Abstract
The endothelial glycocalyx, a glycosaminoglycan layer located on the apical surface of vascular endothelial cells, has been shown to be important for several endothelial functions. Previous studies have documented that the glycocalyx is highly abundant in the mouse common carotid region, where the endothelium is exposed to laminar shear stress, and it is resistant to atherosclerosis. In contrast, the glycocalyx is scarce or absent in the mouse internal carotid sinus region, an area exposed to nonlaminar shear stress and highly susceptible to atherosclerosis. On the basis of these observations, we hypothesized that the expression of components of the endothelial glycocalyx is differentially regulated by distinct hemodynamic environments. To test this hypothesis, human endothelial cells were exposed to shear stress waveforms characteristic of atherosclerosis-resistant or atherosclerosis-susceptible regions of the human carotid, and the expression of several components of the glycocalyx was assessed. These experiments revealed that expression of several components of the endothelial glycocalyx is differentially regulated by distinct shear stress waveforms. Interestingly, we found that heparan sulfate expression is increased and evenly distributed on the apical surface of endothelial cells exposed to the atheroprotective waveform and is irregularly present in cells exposed to the atheroprone waveform. Furthermore, expression of a heparan sulfate proteoglycan, syndecan-1, is also differentially regulated by the two waveforms, and its suppression mutes the atheroprotective flow-induced cell surface expression of heparan sulfate. Collectively, these data link distinct hemodynamic environments to the differential expression of critical components of the endothelial glycocalyx.
Keywords: shear stress, endothelium, glycocalyx, heparan sulfate, syndecan-1
the vascular endothelium is a single layer of cells lining the inner surface of blood vessels. This cellular layer not only serves as an interface between the blood and surrounding tissues, but it also plays important roles in inflammation (28), angiogenesis (5), blood coagulation (9), and control of vascular tone (27). Endothelial dysfunction, the state in which the endothelial cells can no longer respond to external stimuli to preserve homeostasis, is frequently followed by deterioration of vascular function and development of several vascular pathologies, including atherosclerosis (17). Interestingly, multiple studies have shown, in experimental animals and human subjects, that the location where early lesions of atherosclerosis develop is highly focal in nature. For example, in the carotid artery, early atherosclerotic lesions are more frequently observed at the carotid sinus bifurcation, where the endothelium is exposed to disturbed flow and displays several distinct cell biological features, including scarce expression of the endothelial glycocalyx layer (EGL) (10, 18, 22, 55, 56). In contrast, the common carotid region, where the endothelium is exposed to laminar flow and displays abundant expression of the EGL, is resistant to the development of early lesions of atherosclerosis (10, 18, 22, 55, 56). Collectively, these observations have defined a correlation between atheroprotection, shear stress, and EGL expression.
The EGL is a gel-like structure that is present on the apical surface of endothelial cells (13, 46, 59). It is mainly composed of three glycosaminoglycans (GAGs): heparan sulfate, chondroitin sulfate, and hyaluronic acid. Heparan sulfate is bound to proteoglycans, including syndecan-1, -2, and -4 and glypican-1; chondroitin sulfate is bound to biglycan and the above-mentioned syndecans; and hyaluronic acid is electrostatically attached to CD44 (50). All three negatively charged GAGs tangle together and form the characteristic glycocalyx structure observed by electron microscopy (48, 55, 59).
One of the main functions of the glycocalyx is to regulate vascular permeability and modulate binding of molecules that interact with the endothelial receptors. This process is achieved through two main mechanisms: 1) the EGL can directly bind to some biomolecules and increase their local concentration, and 2) the EGL can also selectively block some other biomolecules and prevent them from reaching the endothelial surface (1, 3, 33). There is also evidence that the endothelial glycocalyx may act as a mechanosensor. When endothelial cells are subjected to flow, the cells adapt to the mechanical stimuli by reorganizing the cytoskeleton (24, 34, 53), aligning to the direction of the flow (42), releasing the vasodilator nitric oxide (NO) (43), suppressing the proliferation rate (34), and decreasing the migration speed (25). Interestingly, these flow-mediated responses are impaired when the EGL is compromised. For example, studies in the laboratory of J. Tarbell have demonstrated that cultured endothelial cells pretreated with heparanase or hyaluronidase to remove heparan sulfate or hyaluronic acid from the EGL no longer reorganize the cytoskeleton or produce NO in response to flow (14, 35, 53). Previous work by our laboratory and others also showed that, after heparanase-based removal of heparan sulfate proteoglycans, the endothelial cells lose the ability to sense flow and modulate its migration speed and proliferation rate (32, 60). Finally, recent studies by Constantinescu et al. (8) and Mulivor and Lipowsky (33) established a correlation between damaged EGL and leukocyte adhesion, linking the presence of EGL to endothelial activation. Collectively, these studies document that EGL expression is critical for the endothelium to properly respond to the hemodynamic environment and to function as a protective barrier in the context of inflammation.
The abundance of EGL is dynamically controlled by production and degradation: transport of newly produced proteoglycans from the endoplasmic reticulum/Golgi to the cell membrane and endocytic degradation or shedding of the proteoglycans from the cell surface by matrix metalloproteinase (MMP) family proteins and other enzymes such as heparanase, chondroitinase, and hyaluronidase. Several in vivo measurements of EGL thickness have been made. The pioneering intravital microscopy work by Vink and Duling (58) showed the EGL height in hamster muscle capillaries to be 0.4–0.5 μm. A similar value was found by Potter and Damiano (38), who used microparticle image velocimetry to measure mouse cremaster venules, and Chappell et al. (6), who used transmission electron microscopy to study the human umbilical vein. Recently, using confocal laser scanning microscopy, van den Berg et al. (54) demonstrated that the EGL in mouse aorta can be as thick as 2–4 μm. The conclusion from these in vivo measurements is that the EGL is 0.4–4 μm thick. However, whether the glycocalyx layer is also present in cultured endothelial cells remains a major question in the field. Several studies have demonstrated that the EGL is almost absent in various cultured endothelial cell types, including human umbilical vein endothelial cells (HUVEC) and bovine aortic endothelial cells (BAEC) (6, 20, 38). In contrast, data from other studies support a similar EGL abundance in vitro and in vivo (11, 49, 53). Recently, using rapid freezing/freeze substitution transmission electron microscopy, Ebong et al. (13) showed an EGL thickness of 11 μm in cultured BAEC. These contradictory data suggest that the integrity of EGL in vitro may be highly sensitive to factors such as cell type, culture condition, and preservation/processing techniques. However, little is known regarding specific biomechanical or biochemical inputs capable of inducing or suppressing the expression of components of the EGL.
