wished to determine which signaling pathway is responsible for MRTF translocation. Hypertonicity has been shown to activate various Rho family GTPases (RhoA, Rac, Cdc42) with varying magnitude and kinetics in different cell types (16, 37, 56, 66, 68). Both RhoA and Rac have been shown to impact MRTF nuclear translocation (9, 43, 61) and both are osmotically sensitive. Because in LLC-PK1 cells hypertonicity elicits only a small and transient (<1 min) Rac activation followed by a suppression (66), while RhoA shows sizable and sustained activation (Ref. 16 and see Fig. 4A), we hypothesized that the latter might be involved in the osmotically induced MRTF translocation. To test this possibility, we used nonrelated and RhoA-specific siRNA, the latter of which essentially eliminated RhoA expression in the monolayer as verified by Western blotting (Fig. 2A). RhoA siRNA did not change MRTF distribution under isotonic conditions, but it completely abolished the osmotically induced nuclear uptake of MRTF (Fig. 2B). Since the Rho effector Rho kinase (ROK) was implicated in osmotically induced actin polymerization (66) and MRTF can undergo Rho-dependent phosphorylation (43), we tested the potential involvement of ROK in MRTF redistribution. The potent ROK inhibitor Y-27632 fully prevented the hypertonicity-provoked nuclear accumulation of MRTF, as demonstrated both by immunostaining (Fig. 2C) and Western blotting of nuclear extracts (Fig. 2D). Together, these data imply that the RhoA/Rho kinase pathway is indispensable for the hypertonicity-induced nuclear translocation of MRTF.
Fig. 4.
RhoA and p38 oppositely regulate nuclear accumulation of MRTF: comparison of the effect of hypertonicity and cell contact disassembly on MRTF-regulating signaling pathways. A and B: to discern why hyperosmolarity is a weaker stimulus than low-calcium-induced contact disassembly for MRTF translocation, we compared the effect of these stimuli on two, potentially MRTF-regulating signaling, events: RhoA activation (A) and p38 phosphorylation (B). LLC-PK1 cells were serum starved and then incubated in hyperosmotic or low-calcium medium for the indicated times and then lysed. In A, active RhoA was measured using the GST-RBD pull-down assay as in Fig 3. In B, phospho-p38 (pp38) and p38 were detected in the total cell lysates. Active/total RhoA as well the pp38/p38 ratios were determined by densitometry, as shown on the graphs above the blots (means ± SE are shown for n = 4 and n = 5 for hyperosmolarity and LCM, respectively). The ratios obtained after 10 min of hypertonic stimulation were taken as unity. Note that hyperosmolarity is a stronger stimulus for both RhoA and p38 activation than LCM. C and D: hyperosmotically induced activation of p38 mitigates the nuclear accumulation of MRTF. LLC-PK1 cells were preincubated with vehicle (DMSO) or 10 μM SB203580 in isotonic Na+ medium for 15 min before exposure to hyperosmotic medium in the absence or presence of the inhibitor, as indicated. C: after 20 min of stimulation, cells were fixed and MRTF translocation was detected by immunostaining. D: hyperosmotic stimulation was terminated after the indicated times, the cells were lysed, and nuclear fractions were prepared as in Fig 1C. Nuclear MRTF content was determined by Western blotting and normalized to histones as nuclear markers. The plots show densitometric quantification for n = 6 experiments. In each experiment, the normalized MRTF content of vehicle-treated (control) samples exposed to hyperosmolarity for 10 min was taken as unity, and other values are expressed relative to this.
Fig. 2.
Hyperosmolarity-induced MRTF translocation is mediated by RhoA and Rho kinase (ROK). A: efficient downregulation of RhoA by specific siRNA. Cells were transfected with control (nonrelated, NR) or RhoA-specific siRNAs (100 nM) for 48 h and then subjected to Western blotting using a RhoA-specific antibody. B: RhoA silencing completely abolishes the hyperosmolarity-induced nuclear accumulation of MRTF. Cells were treated with NR or RhoA siRNA as in A and then exposed to iso- or hypertonic treatment for 20 min, as indicated, and stained for MRTF. Nuclei were visualized by DAPI. C and D: the ROK inhibitor Y-27632 prevents the hyperosmolarity-induced nuclear translocation of MRTF. Cells were pretreated with vehicle or 20 μM Y-27632 for 30 min, followed by a 20-min exposure to iso- or hyperosmotic medium containing vehicle or Y-27632, as indicated. Subsequently, cells were processed for MRTF immunostaining (C) or nuclear extracts were prepared (D) and subjected to Western blotting for MRTF and the nuclear marker lamin, as in Fig 1C.
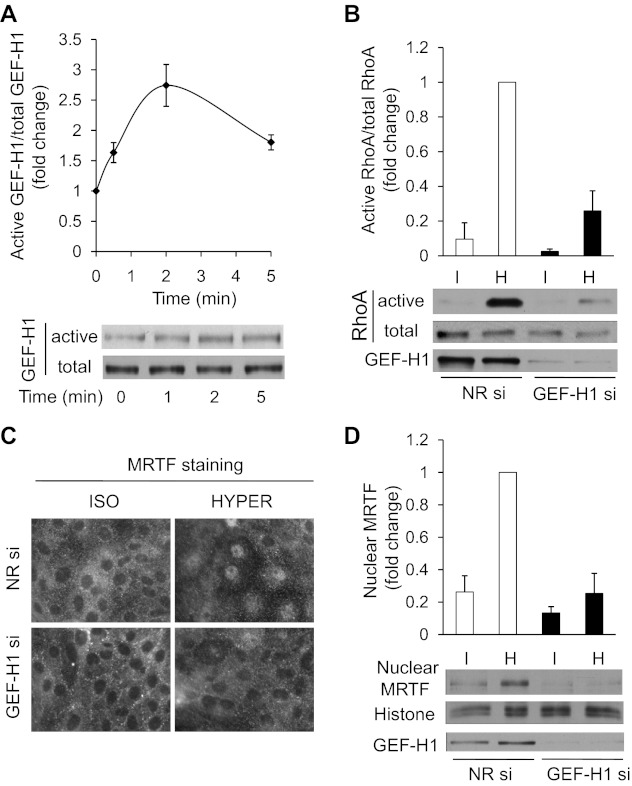
Little is known about the direct upstream mediators of osmotic RhoA activation. Our previous studies have proposed that ezrin-mediated inhibition of Rho guanine nucleotide dissociation inhibitor (Rho-GDI) might be involved in this process in Ehrlich-Lettre ascites cells (56), but no osmotically regulated guanine nucleotide exchange factor (GEF) (i.e., direct RhoA activator) has been so far identified. We considered GEF-H1 as a good candidate, because this key RhoA-activating molecule has been shown to be regulated by mechanical (shear) stress and other physical factors (6, 25, 71), and it can be associated with intercellular contacts (4), which have been proposed to transmit volume-dependent signals (29). To address the potential role of GEF-H1, first we asked whether it is activated by hyperosmolarity. To this end we performed affinity pull-down assays, using a RhoA(G17A) mutant-GST fusion protein, which mimics nucleotide-free RhoA, and therefore specifically binds to activated GEFs (23, 32, 70). GEFs in lysates obtained from iso- and hypertonically treated cells were precipitated with RhoA(G17A)-GST-covered beads, and the precipitates were immunoblotted with an anti-GEF-H1 antibody. Hyperosmolarity induced a rapid increase in the amount of captured GEF-H1, which peaked after 2 min at 2.5-fold over the isotonic level (Fig. 3A). To test the functional significance of the observed GEF-H1 activation, we treated the cells with nonrelated or GEF-H1-specific siRNA and performed RhoA activation assays after iso- and hypertonic exposure (Fig. 3B). GEF-H1 siRNA induced a strong reduction in GEF-H1 expression and concomitantly suppressed basal RhoA activity and prevented its rise over the isotonic (basal) level. The residual RhoA activation in the presence of GEF-H1 siRNA is likely due to the remaining GEF-H1 expression in a small portion of the cell population and might also reflect the contribution of other RhoA-activating pathways. Having seen that GEF-H1 is a key component of the osmotically induced RhoA activation, we tested its impact on MRTF translocation. As expected, GEF-H1 knockdown strongly mitigated the hypertonicity-triggered MRTF translocation, as detected both by immunofluorescence microscopy (Fig. 3C) and Western blotting of nuclear extracts (Fig. 3D).
