Abstract
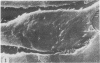
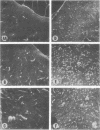
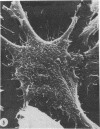
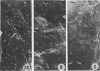
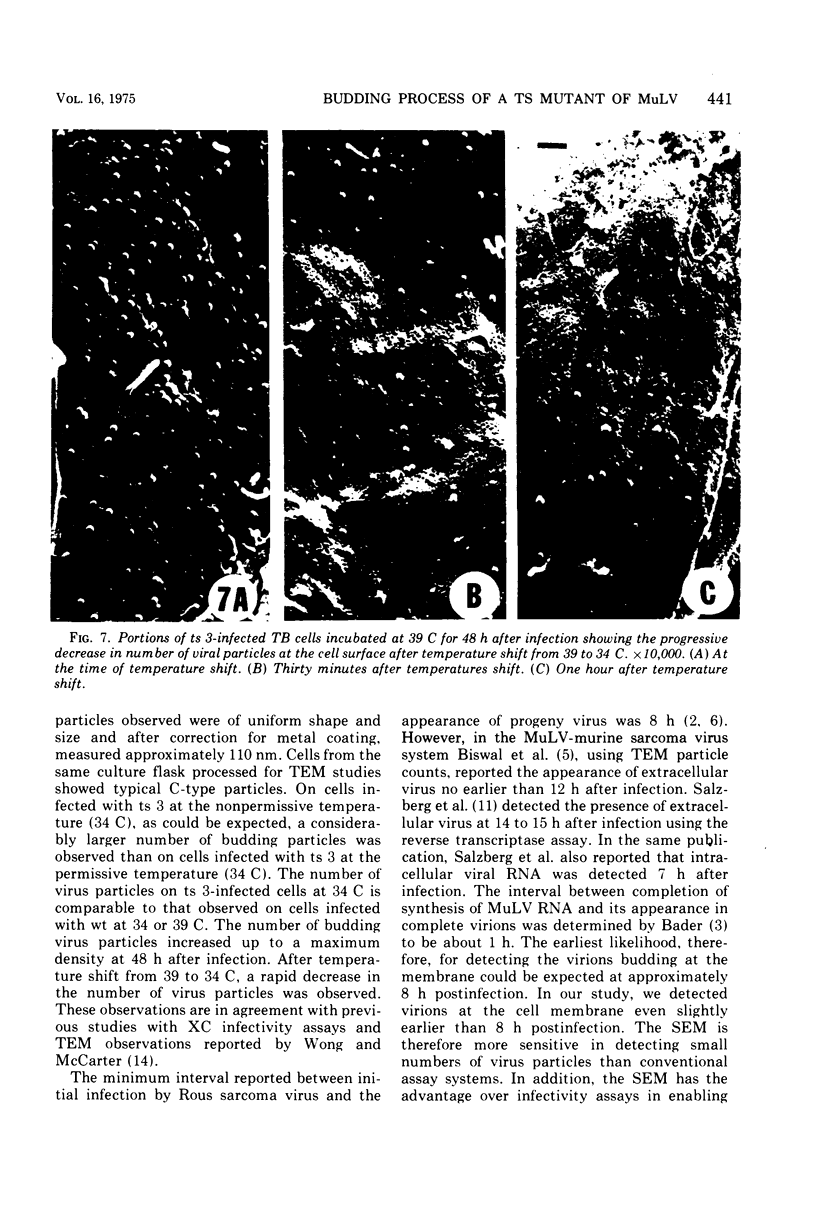
The scanning electron microscope was used to study the budding process of the wild-type Moloney murine leukemia virus and one of its temperature-sensitive mutants, designated ts 3. A considerably larger number of budding particles was observed on TB cells infected with ts 3 at the nonpermissive temperature (39 C) than at the permissive temperature (34 C). No apparent difference was noted between the number of particles on ts 3-infected cells at (34 C) and wild-type-infected cells at 34 or 39 C. Virions were detected at the cell membrane of ts 3-infected cells at 39 C as early as 8 h postinfection. Virion density increased progressively up to 48 h after which no increase was observed. An average of 1,600 virus particles was observed at the cell surface at the peak of virus production. The distribution of these on the cell membrane appeared to be random. The maximum proportion of the cell surface occupied by the viral particles did not exceed 10%. After temperature shift from 39 to 34 C, approximately 90% of the particles had dissociated from the cell membrane within 1 h.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALL J. K., HUH T. Y., MCCARTER J. A. ON THE STATISTICAL DISTRIBUTION OF EPIDERMAL PAPILLOMATA IN MICE. Br J Cancer. 1964 Mar;18:120–123. doi: 10.1038/bjc.1964.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P. Metabolic requirements for infection by Rous sarcoma virus. I. The transient requirement for DNA synthesis. Virology. 1966 Jul;29(3):444–451. doi: 10.1016/0042-6822(66)90220-0. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Biswal N., Grizzard M. B., McCombs R. M., Benyesh-Melnick M. Characterization of intracellular ribonucleic acid specific for the murine sarcoma-leukemia virus complex. J Virol. 1968 Nov;2(11):1346–1352. doi: 10.1128/jvi.2.11.1346-1352.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V. Scanning electron microscopic studies of virus-infected cells. I. Cytopathic effects and maturation of vesicular stomatitis virus in L2 cells. J Virol. 1975 Feb;15(2):355–362. doi: 10.1128/jvi.15.2.355-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic M. K., Carter D. P., Pitelka D. R., Wofsy L. Hapten-sandwich labeling. II. Immunospecific attachment of cell surface markers suitable for scanning electron microscopy. J Cell Biol. 1975 Feb;64(2):311–321. doi: 10.1083/jcb.64.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panem S., Kirsten W. H. Secondary scanning electron microscopy of cells infected with murine oncornaviruses. Virology. 1975 Feb;63(2):447–458. doi: 10.1016/0042-6822(75)90317-7. [DOI] [PubMed] [Google Scholar]
- SVOBODA J., CHYLE P., SIMKOVIC D., HILGERT I. Demonstration of the absence of infectious Rous virus in rat tumour XC, whose structurally intact cells produce Rous sarcoma when transferred to chicks. Folia Biol (Praha) 1963 Apr;9:77–81. [PubMed] [Google Scholar]
- Salzberg S., Robin M. S., Green M. Appearance of virus-specific RNA, virus particles, and cell surface changes in cells rapidly transformed by the murine sarcoma virus. Virology. 1973 May;53(1):186–195. doi: 10.1016/0042-6822(73)90477-7. [DOI] [PubMed] [Google Scholar]
- Springer E. L., Riggs J. L., Hackett A. J. Viral identification by scanning electron microscopy of preparations stained with fluorescein-labeled antibody. J Virol. 1974 Dec;14(6):1623–1626. doi: 10.1128/jvi.14.6.1623-1626.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., McCarter J. A. Studies of two temperature-sensitive mutants of Moloney murine leukemia virus. Virology. 1974 Apr;58(2):396–408. doi: 10.1016/0042-6822(74)90075-0. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Russ L. J., McCarter J. A. Rapid, selective procedure for isolation of spontaneous temperature-sensitive mutants of Moloney leukemia virus. Virology. 1973 Feb;51(2):424–431. doi: 10.1016/0042-6822(73)90441-8. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]