In this study, we hypothesized that the expression of critical components of the EGL is regulated by specific shear stress waveforms present in atherosclerosis-resistant or atherosclerosis-susceptible regions of the human vasculature.
MATERIALS AND METHODS
Endothelial cell culture.
Primary HUVEC were isolated in the Department of Pathology, Center for Excellence in Vascular Biology, Brigham and Women's Hospital, as previously described (16). HUVEC were cultured in Medium 199 containing 20% calf serum, 1% l-glutamine, 1% penicillin-streptomycin, 50 μg/ml endothelial cell growth supplement (Biomedical Technologies, Stoughton, MA), and 100 μg/ml heparin. Cells were incubated in a humidified incubator at 37°C and 5% CO2. After reaching confluence, cells were detached with 1% trypsin and plated onto a 0.1% gelatin-coated 10.8-cm polystyrene plate surface (Plaskolite, Columbus, OH) for shear stress experiments.
Hemodynamic shear stress in vitro system.
The shear experiments implementing the atheroprotective and atheroprone shear stress waveforms were conducted in a modified dynamic flow system, as previously described (10). For these experiments, the culture medium was supplemented with 1.7% dextran (1.5–2.8 × 106 mol wt; Sigma-Aldrich, St. Louis, MO) to increase viscosity of the medium to 2.1 cP. The flow system was kept at 37°C and 5% CO2 in humidified air.
Flow cytometry analysis.
Surface expression of GAGs and their protein carriers was measured using flow cytometry. The following primary antibodies were used: anti-syndecan-1 antibody (1:10 dilution, biotin-conjugated mouse IgG1; clone B-A38, Abcam, Cambridge, MA), anti-syndecan-2 antibody (1:10 dilution, allophycocyanin-conjugated rat IgG2B; R & D Systems, Minneapolis, MN), anti-syndecan-4 antibody (1:5 dilution; biotin-conjugated goat IgG, R & D Systems), anti-glypican-1 antibody (1:5 dilution; biotin-conjugated goat IgG, R & D Systems), anti-CD44 antibody (1:5 dilution, biotin-conjugated mouse IgG2B; BD Biosciences, Bedford, MA), anti-heparan sulfate antibody (1:100 dilution, mouse IgM; clone 10E4, US Biological, Swampscott, MA), and anti-chondroitin sulfate antibody (1:50 dilution, mouse IgG2A; BD Pharmingen, San Diego, CA). The primary antibodies were coupled with the appropriate Alexa Fluor 488-conjugated secondary antibodies: goat anti-mouse IgM- and goat anti-mouse IgG-streptavidin (1:400 dilution; Invitrogen, Carlsbad, CA). Briefly, enzyme-free PBS-based cell dissociation buffer (Invitrogen) was used to detach HUVEC from the surface. The isolated cells were resuspended in PBS containing 15% fetal calf serum and 0.2 mM EDTA and blocked for 30 min at 4°C. The appropriate primary antibody was subsequently added to the cell suspensions and incubated at 4°C for 45 min. The cells were then centrifuged at 1,200 relative centrifugal force, washed once with PBS, and incubated with the secondary antibody and 7-amino-actinomycin (1:20 dilution; BD Pharmingen) for 30 min. Cells were washed an additional two times before the fluorescent signal was measured by fluorescein-activated cell sorting (FACS) using FACSCablibur (BD Biosciences). The data were analyzed and quantified with FlowJo version 9.1 (FlowJo, Ashland, OR).
Immunofluorescence microscopy.
Immediately after flow exposure, cells were fixed in 4% paraformaldehyde for 5 min, washed three times with PBS at room temperature, and blocked in 1% BSA for 30 min. The samples were then incubated with anti-heparan sulfate antibody (1:100 dilution; catalog no. 10E4, US Biological), anti-syndecan-1 antibody (1:50 dilution; catalog no. B-A38, Abcam), or anti-hyaluronic acid antibody (1:100 dilution; Abcam) for 1 h, washed three times with PBS, and then incubated with the appropriate Alexa Fluor 488 secondary antibody for 1 h. For nuclear staining, the cells were incubated with 600 nM 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) for 5 min or permeabilized with 0.2% Triton X-100 in PBS for 10 min and then incubated with 1 μM TO-PRO-3 dye (Invitrogen) for 15 min. The coverslips were mounted with Gel Mount mounting medium (BioMeda, Foster City, CA). Images were taken using a Nikon Eclipse Ti microscope. Hyaluronic acid signal was quantified using ImageJ. The relative hyaluronic acid signal was calculated by subtracting the mean signal intensity of the background (secondary antibody-only control) from that of the samples. Three fields from a sample were used to derive the average signal intensity. The histogram and statistics were generated from samples collected from three independent experiments.
Confocal immunofluorescence microscopy.
The UltraView RS spinning disk confocal imaging system (PerkinElmer, Waltham, MA) was used to obtain z stacks (0.05 μm apart) of the sample. Deconvolution of the images was performed using AutoQuant deconvolution software (Media Cybernetics, Bethesda, MD), and the three-dimensional reconstruction was made using ImageJ (Image 3D Viewer Toolbox).
Quantitative analysis of glycocalyx coverage and the glycocalyx thickness.
Percent coverage of the glycocalyx was calculated by determining the acquired fluorescent image's percentage of pixels in which heparan sulfate signal intensity is above a specific threshold. This threshold was set at the value where the corresponding secondary antibody-only control had <0.5% “positive” pixels. Three fields (432 × 329 μm) from a sample were used to derive the glycocalyx coverage.
Short-hairpin RNA experiments.
HUVEC were transfected at 50% confluence with a lentiviral short-hairpin RNA (shRNA) targeting human syndecan-1 [multiplicity of infection (MOI) 5; catalog no. TRCN000072580, Sigma-Aldrich] or a lentiviral nontarget shRNA control (MOI 5; catalog no. SHC016V, Sigma-Aldrich) in medium supplemented with hexadimethrine bromide (8 μg/ml). Lentiviral particles were washed out after 24 h, and the cells were grown to confluence. The cells were then treated with puromycin (5 μg/ml; Sigma-Aldrich) for 72 h to select for transfected HUVEC and then incubated in medium without puromycin for 48 h before the flow experiments were conducted.
RNA isolation and quantitative PCR analysis.
Cells were lysed for RNA isolation, and real-time TaqMan PCR was performed as previously described (36).
Statistics.