Fig. 3.
Hyperosmolarity activates GEF-H1, which in turn is a major contributor to the ensuing RhoA activation and MRTF translocation. A: LLC-PK1 cells were treated with iso- or hyperosmotic medium for the indicated times, after which cells were lysed and active GEFs were precipitated using GST-RhoA(G17A). GEF-H1 in the precipitates (active) and in an aliquot of the input cell lysates (total) was detected by Western blotting. The graph shows densitometric quantification. The amount of precipitated GEF-H1 was normalized to the corresponding total GEF-H1, and the ratio obtained in the isotonic samples was set to 1. The graph shows means ± SE from n = 3 experiments. B: LLC-PK1 cells were transfected with nonrelated or GEF-H1-specific siRNA and 48 h later were exposed to iso- or hyperosmotic medium for 5 min. The cells were then lysed and active RhoA was precipitated using GST-RBD. RhoA in precipitates (active) and in an aliquot of the input cell lysates (total) was detected using Western blotting. For each sample, active RhoA was normalized to the corresponding total RhoA. In each experiment, the normalized RhoA activity measured in the 5-min hypertonic samples was taken as 1, and all other values are expressed accordingly. The graph above the blot shows the cumulative data (means ± SE) for n = 4 separate experiments. While the difference between the NR and GEF-H1 siRNA-treated isotonic samples is not significant (P > 0.4), the difference between the hypertonic NR and GEF-H1 siRNA-treated samples is highly significant (P < 0.001). C: cells were treated with NR or GEF-H1 siRNA as in B and then exposed to iso- or hypertonicity for 20 min, and subsequently immunostained for MRTF. D: cells were pretreated with NR or GEF-H1-specific siRNA and exposed to iso- or hypertonic medium as in B, except the treatment was terminated after 10 min. Cells were then lysed and nuclear extracts were prepared as in Fig 1C and probed for MRTF and histones as nuclear marker. GEF-H1 downregulation was verified in an aliquot of the corresponding total cell lysates. Data were normalized to the nuclear MRTF/histone ratio, which was set as 1 for the NR siRNA hypertonic samples. The graph shows data for n = 6 experiments. P < 0.001 for the difference between the NR and GEF-H1 siRNA-treated hypertonic samples.
Taken together, these results imply that the RhoA-ROK pathway is indispensable for the hypertonicity-induced MRTF translocation and that GEF-H1 is activated by hyperosmolarity and significantly contributes to the ensuing RhoA activation and consequent MRTF redistribution.
RhoA and p38 MAPK oppositely regulate nuclear accumulation of MRTF after hypertonic stimulation.
The fact that hyperosmolarity-induced MRTF translocation, while substantial, was significantly less than that induced by low-calcium medium (Fig. 1E) prompted us to consider whether this difference is due to a proportional difference in RhoA activation or may reflect additional regulatory inputs. To address this question, first we compared RhoA activation elicited by the two stimuli. Hypertonicity provoked robust RhoA activation that at every time point between 0 and 60 min was either larger than (0–10 min) or equal to (later times) the response seen upon LCM stimulation (Fig. 4A). In agreement with our previous studies, these data show that hyperosmotic stress is a strong and sustained activator of RhoA in LLC-PK1 cells, while LCM is a weaker stimulus. These findings suggested that other factors than RhoA must be responsible for the observed differences. Since RhoA activation is an absolute requirement for nuclear MRTF uptake with regard to both stimuli, we surmised that differences in pathways regulating nuclear retention/efflux of MRTF might underlie the observed difference in MRTF accumulation. Relevantly, members of the MAPK family were reported to phosphorylate MRTF (43, 46), which in turn facilitates its nuclear efflux (46). Since hyperosmolarity activates predominantly p38 MAPK (p38) in tubular cells, we compared the effect of hypertonicity and LCM on p38 phosphorylation (an accurate marker of p38 activation). Hyperosmolarity induced much stronger and longer lasting p38 activation than LCM (Fig. 4B). To discern whether p38 activation could indeed influence MRTF trafficking, we pretreated cells with SB203580, a potent p38 MAPK inhibitor, and followed MRTF distribution by immunofluorescence microscopy (Fig. 4C) and Western blotting of nuclear extracts (Fig. 4D). While SB203580 had no significant effect on MRTF distribution under isotonic conditions, osmotic stress induced markedly stronger nuclear MRTF accumulation in the presence of the inhibitor (Fig. 4C). Time-dependent monitoring of nuclear MRTF content revealed that SB203580-treated cells had more nuclear MRTF at every time point after stimulation, but the most pronounced (more than twofold) and highly significant difference was observed after 30 min of exposure to hypertonicity. Thus inhibition of p38 promoted nuclear accumulation of MRTF, presumably by mitigating or delaying MRTF efflux from the nucleus. Nonetheless, MRTF eventually left the nucleus in SB203580-treated cells as well, indicating the additional involvement of other mechanisms. In summary, the data indicate that p38 contributes to the removal of MRTF from the nucleus under hypertonic conditions, suggesting that hyperosmotic stress has a dual effect on MRTF traffic, by stimulating both RhoA-dependent nuclear uptake and p38-promoted release of MRTF.
The fate and role of MRTF under hyperosmotic conditions.
