Abstract
The role of SMA and SMB smooth muscle myosin heavy chain (MHC) isoforms in tonic and phasic contractions was studied in phasic (longitudinal ileum and stomach circular antrum) and tonic (stomach circular fundus) smooth muscle tissues of SMB knockout mice. Knocking out the SMB MHC gene eliminated SMB MHC protein expression and resulted in upregulation of the SMA MHC protein without altering the total MHC protein level. Switching from SMB to SMA MHC protein expression decreased the rate of the force transient and increased the sustained tonic force in SMB(−/−) ileum and antrum with high potassium (KPSS) but not with carbachol (CCh) stimulation. The increased tonic contraction under the depolarized condition was not through changes in second messenger signaling pathways (PKC/CPI-17 or Rho/ROCK signaling pathway) or LC20 phosphorylation. Biochemical analyses showed that the expression of contractile regulatory proteins (MLCK, MLCP, PKCδ, and CPI-17) did not change significantly in tissues tested except for PKCα protein expression being significantly decreased in the SMB(−/−) antrum. However, specifically activating PKCα with phorbol dibutyrate (PDBu) was not significantly different in knockout and wild-type tissues, with total force being a fraction of the force generation with KPSS or CCh stimulation in SMB(−/−) ileum and antrum. Taken together, these data show removing the SMB MHC protein expression with a compensatory increase in the SMA MHC protein results in enhanced sustained KPSS-induced tonic contraction with a reduced rate of force generation in these phasic tissues.
Keywords: smooth muscle, tonic contraction, phasic contraction, second messenger pathways, myosin, SMA, SMB
the contractile properties of smooth muscle are broadly classified as tonic and phasic. Upon stimulation, tonic smooth muscle is able to maintain a sustained elevated force whereas phasic smooth muscle exhibits a transient rapid force generation followed by a decrease in force to an intermediate level (55). The differences between tonic and phasic contractile patterns cannot be explained by the time course of intracellular Ca2+ concentration ([Ca2+]i) or LC20 phosphorylation upon stimulation. In both phasic and tonic smooth muscles, the [Ca2+]i and LC20 phosphorylation increase rapidly upon stimulation followed by a decrease to an intermediate steady state (7, 50). Similarly in both tissue types, maximal shortening velocity (Vmax) rapidly increases to the maximal value upon stimulation and then decreases to an intermediate level. In phasic smooth muscle, force generation patterns follow the [Ca2+]i and level of LC20 phosphorylation. However, in tonic smooth muscle, after initial force activation, despite decreases in [Ca2+]i and LC20 phosphorylation to intermediate levels, force can be maintained at a sustained high level. This force maintenance with low [Ca2+]i, LC20 phosphorylation, and Vmax has been referred to as the “latch state” (7, 50). The mechanism underlying the latch state is unknown. Several regulatory mechanisms have been hypothesized including altered kinetics of phosphorylated vs. dephosphorylated myosin cross bridges (7, 21), cytoskeletal remodeling (19, 26, 35, 41, 45), calponin- or caldesmon-dependent actin-to-myosin cross-links (57, 59), second messenger pathway regulation of MLCK/MLCP activity (24, 28, 47, 48, 53, 63), and the kinetic properties of actomyosin ATPase of different myosin isoforms [nonmuscle (NM) and smooth muscle (SM) myosin II isoforms; Refs. 16, 32, 33, 37–39, 42, 51, 65]. The kinetic properties of the actomyosin ATPase from different myosin isoforms in smooth muscle are of particular interest because of the reported capability of myosin II isoforms with slower ATPase activity [NM vs. SM myosin heavy chain (MHC) and SMA vs. SMB MHC] to maintain force, and the preferential expression of SMA vs. SMB SM MHC isoforms in tonic and phasic smooth muscle, respectively (10, 11). In addition, the expression of SM and NM myosin is developmentally regulated and tissue specific (11–13, 46, 61). For NM MHC, in the adult wild-type mouse bladder where the expression of NM myosin decreases to little or none, the tonic contraction continues to be present. In adult rabbit tissues where the expression of NM myosin is undetectable, the tonic contractions are still present (10, 20, 23). Thus the NM myosin isoforms may not be the major regulator for tonic contractions in adult animals.