A two-tailed Student's t-test was used to determine statistical significance between two groups. Difference at P < 0.05 was considered statistically significant.
RESULTS
Expression of components of the endothelial glycocalyx on the cell surface of static cultured HUVEC.
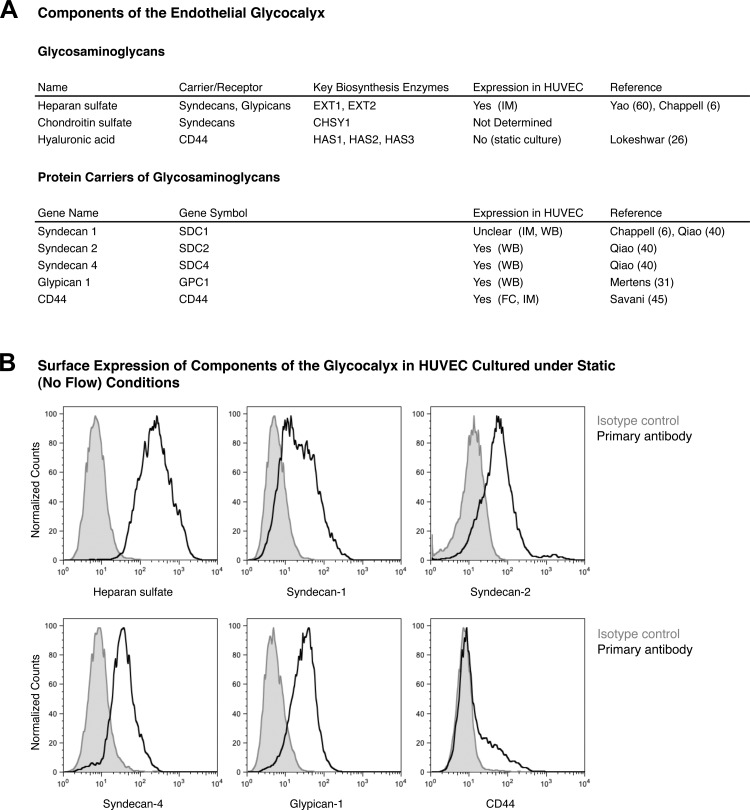
The abundance and composition of the EGL varies across different endothelial cell types. In this study, we focused on HUVEC, since these cells have been extensively used by our laboratory and others to assess the role of biomechanical forces in endothelial gene expression (10, 12, 16, 36, 47). Interestingly, of the three GAGs commonly present on the endothelial surface, only heparan sulfate has been previously shown to be expressed in HUVEC (Fig. 1A) (6, 60). Hyaluronic acid, on the other hand, was reported to be absent in one study (26). The status of chondroitin sulfate is unclear, since no study has directly measured the abundance of chondroitin sulfate expressed in HUVEC. As for the protein anchors of the GAGs, the major carriers of heparan sulfate and chondroitin sulfate are the syndecan and glypican family proteins. Among the syndecan family members, syndecan-3 is expressed only in the neuronal cells, and syndecan-1, -2, and -4 have been shown to be expressed in endothelial cells (3). Among the glypican family members, only glypican-1 is present in endothelial cells (3, 15). Hyaluronic acid, on the other hand, is noncovalently bound to the cell surface glycoprotein CD44. Expression of syndecan-2, syndecan-4, glypican-1, and CD44 has been previously documented in HUVEC (Fig. 1A). There are studies supporting the presence and absence of syndecan-1 in HUVEC (6, 40).
Fig. 1.
Components of the endothelial glycocalyx in human umbilical vein endothelial cells (HUVEC). A: list of protein carriers and glycosaminoglycans (GAGs) previously documented to be part of the endothelial glycocalyx and their expression in HUVEC. CHSY1, chondroitin sulfate synthase 1; HAS1, hyaluronan synthase 1; EXT1, exostosin 1; IM, immunostaining; WB, Western blotting; FC, flow cytometry. B: representative flow cytometry analysis of endothelial glycocalyx components present in our static cultured HUVEC.
Because the expression and subcellular localization of GAGs and glycoproteins may be affected by the specific culture conditions, we first examined the expression of components of the EGL in our static (no-flow) primary cultured HUVEC. We used FACS analysis to directly assess the surface expression of these EGL components in these cells. These analyses confirmed the cell surface expression of heparan sulfate, syndecan-1, -2, and -4, glypican-1, and CD44 (Fig. 1B). However, signals from chondroitin sulfate and hyaluronic acid were very weak. Therefore, we conclude that the EGL in static cultured HUVEC comprises heparan sulfate anchored by syndecan-1, -2, and -4 and glypican-1.
Expression of heparan sulfate and hyaluronic acid is regulated by specific shear stress waveforms.