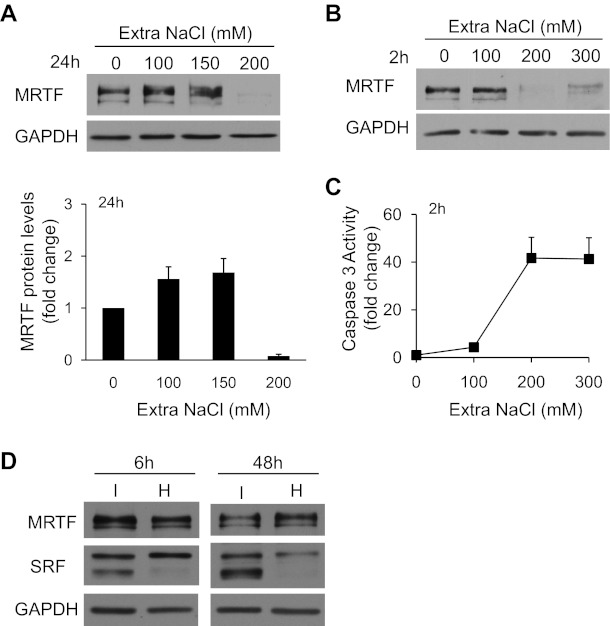
In the following experiments we wished to investigate whether hyperosmolarity could trigger MRTF-dependent transcription and whether MRTF might mediate osmoprotective effects. As an introductory study, we tested whether long-term exposure to various osmolarities impacts MRTF protein expression itself. Supplementing the isotonic medium with 100 or 150 mM NaCl for 24 h resulted in a slight increase in MRTF protein levels, whereas further elevation of the added concentration of NaCl caused a dramatic reduction in the MRTF-immunoreactive band (Fig. 5A). To better characterize this surprising effect, we applied mild or strong hyperosmolarity for a much shorter time (2 h). Again, after the addition of ≥200 mM NaCl we observed a near-complete elimination of the MRTF band (Fig. 5B), indicating that strong osmotic stress may cause acute MRTF degradation, or alternatively elicit posttranslational modifications that mask the immunoreactive epitopes (see below). Addition of 200 mM NaCl (700 mosM total) or more also induced robust activation of caspase-3 (Fig. 5C), indicating that acute exposure of LLC-PK1 to an osmotic concentration of 700 mosM or higher initiates apoptosis concomitant with the loss of the MRTF protein. Thus MRTF expression is sensitive to osmolarity, and the destabilization of the molecule occurs at a rather sharp threshold, which is similar to the threshold of apoptosis induction. Furthermore, these experiments instructed us that any potential transcriptional or protective effect should be tested at relatively mild hyperosmolarity (e.g., 600 mosM in total) and for shorter exposures. Therefore we checked the impact of 150 mM NaCl (600 mosM total, our standard conditions in the translocation experiments) on MRTF and SRF at 6 and 48 h (Fig. 5D). At this level of hyperosmolarity MRTF was stable (albeit after 48 h we occasionally detected a decrease). SRF manifested as two immunoreactive bands. A 6-h exposure to extra 150 mM NaCl reduced the intensity of the lower band, whereas after 48 h usually both bands were suppressed (see discussion).
Fig. 5.
Fate of MRTF under strongly hyperosmotic conditions. A: following serum depletion, LLC-PK1 cells were incubated in serum-free isotonic or hypertonic DMEM. The osmolarity of the medium was set between 500 and 700 mosM (total) by the addition of extra NaCl at the indicated concentrations. After 24 h of exposure, the cells were lysed and whole cell lysates were subjected to immunoblotting for the indicated proteins. The graph below the blot shows densitometric quantification of MRTF protein levels normalized to the corresponding GAPDH levels (means ± SE, n = 3). B: cells were incubated for 2 h in serum-free isotonic or hypertonic DMEM supplemented with extra NaCl at the indicated concentrations and then lysed and processed as in A. Note the robust decrease in the MRTF signal at and above a total osmolarity of 700 mosM. C: short-term exposure to strong hyperosmolarity (≥700 mosM) activates caspase-3 in LLC-PK1 cells. Cells were treated for 2 h with the indicated extra concentrations of NaCl added into the isotonic Na+ medium. Cells were harvested and lysed, and the obtained lysates were assayed for caspase-3 activity, using a fluorigenic substrate as described in materials and methods. D: cells were treated for 6 or 48 h in isotonic or hypertonic conditions (150 mM extra of NaCl, 600 mosM total), and cell lysates were immunoblotted for the indicated protein. While MRTF expression does not show major changes at this osmolarity at either time, serum response factor expression (SRF) is affected.
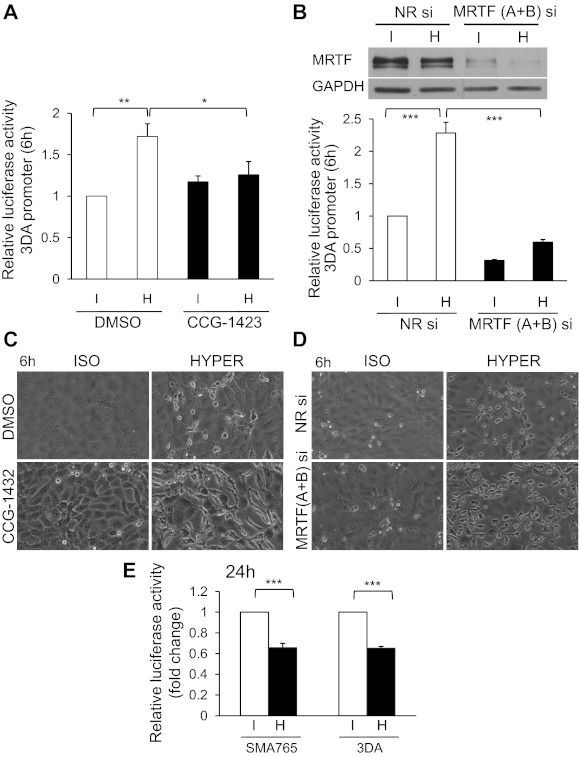
In light of these data we checked the impact of hyperosmolarity (150 mM NaCl, 6-h exposure) on MRTF/SRF-dependent transcription using a CArG box-containing luciferase reporter construct (3DA) (Fig. 6, A and B). Hypertonic exposure caused a ≈1.8-fold increase in CArG-mediated transcription. Importantly, CCG-1423, a compound which inhibits MRTF/SRF interaction (20), completely prevented the hypertonicity-provoked activation of the reporter. As an alternative (nonpharmacologic) approach we used nonrelated siRNA or a mixture of siRNAs against MRTF-A and MRTF-B, the two isoforms of the protein. Transfection of the cells with the MRTF-specific siRNAs for 48 h strongly reduced MRTF expression (Fig. 6B, top), and it concomitantly decreased the activity of the CArG-dependent reporter under isotonic conditions and efficiently suppressed its activation by hypertonicity (Fig. 6B). The difference between the effect of CCG-1423 and MRTF siRNAs on the basal activity of the reporter is likely due to the fact that the pharmacological inhibitor was applied only 30 min before hypertonic stimulation, whereas the transfection with the siRNA was performed 48 h before hypertonic exposure, during which the cumulative expression of the reporter could also be suppressed. Taken together, these data clearly imply that moderate, intermediate duration hyperosmolarity activates MRTF/SRF-dependent transcription in tubular cells. Since the accumulation of MRTF in the nucleus is transient (see Fig. 1, C and D), these results also suggest that an early rise in nuclear MRTF concentration is sufficient to initiate or prime for a subsequent transcriptional response that manifests at a time when the bulk MRTF concentration is no longer detectably elevated. This protracted mode of action is similar to our previous results obtained with LCM as a stimulus (40).