The SMA and SMB MHC isoforms are the result of alternative splicing from the same myosin gene. The differences between SMA and SMB MHC are located in the S1 head region of SM MHC where the SMB isoform has an additional seven [QGPSFAY(mouse and rat)] amino acids compared with SMA (1, 22, 31). The seven amino-acid insertion in the S1 head of SMB MHC makes ATPase activity approximately twofold greater than SMA, which is consistent with the more rapid force transient (31). The slower ATPase activity of SMA MHC is also consistent with the kinetics of tonic contraction with a five times higher MgADP affinity and a three times lower second-order rate constant for MgATP than SMB (7, 56). Furthermore, SMA and SMB MHC isoforms are preferentially expressed in tonic and phasic tissues, respectively (9), even within a single organ (in the rabbit stomach, ∼90% of phasic antrum SM MHC mRNA is the SMB isoform, and ∼90% of tonic fundus SM MHC mRNA is the SMA isoform) (11). Thus it seems reasonable to hypothesize the existence of physiological relevance of SMA and SMB MHC to tonic and phasic contractile patterns in corresponding smooth muscle tissues.
In an effort to investigate SM MHC function, multiple research articles have been published based on a SMB knockout mouse model (3). SMB MHC knockout bladder and trachea tissues produce significantly less force and have a slower shortening velocity than wild-type controls (3, 60). Studies from the same group (3) but using different tissues (aorta and mesenteric vessels) demonstrate that the SMB knockout tissues produce significantly more force than wild-type tissues with decreased shortening velocity compared with wild-type controls (4). However, since SMB MHC protein expression is negligible in wild-type large arterial tissue such as the aorta, the SMB knockout in this tissue does not result in any significant SMA/B MHC protein expression shift. It was concluded that the decreased contractility in the SMB MHC knockout bladder is due to the increased expression of the thin filament regulatory proteins calponin and caldesmon, and activation of MAPK (2, 4). Studies using h1-calponin and SMB MHC double knockout mice suggest that upregulation of h1-calponin may contribute to the decreased force production in SMB knockout bladder and mesenteric vessels but is confounded by the significantly reduced smooth muscle α-actin levels in SMB(−/−) tissues (2). Results from Patzak et al. (44) using the same animal model report that there is no correlation between SMB MHC protein expression and the rate of force generation in perfused afferent and efferent renal arterioles. Hypolite et al. (27) reported that compared with the wild type the loss of SMB MHC protein in detrusor smooth muscle increases contraction force due to the upregulation of PKC-mediated signaling pathway. In spite of the studies on smooth muscle tissue function using this SMB MHC knockout mouse model, it remains unclear if and how SMA/B MHC protein expression relates to phasic and tonic muscle mechanics. The focus of the above studies using the SMB MHC knockout mouse model was primarily on the role of SMA/B MHC isoforms in maximal force generation and shortening velocity of smooth muscle tissues. They did not investigate the relationship between SMA/B MHC protein isoforms and sustained force maintenance in tonic contraction, which are the major characteristics distinguishing tonic from phasic contractions. Thus the question of the contractile patterns (tonic and phasic) and their relationship with SMA/B MHC protein isoform expression remains to be addressed. The SMB MHC knockout mouse is a good model to examine this question.
The objective of this study was to test the hypothesis that the expression of SMA and SMB SM MHC protein determines the extent of the tonic or phasic contraction in smooth muscle. Our results show that switching from SMB to SMA MHC protein isoform expression significantly decreases the rate of the force transient. Sustained tonic force is significantly increased in phasic tissues (ileum and stomach antrum) but remains unaltered in tonic tissue (stomach fundus) of SMB(−/−) mice. In addition, our data show that the increased tonic contraction in SMB(−/−) ileum and antrum are not through changes in LC20 phosphorylation levels or through changes in the established regulatory pathways (PKC/CPI-17 or Rho/ROCK signaling pathway). These data together suggest that SMA MHC is involved in regulating the level of tonic contraction and is unable to generate the rapid force transient that can occur with SMB MHC expression.
METHODS
Animals.
B6/129 wild-type and SMB(−/−) mice (3) 22–25 wk old were used for all experiments according to the appropriate animal guidelines and welfare regulations as approved by the Marquette University Institutional Animal Care and Use Committee. Heterozygous SMB MHC knockout mice (3) were bred, and homozygous wild-type and knockout offspring were identified for use in the study by long distance PCR using primer sets specific for the wild-type and knockout allele (3).
Tissue handling.
Mice were euthanized by carbon dioxide (CO2). Stomach tissues (fundus, antrum) and ileum were isolated; cleaned of blood, adipose, and chyme; and kept in physiological salt solution [PSS: 4.7 mM KCl, 140 mM NaCl, 1.2 mM Na2HPO4, 2.0 mM 3-(N-morpholine) propanesulfonic acid (MOPS), 0.02 mM EDTA, 1.2 mM MgSO4, 1.6 mM CaCl2, and 5.6 mM glucose pH 7.4 at 37°C] at 4°C until processed (no more than 24 h).
Tissue histology.
Eight-micrometer-thick frozen tissue sections (Leica CM1900) picked up on glass slides were thawed at room temperature and stained with hematoxylin and eosin (9, 34) to examine general morphology. Wall thickness of ileum, antrum, and fundus was measured based on the average of three measurements from four representative cross sections using a ×40 objective for ileum and fundus and ×10 objective for antrum (Olympus IX70) and IPLab software (Scanalytics, Fairfax, VA).