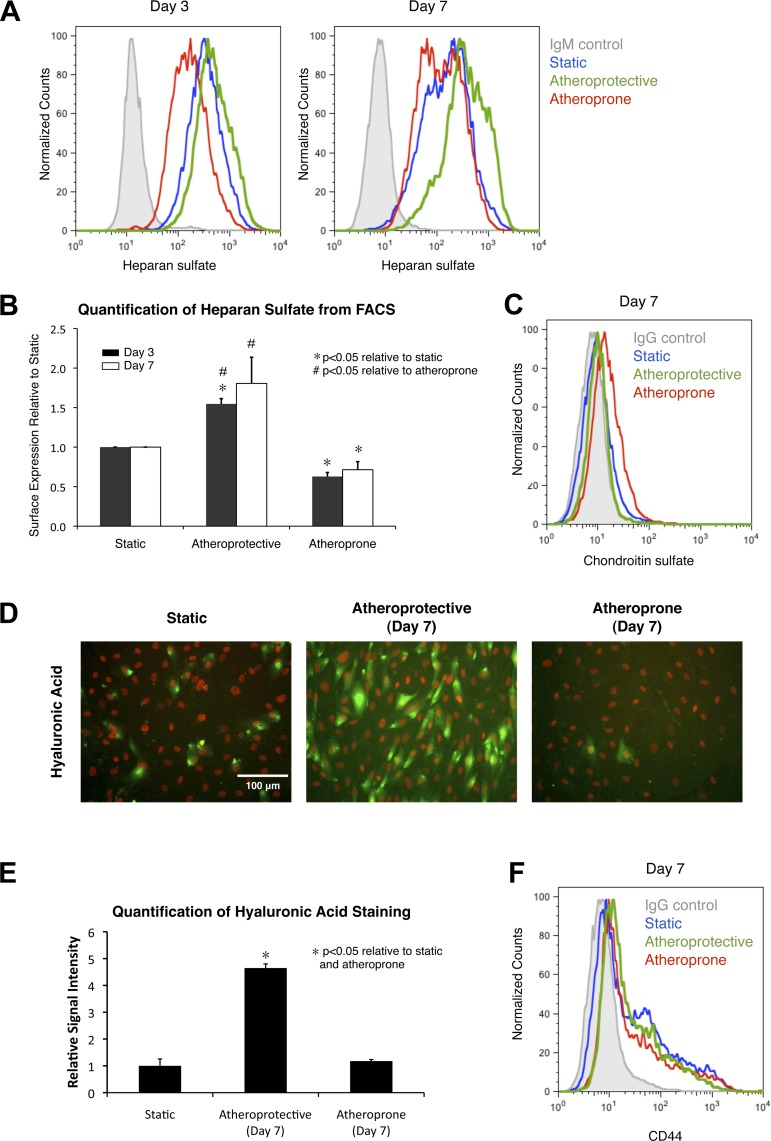
Given that heparan sulfate is the predominant GAG in HUVEC, we first sought to test the hypothesis that shear stress regulates its expression by quantitatively measuring heparan sulfate expression in endothelial cells exposed to two well-characterized shear stress waveforms, namely, the atheroprotective waveform and the atheroprone waveform (10). Previous studies by Potter et al. (39) demonstrated that the time scale for glycocalyx growth is on the order of days. When the glycocalyx was enzymatically degraded, significant recovery was observed as soon as 3 days, and a full recovery was observed after 5–7 days. These data suggest that 2–3 days are required for the generation of the glycocalyx. Therefore, we maintained endothelial cells under static (no-flow) conditions or exposed them to the atheroprotective or atheroprone waveforms for 3 and 7 days and then measured heparan sulfate surface expression via FACS. After 3 and 7 days of exposure to atheroprotective flow, heparan sulfate expression was significantly increased by 54.6 ± 6.7% and 80.6 ± 33.0%, respectively (Fig. 2, A and B). In contrast, atheroprone flow suppressed heparan sulfate expression and caused a 37.2 ± 5.1% and 28.5 ± 10.2% decrease after 3 and 7 days, respectively. These data are consistent with our hypothesis that expression of this GAG is regulated by the specific shear stress waveform and demonstrate that the differential expression is time-dependent.
Fig. 2.
Exposure to atheroprotective waveform leads to induced expression of heparan sulfate and hyaluronic acid, but not chondroitin sulfate. A: representative flow cytometry histogram of surface heparan sulfate expression on cell cultured under static condition or cell exposed to atheroprotective or atheroprone flow for 3 or 7 days. B: quantitative analysis of flow cytometry data from 3 independent experiments. Values are means ± SE (n = 3). C: flow cytometry histogram of surface expression of chondroitin sulfate on cell cultured under static condition or cell exposed to atheroprone or atheroprotective shear stress waveform for 7 days. D: representative immunostaining images of hyaluronic acid in cell cultured under static condition or cell exposed to atheroprotective or atheroprone flow for 7 days. Hyaluronic acid is shown in green, and nucleus [4′,6-diaminido-2-phenylindole (DAPI)] is shown in red. E: quantitative analysis of hyaluronic acid staining signal based on immunostaining images in D. Values are means ± SE (n = 3). F: flow cytometry histogram of surface expression of CD44 on cell cultured under static condition or exposed to atheroprotective or atheroprone shear stress waveform for 7 days.
Knowing that expression of heparan sulfate can be significantly regulated by shear stress after 7 days, we next sought to find whether chondroitin sulfate and hyaluronic acid expression, although absent in static in vitro cultures, can also be induced by flow. HUVEC were similarly exposed to atheroprotective or atheroprone flow for 7 days; then chondroitin sulfate surface expression was measured by FACS, and hyaluronic acid expression was evaluated by immunostaining (due to electrostatic binding to its protein carriers). Our data indicate that chondroitin sulfate is still absent after exposure to either flow condition (Fig. 2C). Interestingly, expression of hyaluronic acid was strongly upregulated by the atheroprotective waveform (Fig. 2D). Quantification of the immunostaining data (Fig. 2E) showed an increase to 464.7 ± 15.6% relative to the static culture. Finally, we assessed if the atheroprotective shear stress-induced hyaluronic acid upregulation is correlated with expression of the hyaluronic acid-binding protein CD44. FACS data indicate that CD44 expression is not shear stress-dependent (Fig. 2F), suggesting that the increase in hyaluronic acid under the atheroprotective condition may be due to a higher rate of hyaluronic acid synthesis. Collectively, our experimental data demonstrate that the two major GAGs of the endothelial glycocalyx, heparan sulfate and hyaluronic acid, are strongly promoted by the shear stress waveform found in the atherosclerosis-resistant regions of human arteries.
Distribution of heparan sulfate at the apical endothelial surface is changed after prolonged exposure to the atheroprotective waveform.