Fig. 6.
Hyperosmolarity regulates MRTF-dependent transcription and MRTF exerts osmoprotective effects against moderate osmotic stress. A: LLC-PK1 cells were cotransfected with the firefly luciferase under the control of a triple CArG box promoter (3DA) and a control, enhancer-less Renilla luciferase plasmid and 24 h later were exposed to iso- or hypertonic (+150 mM NaCl = 600 mosM total) treatment in serum-free medium for 6 h. Where indicated, cells were pretreated with vehicle (DMSO) or CCG-1423 (3 μM) for 30 min and then exposed to iso- or hyperosmolarity. The corresponding agents remained present throughout the entire experiment. Firefly luciferase activity was normalized to Renilla luciferase activity in the same sample. Normalized promoter activity is expressed relative to the level measured in isotonic control cells (*P < 0.05; **P < 0.01). B: LLC-PK1 cells were transfected with nonrelated NR or MRTF (A+B) siRNA for 24 h, followed by transfection with triple CArG box promoter and a control, enhancer-less Renilla luciferase plasmid for an additional 24 h. Cells were then serum deprived for 3 h and exposed to iso- or hypertonic treatment (as above) for 6 h. Relative luciferase activity was measured as in A. Western blot verifying the efficacy of MRTF (A+B) siRNA is shown above the graph. ***P < 0.001. C and D: LLC-PK1 cells were processed as described in A and B. Representative phase-contrast photomicrographs obtained at ×40 magnification are shown. Note that after inhibition (C) or downregulation of MRTF (D), hyperosmolarity provokes robust morphological changes in the epithelium. E: longer exposure to even moderate hyperosmolarity reduces CArG-dependent transcription. LLC-PK1 cells were cotransfected with either the 3DA or the wild-type p765-SMA-Luc promoter in combination with a control, enhancer-less Renilla luciferase plasmid and exposed to iso- or hypertonic treatment (+150 mM NaCl = 600 mosM total) in serum-free medium for 24 h. Relative luciferase activity was measured as in A.
We next tested whether MRTF might be involved in osmoprotection under these conditions. Inhibition (by CCG-1423) or downregulation (by siRNAs) of MRTF did not seem to cause gross morphological changes under isotonic conditions (Fig. 6, C and D). Dramatically different observations were made after a hypertonic challenge. In the control monolayers, 6 h of exposure to hyperosmolarity provoked only occasional rounding or detachment, seen only in a small fraction of cells; in contrast, hypertonicity caused major morphological alterations in MRTF-inhibited or -depleted cells, characterized by the acquisition of elongated and irregular shapes, formation of dendrite-like processes or peripheral blebs and shriveled appearance, i.e., often the complete loss of the cobblestone-like epithelial character. These findings suggest that MRTF expression and activity are critical for withstanding osmotic stress.
We also investigated the effect of longer-lasting osmotic exposure on transcription. Interestingly, after a 24-h hypertonic treatment, there was a moderate but highly significant decrease in the CArG-dependent luciferase activity (Fig. 6E). Similar observations were made using another reporter, a 765-bp segment of the SMA promoter, which contains two CArG boxes among other cis-elements (40). These findings suggest that the MRTF-dependent transcriptional effect is relatively short-term in hypertonically challenged tubular cells, and it is followed by a reversal, a phenomenon that may be related to the destabilization of SRF under these conditions.
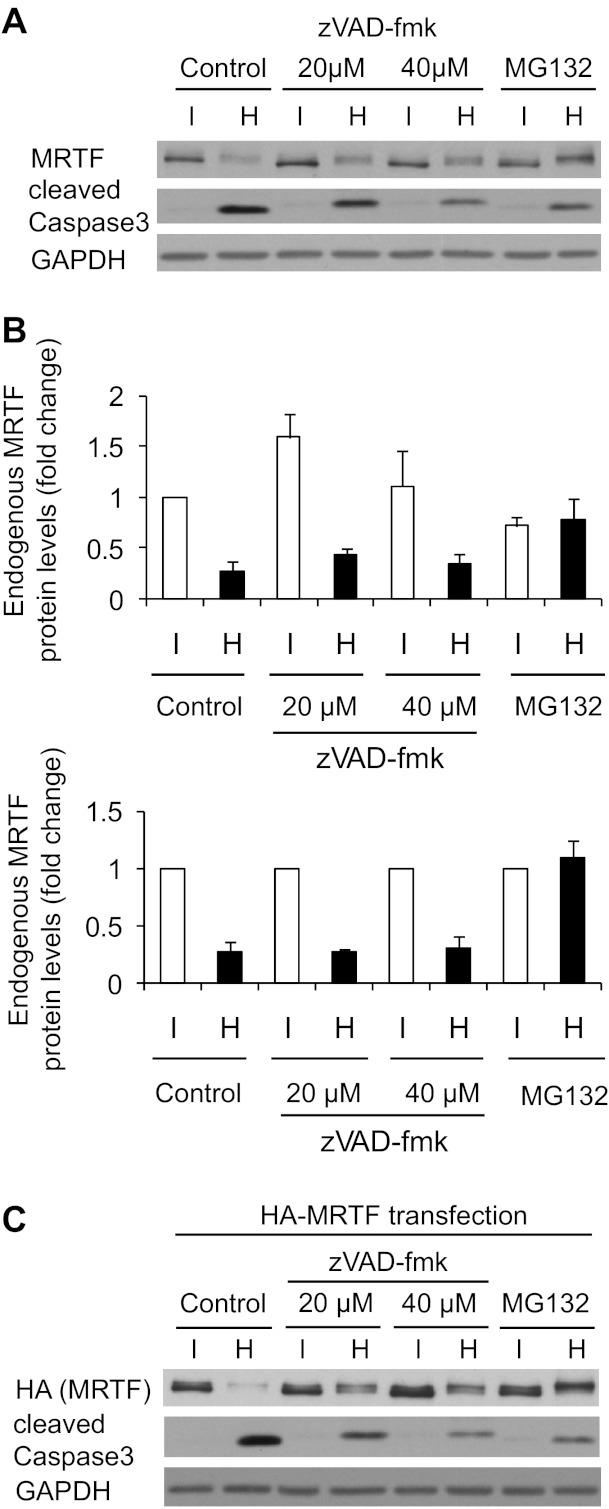
Finally, we sought to obtain insight into the mechanism underlying the downregulation of MRTF under strongly hyperosmotic conditions. Exposure to 200 mM extra NaCl for 2 h caused a substantial drop both in endogenous MRTF and in the heterologously expressed, HA-tagged MRTF, as verified by Western blotting using anti-MRTF and anti-HA antibodies (Fig. 7, A–C). The latter observation is informative in that it excludes the possibility that epitope masking (due to some posttranslational modification) is the main (or exclusive) reason for the observed drop in the MRTF signal. Furthermore, these results also suggest that an inhibition of MRTF transcription is unlikely to be the major cause of the decreased expression either, since the expression of HA-MRTF is driven by the artificial pCMV promoter. Instead, these findings together with the fast decrease in MRTF protein are consistent with enhanced MRTF degradation under highly hyperosmotic conditions. Since MRTF degradation was concomitant with caspase-3 activation, we considered that apoptosis-inducing caspases might be involved in this process. The pan-caspase inhibitor zVAD-fmk caused a strong (albeit not complete) reduction in caspase-3 activation but failed to prevent MRTF degradation (Fig. 7, A–C). zVAD-fmk slightly elevated the steady-state level of MRTF under isotonic conditions (Fig. 7B, top); however, the hypertonicity-induced relative (%) decrease in the endogenous MRTF level was unchanged (Fig. 7B, bottom). High concentration of zVAD-fmk seemed to exert a marginal protective effect with respect to the degradation of the heterologously expressed HA-MRTF. We then asked whether hyperosmolarity might trigger proteasomal MRTF degradation. To assess this possibility, cells were pretreated with the proteasome inhibitor MG132. This compound prevented the osmotically induced decrease in both the endogenous and the heterologously expressed MRTF protein (Fig. 7, A and C). Taken together, the data indicate that strong osmolarity destabilizes MRTF by facilitating its proteasomal degradation.