SDS-PAGE and Western blot analysis.
Proteins were denatured by homogenizing tissues in 1 ml sample buffer [0.0625 M Tris pH 6.8, 0.1% (vol/vol) SDS, 15% (wt/vol) glycerol, 0.01% (wt/vol) bromophenol blue, and 15 mM dithiothreitol], boiled for 3 min, and separated by SDS-PAGE (10% for SMA, SMB, total MHC, NM myosin, MLCK, MYPT1, PKCα, and PKCδ and 15% for β actin, CPI-17, LC20, and phosphorylated LC20). The amount of protein loaded for quantitative Western blots analysis was within the linear range of predetermined standard protein loading curves (data not shown). Separating gels were equilibrated at room temperature for 1 h in MHC transfer buffer (25 mM Tris·HCl, 192 mM glycine, 20% methanol, and 0.1% SDS pH 8.3; Ref, 64) before transfer to nitrocellulose membrane (0.2 μm; Amersham Hybond ECL; GE Healthcare, Buckinghamshire, UK) at 200 mA for 4.5 h at 4°C. For transfer of β actin, CPI-17, LC20, and phosphorylated LC20, gels were equilibrated for 30 min in myosin light chain transfer buffer (10 mM N-cyclohexyl-3-aminopropanesulfonic acid pH 11 and 10% methanol) and then transferred to nitrocellulose at 220 mA for 3 h at 4°C. Membranes were blocked with 5% nonfat dry milk in Tris buffered-saline containing Tween 20 (TBST: 20 mM Tris·HCl pH 7.5, 137 mM NaCl, 3 mM KCl, and 0.05% Tween 20) for 1 h at room temperature and then incubated with primary antibodies in 1% nonfat dry milk TBST for 4 h at the following concentrations PKCα at 1:500; PKCδ at 1:200; MYPT1 at 1:500; LC20 at 1:250; MLCK at 1:500; and CPI-17 at 1:200. All primary antibodies above were purchased from Santa Cruz biotechnology (Santa Cruz, CA). Other antibodies were used at the following concentrations: SMA or SMB MHC antibodies (9) at 1:1,000; total MHC (SM + NM MHC) at 1:2,000, NM myosin at 1:500 (BTI biomedical Technologies, Stoughton, MA); phosphorylated LC20 at Ser19 (Millipore, Temecula, CA) at 1:1,000; and β-actin (Sigma, St Louis, MO) at 1:1,000. Membranes were washed in TBST three times for 5 min each and then incubated in the secondary antibodies, goat anti-rabbit, or goat anti-mouse IgG-horseradish peroxidase (1:10,000 dilution in 1% nonfat dry milk TBST; Jackson ImmunoResearch Laboratories, West Grove, PA) or donkey anti-goat IgG-horseradish peroxidase (1:5,000 dilution in 1% nonfat dry milk TBST; R&D Systems, Minneapolis, MN) for 2 h. The membranes were washed again three times for 5 min each in TBST. Chromomeric substrate-3.3′-diaminobenzidine (DAB) was used to detect proteins. The protein bands on the membranes were scanned using an EPSON expression 1600 scanner and quantified by densitometric analysis using the software Image J (http://rsbweb.nih.gov/ij/). The densitometric protein expression values for a particular protein from the SMB(+/+) or SMB(−/−) tissues were calculated as a percentage of the total densitometer readings from the two tissues [SMB(+/+) + SMB(−/−)] and then normalized to β-actin (which was also calculated as a percentage of the total protein from the two tissues). Thus the densitometric values reported are percentages of total protein normalized to β-actin loading for the corresponding SMB(+/+) and SMB(−/−) tissues. This allows differences in protein expression to be detected but does not allow for the actual level of protein expression to be determined (and thus the numbers reported correspond with the relative expression and not absolute band intensity). To minimize variability, tissue samples from a single animal were run in triplicate. Densitometric results of the three runs were averaged, and used as a n = 1 for Western blot analysis.
LC20 phosphorylation measurements.