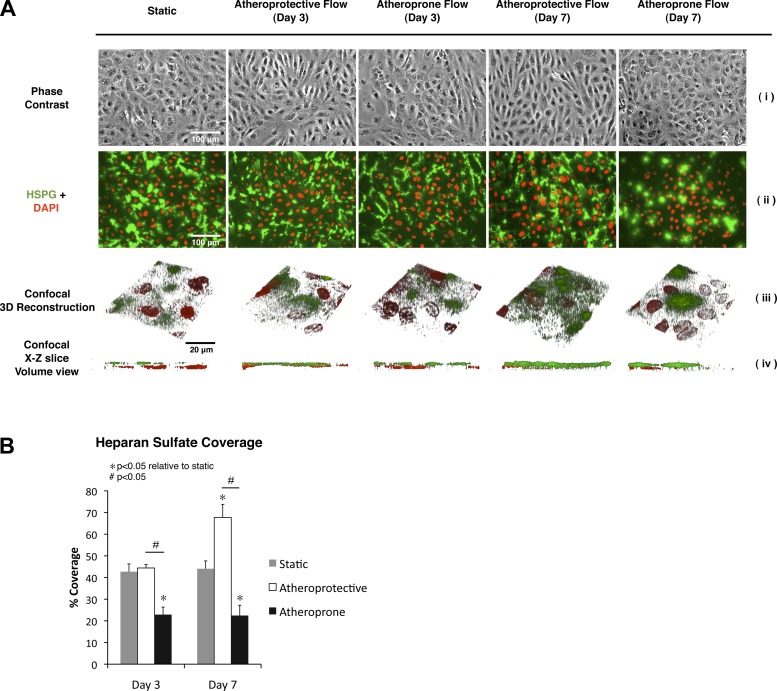
Since heparan sulfate is the predominant GAG constituent of the glycocalyx and multiple studies have demonstrated its functional importance, we further investigated the cell surface distribution of this GAG in the context of the two distinct shear stress waveforms. Confocal immunofluorescence microscopy was performed in cells under the static condition and after 3 or 7 days of exposure to atheroprotective or atheroprone shear stress waveforms. These experiments revealed that heparan sulfate distribution is also shear stress-dependent (Fig. 3A). Under static or atheroprone conditions, heparan sulfate was located mostly in patches, and relatively little heparan sulfate was present on the apical surface. However, a different pattern was observed for the atheroprotective condition: abundant expression of heparan sulfate uniformly distributed on the apical surface of the monolayer. The three-dimensional reconstruction and the x–z volume view of the samples in Fig. 3A were also performed to confirm this finding and validate that only the heparan sulfate on the apical surface was being analyzed. Quantification of the immunostaining images (Fig. 3B) showed that between 42.7% (3 days) and 44.0% (7 days) of the HUVEC monolayer was covered by the EGL under static conditions. After just 3 days of atheroprotective shear stress exposure, there was no significant change in glycocalyx coverage (44.4%). However, we observed a decrease in glycocalyx coverage to 22.9% under the atheroprone waveform. The difference in glycocalyx coverage became more pronounced after 7 days of flow exposure, since coverage in the atheroprotective shear stress condition increased to 66.7%, approximately three times the expression in the atheroprone condition (22.5%). These data document that the hemodynamic shear stress waveform changes not only the quantitative amount, but also the qualitative distribution, of the EGL.
Fig. 3.
Prolonged exposure to atheroprotective flow induces expression of heparan sulfate on the apical surface of endothelial cells. A: representative microscopic images of endothelial cells cultured under static condition or exposed to atheroprotective or atheroprone flow for 3 or 7 days. Phase contrast is shown in i. In ii, heparan sulfate is shown in green, and nucleus (DAPI) is shown in red. HSPG, heparan sulfate proteoglycan. Three-dimensional reconstruction from z stacks obtained by confocal microscopy is shown in iii; heparan sulfate is shown in green, and nucleus (TO-PRO-3) is shown in red. In iv, x–z plane side view of 3-dimensional reconstruction in iii is shown. B: quantitative analysis of percentage of area covered by heparan sulfate based on immunostaining images from 3 independent experiments. Values are means ± SE (n = 3).
Surface expression of syndecan-1 is regulated by the specific shear stress waveforms.
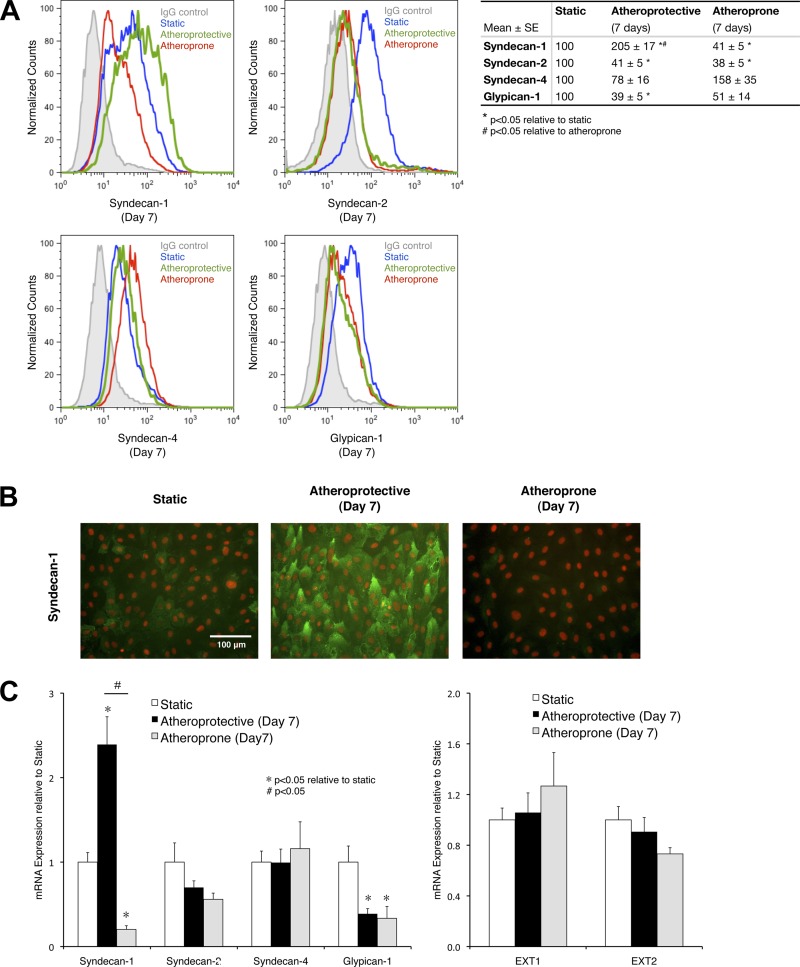
Having established that heparan sulfate expression is regulated by distinct shear stress, we next explored whether its underlying mechanism is through controlling the expression of heparan sulfate proteoglycans or modulating heparan sulfate biosynthesis. First, we measured the change in surface expression of heparan sulfate carrier proteins, namely, syndecan-1, -2, and -4 and glypican-1, after 7 days of exposure to specific shear stress waveforms (Fig. 4A). Expression of syndecan-2 and glypican-1 was decreased by the presence of atheroprotective or atheroprone shear stress. Syndecan-4 seems to be more highly (although not statistically significantly) expressed under the atheroprone than the atheroprotective condition. Syndecan-1 expression, most interestingly, was upregulated after exposure to the atheroprotective flow but downregulated after exposure to the atheroprone flow. These flow cytometry data were confirmed by immunostaining (Fig. 4B) and measurement of mRNA expression (Fig. 4C, left). Immunostaining of syndecan-1 revealed that the atheroprotective flow-mediated expression is suppressed under the atheroprone condition. Experiments measuring the mRNA expression of the heparan sulfate proteoglycan established a high correlation between mRNA expression and protein expression. Syndecan-1 mRNA expression was more than 10 times greater in cells exposed to atheroprotective flow than in cells exposed to atheroprone flow.
Fig. 4.