Fig. 7.
Strong hyperosmolarity promotes rapid proteasomal degradation of MRTF. A: LLC-PK1 cells were serum deprived for 3 h, followed by pretreatment with DMSO, the pan-caspase inhibitor zVAD-fmk (20 or 40 μM), or MG132 (25 μM) for 1 h. Cells were then exposed to iso- or hypertonic (+200 mM NaCl = 700 mosM total) treatment in serum-free medium for 2 h in the presence of the indicated inhibitors. Cell lysates were immunoblotted for endogenous MRTF, cleaved caspase-3, and GAPDH. B: densitometric analysis of the effect of zVAD-fmk and MG132 on endogenous MRTF protein expression under iso- and hypertonic conditions. In the top panel expression is normalized to the untreated isotonic samples, whereas the bottom panel shows the relative changes induced by hypertonicity, with the expression normalized to the isotonic level in each group. The difference between iso- and hypertonic samples is significant for the control and zVAD-fmk-treated samples (P < 0.01) and NS for MG132. C: LLC-PK1 cells were transfected with MRTF-hemagglutinin (HA) plasmid. After 24 h, cells were treated and processed as in A.
DISCUSSION
Cytoskeletal remodeling is an immediate response to osmotic stress, which helps the cell to withstand the ensuing mechanical trauma (17, 36). Our current studies show that hyperosmotically induced, cytoskeleton-regulating signaling pathways and the consequent cytoskeletal changes themselves are not only responsible for the acute structural adaptation, but they also mobilize transcription factors that can impact the expression of cytoskeletal genes. Our recent studies have shown that SRF is phosphorylated and activated upon hyperosmotic stimulation in ELA cells (24). However, SRF is a dual-function transcription factor that can drive both proliferation/survival-promoting early genes and cytoskeleton/muscle differentiation-specific genes (41). Moreover, these two functional modalities were found to be competitive (toggle-switch mechanism), and the selection or ratio between them is governed by the interaction of SRF with distinct transcriptional coactivators, namely with components of the ternary complex (for proliferation) and MRTF (for cytoskeletal control) (7, 41). Therefore we sought to determine whether hyperosmolarity can directly impact the cytoskeletal arm, i.e., MRTF, the activity of which is predominantly regulated through its localization. Our results show that MRTF is an osmosensitive molecule that is rapidly translocated into the nucleus upon hypertonic treatment in a RhoA- and ROK-dependent manner. Consistent with the possibility that the RhoA/ROK pathway acts primarily by inducing net F-actin polymerization, which is a key regulator of MRTF localization, we have shown earlier that the activation of ROK is an important contributor to the osmotically induced rise in F-actin, presumably because ROK mediates cofilin phosphorylation, which in turn reduces the F-actin severing activity of this protein (66). In addition, ROK (directly or indirectly) might induce MRTF phosphorylation as well. This possibility stems from the observations that MRTF was shown to undergo RhoA-dependent phosphorylation (43) and Y-27632 prevents both the stimulus-induced shift in the molecular mass of MRTF (61) and its concomitant translocation. Nonetheless, the role of this phosphorylation in the transport or activity of MRTF remains to be clarified. Finally, cell contractility [increased myosin light chain (MLC) phosphorylation] has also been shown to potentiate MRTF accumulation (21). Since hyperosmolarity provokes RhoA/ROK-dependent MLC phosphorylation in tubular cells (18), this mechanism may also facilitate nuclear MRTF accumulation under hyperosmolar conditions.
Our studies also provide insight into the hitherto unknown upstream mechanisms responsible for hypertonicity-induced RhoA activation. Based on our findings that hyperosmotic stress activates the RhoA exchange factor GEF-H1 and that GEF-H1 downregulation reduces the osmotically provoked RhoA activation and MRTF translocation, we conclude that GEF-H1 is an osmotically sensitive signal transducer and the GEF-H1/RhoA/ROK pathway is a major mediator of the hypertonicity-triggered MRTF translocation. This conclusion is in accord with a recent report showing that GEF-H1 can regulate SRF-dependent transcription (SMA expression) in TGF-β-stimulated retinal pigment cells (67). Furthermore, GEF-H1 has been recently shown to be activated by stretch (6), extracellular matrix stiffening (27), and depolarization (71), which, together with its osmosensitivity documented herein, implies that this molecule is a key mechanotransducer coupling physical changes to RhoA activation. However, GEF-H1 silencing did not completely eliminate RhoA activation and MRTF translocation. While this could be due to some residual GEF-H1 activity, it is likely that GEF-H1 is not the only link between osmotic stress and RhoA. In this regard previous studies have revealed that ezrin is activated by hyperosmotic stress and its downregulation mitigates the shrinkage-induced RhoA activation in ELA cells (56). Since active ezrin counteracts the RhoA-sequestering capacity of Rho-GDI (65), this mechanism may represent a significant permissive input. Another intriguing possibility comes from the elegant studies of Guilley et al. (25), who showed that integrin-mediated force transduction activates two GEFs, GEF-H1 and leukemia-associated Rho GEF (LARG), in parallel and each of these is responsible for approximately half of the ensuing RhoA activation. LARG was activated via integrin-mediated stimulation of the Src-family kinase Fyn. These findings point to LARG as a candidate in the regulation of osmotic RhoA activation as well, because integrins were proposed to transmit cell volume-dependent signals (60) and previous studies by us (34, 35) and others (11) have shown that hyperosmotic stress selectively activates Fyn (and in certain cell types Yes) but not p60 Src, the third ubiquitous member of the family.