Prolonged exposure to atheroprotective flow induces expression of syndecan-1 on the apical surface of endothelial cells. A: flow cytometry histograms of syndecan-1, -2, and -4 and glypican-1 surface expression on cells cultures under static condition or exposed to atheroprotective or atheroprone shear stress waveform for 7 days and quantitative analysis of flow cytometry data from 3 independent experiments. Values are means ± SE (n = 3). B: representative immunostaining images of syndecan-1 in cells cultured under static condition or exposed to atheroprotective or atheroprone flow for 7 days. Syndecan-1 is shown in green, and nucleus (DAPI) is shown in red. C: mRNA expression of heparan sulfate protein carriers (left) and key enzymes specifically responsible for heparan sulfate chain biosynthesis (right). Values are means ± SE (n = 3).
We also assessed whether shear stress affects the expression level of exostosin (EXT) 1 and EXT2, two enzymes responsible for heparan sulfate biosynthesis. There was no significant change in EXT1 and EXT2 expression under the two shear stress conditions (Fig. 4C, right). These data suggest that expression of EXT1 and EXT2 is shear stress-independent and may not be critical for the flow-mediated expression of heparan sulfate. Therefore, syndecan-1 transcriptional regulation may play the most important role in shear stress-regulated heparan sulfate expression.
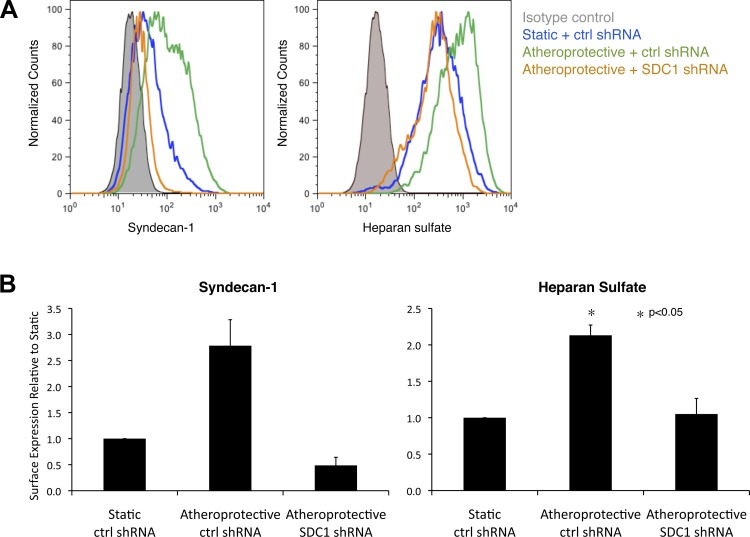
Syndecan-1 silencing blocks the shear-stress-induced heparan sulfate expression.
Having demonstrated a high correlation between the quantitative expression of heparan sulfate and syndecan-1 in a distinct hemodynamic environment, we next tested the hypothesis that the atheroprotective shear stress-induced heparan sulfate expression is mediated through syndecan-1 regulation. HUVEC were transfected with syndecan-1 shRNA to silence syndecan-1 expression and compared with cells transfected with control (nontarget) shRNA under static (no flow) condition or exposed to atheroprotective shear stress for 7 days. The use of syndecan-1 shRNA led to an average 78.6% syndecan-1 silencing under atheroprotective shear stress (Fig. 5). Notably, the atheroprotective shear stress-mediated increase in expression of cell surface heparan sulfate was completely abolished by suppression of syndecan-1 expression. These data demonstrate that syndecan-1 is the major heparan sulfate carrier regulated by atheroprotective flow and that syndecan-1 upregulation is a critical mechanism behind the atheroprotective shear stress-induced heparan sulfate expression.
Fig. 5.
Silencing syndecan-1 expression in endothelial cells blocks atheroprotective flow-induced heparan sulfate expression. A: representative flow cytometry histogram of syndecan-1 and heparan sulfate surface expression in cells treated with control (nontargeting) or syndecan-1 (SDC1) short-hairpin RNA (shRNA) and then maintained in a static condition or exposed to atheroprotective flow for 7 days. B: quantitative analysis of flow cytometry data from 3 independent experiments. Values are means ± SE (n = 3).
DISCUSSION
The abundance of endothelial glycocalyx has been associated with the vasoprotective phenotype of the endothelium (7, 8, 33, 54). Although there are many studies investigating the numerous functions of the EGL, few focus on how EGL expression is regulated. The data presented here demonstrate that expression of some components of the EGL is directly regulated by specific shear stress waveforms. In particular, we found that expression of heparan sulfate, the major GAG of the EGL, is upregulated after prolonged atheroprotective shear stress and downregulated after exposure to the atheroprone waveform. Moreover, we documented that heparan sulfate is present at the apical surface in cultured endothelial cells exposed to the atheroprotective waveform. Besides heparan sulfate, hyaluronic acid expression is also induced by the atheroprotective waveform, establishing that the hemodynamic shear stress waveform found in atherosclerosis-resistant regions is a direct cause of abundant EGL expression.
In the past 10 years, several studies have used in vitro cultured endothelial cells to gain a better understanding of the EGL. Recent reports of Potter et al. (38) and Chappell et al. (6) that the glycocalyx structure observed in vivo is absent in vitro question the conclusion derived from experiments using in vitro models. Using microparticle image velocimetry, Potter et al. measured EGL thickness and found that the glycocalyx is much more abundant in mouse cremaster muscle venules (∼520 nm) than in cultured BAEC (20 nm) or cultured HUVEC (30 nm). Their data are further supported by electron microscopy studies by Chappell et al., who measured the thickness of the glycocalyx in ex vivo human umbilical vein endothelium (878 nm) and cultured HUVEC (29 nm). However, a recent study by Ebong et al. (13) argued that these in vitro estimations may be flawed because of artifact from alcohol dehydration, substrates used to coat the surface, or media supplements of high-concentration dextran (4%). Importantly, our data indicate the EGL expression for HUVEC under static conditions is irregular and discontinuous, suggesting that the EGL distribution is highly heterogeneous, and, depending on the area chosen for measurement, its measured dimensions (e.g., thickness) can be dramatically different.
Several earlier studies indicate that shear stress may play an important role in regulating EGL expression: in endothelial cells subjected to flow, GAG synthesis was increased, hyaluronic acid incorporation into EGL was induced, and hyaluronan synthase 2 expression was upregulated (2, 19, 30). Our study has further shown that exposure to the atheroprotective shear stress waveform for 7 days is necessary for the high and uniform apical cell surface expression of some components of the glycocalyx, such as heparan sulfate and hyaluronic acid, in cultured endothelial cells.
Having demonstrated that the atheroprotective shear stress enhances heparan sulfate expression, an interesting question arises: what is the mechanism behind this process? The abundance of the EGL is controlled by several factors: 1) the abundance of its protein carriers, 2) the expression and activity of enzymes responsible for biosynthesis of GAGs, 3) degradation following endocytosis, and 4) the expression and activity of the MMP responsible for shedding of the glycoproteins. Here we report on some of these possible mechanisms that influence the cell surface expression of heparan sulfate. We measured the mRNA and protein expression of all HUVEC heparan sulfate anchors under static and different flow conditions and found syndecan-1 to be uniquely upregulated by the atheroprotective waveform. Furthermore, silencing of syndecan-1 under flow results in the suppression of shear stress-induced heparan sulfate expression, suggesting that, among all heparan sulfate proteoglycans, syndecan-1 plays a critical role in regulating the cell surface expression of heparan sulfate under atheroprotective shear stress. On the other hand, we did not find the mRNA expression of EXT1 and EXT2, the two key enzymes responsible for heparan sulfate biosynthesis, to be shear stress-dependent. We cannot rule out that the enzymatic activities of these two proteins are regulated through posttranslational modifications, leading to an increase in their specific activities, nor can we rule out a role for changes in endocytic degradation in controlling the surface expression of heparan sulfate.
Shedding of the proteoglycans is catalyzed by the MMP family proteins. MMP1, MMP2, MMP3, and MMP9 have been shown to take part in shedding of the glycocalyx protein carriers (4). Magid et al. (29) demonstrated that the activity of MMP9 promoter and the protein level of tissue inhibitor of MMP1 (TIMP-1) can be differentially regulated by shear stress. However, because of limited understanding of the role of shear stress in posttranslational regulation of MMPs and TIMPs, we cannot conclude whether MMPs play an important role in shear stress-induced expression or suppression of components of the glycocalyx. Investigating the relationship between shear stress and glycocalyx shedding may be a critical step for our understanding of the dynamics of glycocalyx expression.
As we have established the cause-effect relationship between shear stress and the expression of the components of the EGL, the next question to ask is as follows: does this change in EGL expression contribute to the atheroprone or atheroprotective behaviors observed in vivo in different ways. First, as previously discussed, the EGL may bind and, thus, increase the local concentration of some macromolecules. One such molecule is the extracellular SOD (ecSOD), an enzyme responsible for controlling the redox state of the cells. The C class ecSOD has been shown to bind tightly to the EGL. This class of ecSOD possesses a protein domain containing many positively charged amino acids, facilitating its binding to the negatively charged heparan sulfate (21, 44). By providing more binding sites to the ecSOD, an increased EGL expression may help reduce the oxidative stress to the endothelium.
The second impact of a more abundant EGL expression is increased sensitivity to shear stress. Previously, theoretical models established that the fluid shear stress acting on the endothelium by flowing blood is fully dissipated in the glycocalyx layer and the apical membrane essentially is not exposed to shear stress forces. To deliver the force into the cell, the stress is transmitted by the glycocalyx to the cell cytoskeleton through proteoglycans such as syndecans or glypicans (51, 59). In this scenario, the heparan sulfate acts as an amplifier that increases the surface area for the core protein carriers exposed to shear stress. These models predict that if heparan sulfate is removed, the ability of the cell to sense the fluid shear stress will be significantly impaired. Indeed, it is evident from previous experimental data that when heparan sulfate is removed through enzymatic treatment, the endothelial cell can no longer respond to the mechanical stimuli by modulating the proliferation and migration rate, reorganizing the cytoskeleton, and aligning to the direction of the flow (32, 53, 60). Some studies also reported that heparan sulfate and hyaluronic acid are also important for flow-induced NO production, and enzymatic removal of these two GAGs attenuates this effect (14, 35). However, this view has been challenged by a recent report that suppression of flow-mediated NO production by enzymatic removal of heparan sulfate can be rescued by addition of tempol, a radical scavenger. These data suggest that increased NO production under flow is contributed by heparan sulfate hosting the ecSOD and, thereby, increasing the NO half-life, rather than by heparan sulfate acting as a mechanosensor. This study, on the other hand, confirmed that hyaluronic acid is indeed a mechanosensor for NO production (23). These theoretical models and experimental data suggest that the presence of EGL is critical to the activation of shear stress-regulated mechanotransduction pathways.
The third impact of a thicker EGL is prevention of leukocyte adhesion, an early step in atherogenesis. Constantinescu et al. (7, 8) demonstrated that addition of oxidized LDL or enzymatic removal of heparan sulfate in mouse cremaster venules induces leukocyte adhesion. Perfusion of the venules with heparan sulfate or heparin, however, reverses this effect. van den Berg et al. (54) further showed that accumulation of oxidized LDL in the intimal layer beneath the EGL is in fact inversely correlated to the EGL abundance. Mulivor and Lipowsky (33) also found that the chemoattractant N-formyl-Met-Leu-Phe leads to glycocalyx shedding and leukocyte attachment in the rat right internal jugular vein due to MMP activation. Addition of doxycycline, a MMP inhibitor, attenuates this process. Some investigators have attributed these experimental observations to steric hindrance (41). It is argued that the length of the cell adhesion molecules such as P-selectin (∼38 nm) is significantly small compared with the thickness of the glycocalyx (∼400–4,000 nm). Therefore, the heparan sulfate and other GAGs shield the cell adhesion molecules from interacting with the nearby leukocytes (41). In one study supporting this idea, it was observed that when P-selectin length is shortened by decreasing the number of consensus repeats, it is less likely that cultured cells will recruit neutrophils (37). Several other studies can further be used to interpret our data to suggest that syndecan-1 might be the most prominent heparan sulfate anchor and might play the most important role in shear stress-induced heparan sulfate expression. Previous studies have demonstrated that syndecan-1 knockout mice are healthy under normal conditions, but a more dramatic pathological phenotype is shown after challenge with pathological stimuli (52). For example, when myocardial infarction was induced in syndecan-1 knockout mice by permanent ligation of the left coronary artery, enhanced leukocyte adhesion and transendothelial migration were observed (57). Since the plasma B cell is the only type of leukocyte presenting syndecan-1 under normal conditions, syndecan-1 expression on endothelial cells may inhibit leukocyte adhesion (52). Collectively, these studies provide evidence that a thicker EGL layer may be a stronger steric protection against leukocyte attachment.