Although the investigation of the upstream mechanisms that connect the osmotic insult (or other mechanical stimuli) to GEF-H1 activation is beyond the scope of the current work, considering some potential mechanisms may facilitate future studies in this direction. GEF-H1 can be stimulated by its release from microtubules or the tight junctions (1, 12) [either due to microtubule disassembly or possibly due to GEF-H1 phosphorylation (10, 73)] and by enhancing its intrinsic activity (again via phosphorylation). A variety of kinases (FAK, ERK, PAK family members, etc.) have been implicated in GEF-H1 regulation (22, 25, 32, 73), and several of these are also affected by osmotic shock (see Ref. 29). Future studies are warranted to test their involvement.
The discrepancies between the magnitude of RhoA activation and MRTF translocation by various stimuli prompted us to consider the role of additional regulatory inputs. We found that inhibition of p38 facilitated the nuclear accumulation of MRTF, an effect that may be due to p38-mediated phosphorylation of MRTF. This possibility is supported by the facts that MRTF contains potential p38 target sites (some of which are ERK targets as well), and ERK-mediated MRTF phosphorylation was shown to increase MRTF's affinity for actin, which in turn enhances nuclear export and may inhibit import (46). Irrespective of the exact mechanism, the observed effect was selective for p38 inhibition since pharmacological suppression of the ERK pathway did not detectably influence the hypertonicity-triggered MRTF accumulation (data not shown). This observation is consistent with the fact that hyperosmolarity is a weak and transient stimulus for ERK in many cells, while in others it actually inhibits this kinase (Ref. 24; see also Ref. 29). Furthermore, this finding also implies that ERK, which has been shown to mediate GEF-H1 activation in response to various stimuli (25, 32, 71), is unlikely to fulfill such a role with regard to hyperosmolarity (present study) or membrane stiffness (27). While p38 activity mitigates MRTF's nuclear accumulation during osmotic stress, its effect on MRTF transport is stimulus dependent and its overall impact on SRF-driven transcription is complex. Specifically, LCM-triggered MRTF translocation is suppressed by p38 inhibition, indicating that p38 is needed for upstream signaling steps induced by contact disruption (61). More importantly, SRF activity itself can be enhanced by p38, as indicated by the observation that p38 inhibition significantly reduced the hypertonicity-provoked activation of the SRF reporter in ELA cells (24). The likely mechanism underlying this finding is that p38 activates MAPKAP kinase-2, which directly phosphorylates and activates SRF (28, 58). This scenario raises the interesting possibility that p38 might modulate SRF activity during osmotic stress by enhancing it towards early genes (ternary factors) but mitigating it towards the cytoskeletal (MRTF-dependent) genes. A further level of complexity is that p38 has four isoforms, of which hyperosmolarity activates p38α and p38δ, both of which are inhibited by SB203580 but exert opposite effects on the activity of TonEBP (74). Clearly, the potential isoform-specificity as well as the exact mechanism(s) whereby p38 fine-tunes the activity of MRTF and SRF await elucidation.
Our studies also revealed the functional relevance of the osmotically induced MRTF translocation. We showed that pharmacological or genetic interference with MRTF suppresses the hyperosmolarity-induced increase in CArG-dependent transcription. Moreover, elimination or inhibition of MRTF increased the susceptibility of the cells to hyperosmotic damage, which manifested in gross morphological alterations at osmolarities that did not bring about major structural changes in control cells. Shortly before the submission of this work, Nie et al. (47) reported that downregulation of GEF-H1 augmented hyperosmolarity-induced lactate dehydrogenase release (necrotic damage) in MDCK cells, a finding consistent with the proposed osmoprotective role of the RhoA/GEF-H1/MRTF pathway. Interestingly, in various systems, MRTF has been reported to act either as an inhibitor or as a potential inducer of apoptosis. Specifically, MRTF overexpression protected cells against TNF-α- or hypoxia-induced apoptosis (27, 59), and the SRF/MRTF inhibitor CCG-1423 promoted apoptosis in melanoma cells (20). In contrast, activated MRTF was reported to drive the expression of proapoptotic Bcl family members (62). In light of these findings we sought to investigate whether MRTF could alter the level of osmotically provoked apoptosis. However, an unexpected phenomenon precluded the assessment of this question: we found that at apoptosis-inducing osmolarities, MRTF itself is rapidly degraded. Since osmotic stress activates caspases and SRF has been reported to be a target of caspase-3 (5, 19), it was conceivable that the decrease in MRTF protein might be a caspase-mediated process. However, the inability of the pan-caspase inhibitor zVAD-fmk to prevent MRTF degradation strongly argues against this possibility. Nonetheless, contribution of caspase activation cannot be ruled out since caspase-3 cleavage was not completely abolished by the inhibitor. Importantly, the efficient reduction in MRTF degradation by MG132 indicates that the proteasomal pathway is a main mechanism in the osmotically induced MRTF degradation. As mentioned, high osmolarities destabilize SRF as well. Since osmotic stress has been shown to induce SRF phosphorylation (24), the initial up-shift in the molecular mass of SRF observed after hypertonic treatment may reflect such modification. The osmotically induced phosphorylation may prime SRF for subsequent degradation, a concept that remains to be assessed in future studies. Overall, these observations suggest that while MRTF is necessary for adaptation to moderate osmotic stress, its activity and expression are very tightly controlled under hypertonic conditions. Accordingly, its translocation is transient and relatively modest, its RhoA-dependent nuclear accumulation is mitigated by p38-dependent and -independent mechanisms, and at higher osmolarities its stability (and that of SRF) is compromised. In keeping with this, the ensuing transcriptional effects are moderate and short term, present at 6 but not 24 h after stimulation. These observations indicate that under strongly hyperosmotic conditions, the “preferable” cell fate is death over epithelial-mesenchymal transition (EMT). Indeed, we never detected SMA expression, the hallmark of the myofibroblast phenotype, after stimulation with hyperosmolarity either alone or in combination with TGF-β (not shown). This is in sharp contrast with the combined effect of LCM and TGF-β, which induce robust, MRTF-dependent SMA expression and epithelial-myofibroblast transition although none of these stimuli can induce SMA expression in itself in our cells (39, 40). The above-mentioned MRTF-inhibiting mechanisms can ensure that under hyperosmotic conditions the activation of RhoA, which is necessary for cytoskeletal rearrangement and the maintenance of functional integrity, can be uncoupled from EMT induction.
Our experiments were performed on LLC-PK1 proximal tubule cells, because our earlier studies have thoroughly characterized both the hypertonicity-induced changes in the activity of Rho GTPases (16, 66) and MRTF/SRF-dependent EMyT (14, 21, 39, 40) in this cell type. However, proximal tubule cells do not reside in a hyperosmotic milieu in vivo. Thus while these cells are appropriate for studying the effect of acute osmostress on the epithelium, the role and behavior of MRTF should also be investigated in cells originating from the distal nephron, or in cells that have been adapted to chronic hyperosmolarity. Such future studies will show whether MRTF can be preserved in adapted cells and whether MRTF signaling plays a role in the regulation of the cytoskeleton and integrity in cells functioning in a chronically hyperosmotic environment.