In conclusion, this study establishes a cause-effect relationship between distinct shear stress waveforms and the expression of components of the endothelial glycocalyx, revealing a specific environmental cue that may be responsible for the previously documented relationship between the glycocalyx and atheroprotection.
GRANTS
This study was funded by National Institutes of Health Grants T32 EB-006438, R01 HL-090856, and R01 HL-089940. We also acknowledge support from the Singapore-MIT Computational and Systems Biology Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K., C.F.D., and G.G.-C. are responsible for conception and design of the research; A.K. performed the experiments; A.K., C.F.D., and G.G.-C. analyzed the data; A.K., C.F.D., and G.G.-C. interpreted the results of the experiments; A.K., C.F.D., and G.G.-C. prepared the figures; A.K., C.F.D., and G.G.-C. drafted the manuscript; A.K., C.F.D., and G.G.-C. edited and revised the manuscript; A.K., C.F.D., and G.G.-C. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Kay Case and Vannessa Davis for isolation and culture of human endothelial cells. We also thank Alan J. Grodzinsky for discussing and commenting on this study. We thank Syuan-Min Guo and Mark Bathe for their help and the use of their confocal microscopy facility.
REFERENCES
- 1. Abrahamsson T, Brandt U, Marklund SL, Sjöqvist PO. Vascular bound recombinant extracellular superoxide dismutase type C protects against the detrimental effects of superoxide radicals on endothelium-dependent arterial relaxation. Circ Res 70: 264–271, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann NY Acad Sci 748: 543–554, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68: 729–777, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4: 197–250, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389–395, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res 104: 1313–1317, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Constantinescu AA, Vink H, Spaan JA. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol 280: H1051–H1057, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 23: 1541–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Dahlbäck B. Blood coagulation. Lancet 355: 1627–1632, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, García-Cardeña G, Gimbrone MA. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101: 14871–14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devaraj S, Yun JM, Adamson G, Galvez J, Jialal I. C-reactive protein impairs the endothelial glycocalyx resulting in endothelial dysfunction. Cardiovasc Res 84: 479–484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science 243: 1483–1485, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol 31: 1908–1915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Fransson LA, Belting M, Cheng F, Jonsson M, Mani K, Sandgren S. Novel aspects of glypican glycobiology. Cell Mol Life Sci 61: 1016–1024, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA 98: 4478–4485, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gimbrone MA, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci 902: 230–240, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med 259: 393–400, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 290: H458–H462, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Janczyk P, Hansen S, Bahramsoltani M, Plendl J. The glycocalyx of human, bovine and murine microvascular endothelial cells cultured in vitro. J Electron Microsc (Tokyo) 59: 291–298, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Karlsson K, Marklund SL. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J 255: 223–228, 1988 [PMC free article] [PubMed] [Google Scholar]
- 22. Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol 5: 293–302, 1985 [DOI] [PubMed] [Google Scholar]
- 23. Kumagai R, Lu X, Kassab GS. Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free Radic Biol Med 47: 600–607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langille BL, Graham JJ, Kim D, Gotlieb AI. Dynamics of shear-induced redistribution of F-actin in endothelial cells in vivo. Arterioscler Thromb 11: 1814–1820, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Levesque MJ, Nerem RM, Sprague EA. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials 11: 702–707, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Lokeshwar VB. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel and vein-derived human endothelial cells. J Biol Chem 275: 27641–27649, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Luscher TF, Richard V, Tschudi M, Yang ZH, Boulanger C. Endothelial control of vascular tone in large and small coronary arteries. J Am Coll Cardiol 15: 519–527, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Luscinskas FW, Gimbrone MA. Endothelial-dependent mechanisms in chronic inflammatory leukocyte recruitment. Annu Rev Med 47: 413–421, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Magid R, Murphy TJ, Galis ZS. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress. Role of c-Myc. J Biol Chem 278: 32994–32999, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Maroski J, Vorderwulbecke BJ, Fiedorowicz K, Da Silva-Azevedo L, Siegel G, Marki A, Pries AR, Zakrzewicz A. Shear stress increases endothelial hyaluronan synthase 2 and hyaluronan synthesis especially in regard to an atheroprotective flow profile. Exp Physiol 96: 977–986, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J Biol Chem 267: 20435–20443, 1992 [PubMed] [Google Scholar]
- 32. Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol 203: 166–176, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Mulivor A, Lipowsky H. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrix metalloprotease activity with doxycycline. Microcirculation 16: 657–666, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Osborn EA, Rabodzey A, Dewey CF, Jr, Hartwig JH. Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am J Physiol Cell Physiol 290: C444–C452, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 355: 228–233, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 116: 49–58, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel KD, Nollert MU, McEver RP. P-selectin must extend a sufficient length from the plasma membrane to mediate rolling of neutrophils. J Cell Biol 131: 1893–1902, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res 102: 770–776, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res 104: 1318–1325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qiao D, Meyer K, Mundhenke C, Drew SA, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem 278: 16045–16053, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflügers Arch 454: 345–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Remuzzi A, Dewey CF, Jr, Davies PF, Gimbrone MA., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology 21: 617–630, 1984 [DOI] [PubMed] [Google Scholar]
- 43. Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986 [DOI] [PubMed] [Google Scholar]
- 44. Sandstrom J, Carlsson L, Marklund SL, Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J Biol Chem 267: 18205–18209, 1992 [PubMed] [Google Scholar]
- 45. Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 276: 36770–36778, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Secomb TW, Hsu R, Pries AR. Effect of the endothelial surface layer on transmission of fluid shear stress to endothelial cells. Biorheology 38: 143–150, 2001 [PubMed] [Google Scholar]
- 47. Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci USA 91: 4678–4682, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol 136: 239–255, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am J Physiol Lung Cell Mol Physiol 293: L328–L335, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med 259: 339–350, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Tarbell JM, Weinbaum S, Kamm RD. Cellular fluid mechanics and mechanotransduction. Ann Biomed Eng 33: 1719–1723, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol 31: 3–16, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci USA 101: 16483–16488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van den Berg BM, Spaan JA, Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflügers Arch 457: 1199–1206, 2009 [DOI] [PubMed] [Google Scholar]
- 55. van den Berg BM, Spaan JAE, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol 290: H915–H920, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Vanderlaan PA. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 24: 12–22, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Vanhoutte D, Schellings MW, Gotte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortes V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation 115: 475–482, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA 100: 7988–7995, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao Y, Rabodzey A, Dewey CF. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 293: H1023–H1030, 2007 [DOI] [PubMed] [Google Scholar]