In summary, we have identified the GEF-H1/RhoA/ROK/MRTF pathway as a tightly controlled osmosensitive and osmoprotective mechanism that provides a link between the osmotic environment and the transcriptional control of the cytoskeleton.
GRANTS
This work was supported by grants from the National Science and Engineering Research Council (NSERC), the Canadian Institutes of Health Research (CIHR, MOP-86535 and MOP-106625), and the Kidney Foundation of Canada to A. Kapus and CIHR grant to K. Szászi (MOP-97774). F. Waheed is supported by a Li Ka Shing fellowship and K. Szászi is the recipient of an Early Researcher Award from the Ontario Ministry of Innovation and Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.L.L., F.W., M.L., P.S., M.H., and A.K. performed the experiments; D.L.L., F.W., M.L., and A.K. analyzed the data; F.W., M.L., P.S., A.M., H.N., S.F.P., K.S., and A.K. approved the final version of the manuscript; M.L., K.S., and A.K. prepared the figures; M.L., S.F.P., K.S., and A.K. edited and revised the manuscript; A.M., S.F.P., K.S., and A.K. conception and design of the research; H.N. and A.K. interpreted the results of the experiments; A.K. drafted the manuscript.
Footnotes
This article is the topic of an Editorial Focus by Maurice B. Burg and Joan D. Ferraris (7a).
REFERENCES
- 1. Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell 8: 777–786, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol 10: 719–722, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Badid C, Desmouliere A, McGregor B, Costa AM, Fouque D, Hadj Aissa A, Laville M. Interstitial alpha-smooth muscle actin: a prognostic marker in membranous nephropathy. Clin Nephrol 52: 210–217, 1999 [PubMed] [Google Scholar]
- 4. Benais-Pont G, Punn A, Flores-Maldonado C, Eckert J, Raposo G, Fleming TP, Cereijido M, Balda MS, Matter K. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol 160: 729–740, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertolotto C, Ricci JE, Luciano F, Mari B, Chambard JC, Auberger P. Cleavage of the serum response factor during death receptor-induced apoptosis results in an inhibition of the c-FOS promoter transcriptional activity. J Biol Chem 275: 12941–12947, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol 298: L837–L848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene 324: 1–14, 2004 [DOI] [PubMed] [Google Scholar]
- 7a. Burg MB, Ferraris JD. Salt, skeletons, and suicide. Focus on “Hyperosmotic stress regulates the distribution and stability of myocardin-related transcription factor, a key modulator of the cytoskeleton.” Am J Physiol Cell Physiol (October 24, 2012). doi:10.1152/ajpcell.00319.2012 [DOI] [PubMed] [Google Scholar]
- 8. Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Busche S, Descot A, Julien S, Genth H, Posern G. Epithelial cell-cell contacts regulate SRF-mediated transcription via Rac-actin-MAL signalling. J Cell Sci 121: 1025–1035, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci 118: 1861–1872, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Cantore M, Reinehr R, Sommerfeld A, Becker M, Haussinger D. The Src family kinase Fyn mediates hyperosmolarity-induced Mrp2 and Bsep retrieval from canalicular membrane. J Biol Chem 286: 45014–45029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell 19: 2147–2153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol 73: 413–435, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Charbonney E, Speight P, Masszi A, Nakano H, Kapus A. beta-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition. Mol Biol Cell 22: 4472–4485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Ciano-Oliveira C, Lodyga M, Fan L, Szaszi K, Hosoya H, Rotstein OD, Kapus A. Is myosin light-chain phosphorylation a regulatory signal for the osmotic activation of the Na+-K+-2Cl− cotransporter? Am J Physiol Cell Physiol 289: C68–C81, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Di Ciano-Oliveira C, Sirokmany G, Szaszi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A. Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am J Physiol Cell Physiol 285: C555–C566, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Di Ciano-Oliveira C, Thirone AC, Szaszi K, Kapus A. Osmotic stress and the cytoskeleton: the R(h)ole of Rho GTPases. Acta Physiol (Oxf) 187: 257–272, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Di Ciano C, Nie Z, Szaszi K, Lewis A, Uruno T, Zhan X, Rotstein OD, Mak A, Kapus A. Osmotic stress-induced remodeling of the cortical cytoskeleton. Am J Physiol Cell Physiol 283: C850–C865, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Drewett V, Devitt A, Saxton J, Portman N, Greaney P, Cheong NE, Alnemri TF, Alnemri E, Shaw PE. Serum response factor cleavage by caspases 3 and 7 linked to apoptosis in human BJAB cells. J Biol Chem 276: 33444–33451, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Evelyn CR, Wade SM, Wang Q, Wu M, Iniguez-Lluhi JA, Merajver SD, Neubig RR. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther 6: 2249–2260, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Fan L, Sebe A, Peterfi Z, Masszi A, Thirone AC, Rotstein OD, Nakano H, McCulloch CA, Szaszi K, Mucsi I, Kapus A. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell 18: 1083–1097, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujishiro SH, Tanimura S, Mure S, Kashimoto Y, Watanabe K, Kohno M. ERK1/2 phosphorylate GEF-H1 to enhance its guanine nucleotide exchange activity toward RhoA. Biochem Biophys Res Commun 368: 162–167, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Garcia-Mata R, Wennerberg K, Arthur WT, Noren NK, Ellerbroek SM, Burridge K. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol 406: 425–437, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gorbatenko A, Wiwel M, Klingberg H, Nielsen AB, Kapus A, Pedersen SF. Hyperosmotic stress strongly potentiates serum response factor (SRF)-dependent transcriptional activity in Ehrlich Lettre Ascites cells through a mechanism involving p38 mitogen-activated protein kinase. J Cell Physiol 226: 2857–2868, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol 13: 722–727, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hallows KR, Packman CH, Knauf PA. Acute cell volume changes in anisotonic media affect F-actin content of HL-60 cells. Am J Physiol Cell Physiol 261: C1154–C1161, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Heck JN, Ponik SM, Garcia-Mendoza MG, Pehlke CA, Inman DR, Eliceiri KW, Keely PJ. Microtubules regulate GEF-H1 in response to extracellular matrix stiffness. Mol Biol Cell 23: 2583–2592, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, Nordheim A. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem 274: 14434–14443, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Jeon US, Kim JA, Sheen MR, Kwon HM. How tonicity regulates genes: story of TonEBP transcriptional activator. Acta Physiol (Oxf) 187: 241–247, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Jurmeister S, Baumann M, Balwierz A, Keklikoglou I, Ward A, Uhlmann S, Zhang JD, Wiemann S, Sahin O. MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol Cell Biol 32: 633–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, Kohno M, Rotstein OD, Kapus A, Szaszi K. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem 284: 11454–11466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kapus A, Di Ciano C, Sun J, Zhan X, Kim L, Wong TW, Rotstein OD. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites. The role of Fyn and FER kinases. J Biol Chem 275: 32289–32298, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Kapus A, Szaszi K, Sun J, Rizoli S, Rotstein OD. Cell shrinkage regulates Src kinases and induces tyrosine phosphorylation of cortactin, independent of the osmotic regulation of Na+/H+ exchangers. J Biol Chem 274: 8093–8102, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Kuwayama H, Ecke M, Gerisch G, Van Haastert PJ. Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science 271: 207–209, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Lewis A, Di Ciano C, Rotstein OD, Kapus A. Osmotic stress activates Rac and Cdc42 in neutrophils: role in hypertonicity-induced actin polymerization. Am J Physiol Cell Physiol 282: C271–C279, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Malek AM, Xu C, Kim ES, Alper SL. Hypertonicity triggers RhoA-dependent assembly of myosin-containing striated polygonal actin networks in endothelial cells. Am J Physiol Cell Physiol 292: C1645–C1659, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol 165: 1955–1967, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szaszi K, Kapus A. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol 188: 383–399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 35: 577–593, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Mihira H, Suzuki HI, Akatsu Y, Yoshimatsu Y, Igarashi T, Miyazono K, Watabe T. TGF-beta-induced mesenchymal transition of MS-1 endothelial cells requires Smad-dependent cooperative activation of Rho signals and MRTF-A. J Biochem 151: 145–156, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113: 329–342, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Morita T, Mayanagi T, Sobue K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J Cell Biol 179: 1027–1042, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mouilleron S, Guettler S, Langer CA, Treisman R, McDonald NQ. Molecular basis for G-actin binding to RPEL motifs from the serum response factor coactivator MAL. EMBO J 27: 3198–3208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muehlich S, Wang R, Lee SM, Lewis TC, Dai C, Prywes R. Serum-induced phosphorylation of the serum response factor coactivator MKL1 by the extracellular signal-regulated kinase 1/2 pathway inhibits its nuclear localization. Mol Cell Biol 28: 6302–6313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nie M, Balda MS, Matter K. Stress- and Rho-activated ZO-1-associated nucleic acid binding protein binding to p21 mRNA mediates stabilization, translation, and cell survival. Proc Natl Acad Sci USA 109: 10897–10902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 27: 347–376, 2011 [DOI] [PubMed] [Google Scholar]
- 49. Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol 11: 353–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res 100: 633–644, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Pedersen SF, Hoffmann EK. Possible interrelationship between changes in F-actin and myosin II, protein phosphorylation, and cell volume regulation in Ehrlich ascites tumor cells. Exp Cell Res 277: 57–73, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Pedersen SF, Mills JW, Hoffmann EK. Role of the F-actin cytoskeleton in the RVD and RVI processes in Ehrlich ascites tumor cells. Exp Cell Res 252: 63–74, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol 16: 588–596, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277: C1–C9, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int 80: 41–50, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Rasmussen M, Alexander RT, Darborg BV, Mobjerg N, Hoffmann EK, Kapus A, Pedersen SF. Osmotic cell shrinkage activates ezrin/radixin/moesin (ERM) proteins: activation mechanisms and physiological implications. Am J Physiol Cell Physiol 294: C197–C212, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Rizoli SB, Rotstein OD, Parodo J, Phillips MJ, Kapus A. Hypertonic inhibition of exocytosis in neutrophils: central role for osmotic actin skeleton remodeling. Am J Physiol Cell Physiol 279: C619–C633, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Ronkina N, Menon MB, Schwermann J, Arthur JS, Legault H, Telliez JB, Kayyali US, Nebreda AR, Kotlyarov A, Gaestel M. Stress induced gene expression: a direct role for MAPKAP kinases in transcriptional activation of immediate early genes. Nucleic Acids Res 39: 2503–2518, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sasazuki T, Sawada T, Sakon S, Kitamura T, Kishi T, Okazaki T, Katano M, Tanaka M, Watanabe M, Yagita H, Okumura K, Nakano H. Identification of a novel transcriptional activator, BSAC, by a functional cloning to inhibit tumor necrosis factor-induced cell death. J Biol Chem 277: 28853–28860, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Schliess F, Haussinger D. Osmosensing by integrins in rat liver. Methods Enzymol 428: 129–144, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Sebe A, Masszi A, Zulys M, Yeung T, Speight P, Rotstein OD, Nakano H, Mucsi I, Szaszi K, Kapus A. Rac, PAK and p38 regulate cell contact-dependent nuclear translocation of myocardin-related transcription factor. FEBS Lett 582: 291–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shaposhnikov D, Descot A, Schilling J, Posern G. Myocardin-related transcription factor A regulates expression of Bok and Noxa and is involved in apoptotic signalling. Cell Cycle 11: 141–150, 2012 [DOI] [PubMed] [Google Scholar]
- 63. Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 107: 294–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98: 159–169, 1999 [DOI] [PubMed] [Google Scholar]
- 65. Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem 272: 23371–23375, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Thirone AC, Speight P, Zulys M, Rotstein OD, Szaszi K, Pedersen SF, Kapus A. Hyperosmotic stress induces Rho/Rho kinase/LIM kinase-mediated cofilin phosphorylation in tubular cells: key role in the osmotically triggered F-actin response. Am J Physiol Cell Physiol 296: C463–C475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsapara A, Luthert P, Greenwood J, Hill CS, Matter K, Balda MS. The RhoA activator GEF-H1/Lfc is a transforming growth factor-beta target gene and effector that regulates alpha-smooth muscle actin expression and cell migration. Mol Biol Cell 21: 860–870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell'Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol 5: 1104–1110, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316: 1749–1752, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Waheed F, Speight P, Dan Q, Garcia-Mata R, Szaszi K. Affinity precipitation of active Rho-GEFs using a GST-tagged mutant Rho protein (GST-RhoA(G17A)) from epithelial cell lysates. J Vis Exp 61: 3932, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Waheed F, Speight P, Kawai G, Dan Q, Kapus A, Szaszi K. Extracellular signal-regulated kinase and GEF-H1 mediate depolarization-induced Rho activation and paracellular permeability increase. Am J Physiol Cell Physiol 298: C1376–C1387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci USA 99: 14855–14860, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14–3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem 279: 18392–18400, 2004 [DOI] [PubMed] [Google Scholar]
- 74. Zhou X, Ferraris JD, Dmitrieva NI, Liu Y, Burg MB. MKP-1 inhibits high NaCl-induced activation of p38 but does not inhibit the activation of TonEBP/OREBP: opposite roles of p38alpha and p38delta. Proc Natl Acad Sci USA 105: 5620–5625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou X, Izumi Y, Burg MB, Ferraris JD. Rac1/osmosensing scaffold for MEKK3 contributes via phospholipase C-gamma1 to activation of the osmoprotective transcription factor NFAT5. Proc Natl Acad Sci USA 108: 12155–12160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]