A rapid freeze method was used to preserve the phosphorylation status of LC20 (8). Tissue strips (∼3 × 7 mm) were incubated in PSS with 1 μM phentolamine and 1 μM propranolol for 1 h and activated with high potassium containing physiological saline solution (KPSS). Tissue strips were immediately frozen in dry ice-acetone bath (−78°C) before KPSS stimulation or at 5 s, 1 min, and 10 min time points after KPSS stimulation. The frozen tissue strips were stored overnight at −78°C and then removed from the freezer and allowed to gradually reach room temperature in acetone. The dry weights of the tissue strips were measured after the acetone was thoroughly evaporated. The wet weights of tissue strips were estimated to be fivefold greater than the dry weight (water accounted for 80% of the cell weight; Ref. 21). Dried tissue strips (10 mg/ml) were homogenized with sample buffer at 4°C to obtain tissue samples and were boiled at 100°C for 5 min and centrifuged at 13,000 rpm for 10 min. The supernatant was collected and loaded onto 15% SDS-PAGE at 15 μl per lane (based on protein loading curves showing linearity of signal above and below this level) for protein separation. The separated proteins were trans blotted to a 0.2-μm nitrocellulose membrane, where Ser19 phosphorylated LC20 and LC20 were detected with specific antibodies (see Western blot). The ratio of LC20 phosphorylation between the SMB(+/+) or SMB (−/−) tissues was normalized to β-actin and reported as a ratio normalized to LC20 content for comparison. This allows for determining changes in LC20 phosphorylation levels but not the actual LC20 phosphorylation level itself.
Force measurement.
The mucosa and connective tissues were dissected away from underlying smooth muscle tissues (fundus and antrum; Ref. 8). Circular fundus and antrum or longitudinal ileum tissues were cut into strips, which were held at each end by tissue clips (Harvard Apparatus, Holliston, MA) and mounted via two hooks to an isometric force transducer (Harvard Apparatus) and a stationary post for tension measurements. Force signals were processed by an analog to digital converter (PowerLab/8SP; ADInstruments, Sydney, Australia) and analyzed using Chart5 software (ADInstruments) on a personal computer. The strip sizes between the two clips were ∼3 mm in width and 7 mm in length. The mounted tissues were bathed in water-jacketed glass chambers (Radnoti Glass Technology, Monrovia, CA) and aerated with 95% O2-5% CO2 at 37°C throughout the experiment, which was maintained by an immersion circulator (VWR, West Chester, PA). The mounted tissues were equilibrated in PSS for 1 h at 37°C before the start of the experiment. By adjusting strip length, a 0.9-g preloaded force was applied. Tissue strips were contracted using KPSS (109.65 mM KCl, 1.2 mM Na2HPO4, 2.0 mM MOPS, 0.02 mM EDTA, 1.2 mM MgSO4, 5.6 mM glucose, 1.6 mM CaCl2, and 35.1 mM NaCl pH 7.4 at 37°C) for three contraction and relaxation cycles. All subsequent solutions included 1μM phentolamine and 1 μM propranolol to eliminate potential effects of adrenergic neurotransmitter activity. Force traces were recorded in response to KPSS or 5 μM CCh for at least 10 min [1 μM phorbol dibutyrate (PDBu) contractions for 30 min] followed by relaxation via three PSS washes to allow a return of force to baseline. For inhibition of second messenger pathways, tissue strips were incubated in PSS with inhibitors (PKCα inhibitors: 1 μM Gö 6976 or GF 109203X; and ROCK inhibitors: 10 μM Y27632 or 0.1 μM H1152; EMD Millipore) for 30 min and then stimulated in KPSS with the inhibitor for a minimum of 10 min followed by three PSS washes for 30 min to completely remove inhibitors. At the end of the experiments, KPSS was applied a final time to test tissue viability. Stress was calculated according to Herlihy et al. (25). Briefly, wet weight and strip length between the tissue clips were measured for each strip. The force produced per cross-sectional area (stress) was calculated as F/A (mN/cm2) = [F (g) * 9.8 (mN/g)]/{wet wt (g)/[length (cm) * 1.05 (g/ml)]}.
Analysis of tonic component of contraction.
The area under the active force curve (total force minus passive force) and the ratios of submaximal forces at the 10-min time point relative to the peak force (force at 10 min/peak force) from the same active force trace were measured to quantitatively compare the tonic contractile component produced by the tissues. To standardize the comparison, the force values for a specific agonist were normalized to the peak force values for KPSS contractions for that tissue in both the wild-type and SMB(−/−) animals. The normalized active force trace from the peak force to 10-min force (force at 10 min after peak force) was selected, and the area under that part of the trace was calculated by summing up all the force values over the time (integral calculation).
Analysis of force transient.
Time to peak tension (from force initiation to peak force), the area under the active force curve (from force initiation to peak force), and the slope of the rapid force transient (steepest slope during initial force generation, dp/dt) were measured to quantitatively compare the initial fast force transient induced by KPSS or CCh. The area under the curve was calculated by summing up all the force values over the time (min) (integral calculation).
Reagents.
Gö 6976, GF 109203X, Y27632, H1152, and PDBu were from EMD Millipore (Billerica, MA), and all other chemicals were from Sigma (St Louis, MO).
Statistics.
For comparison of KPSS-, CCh-, or PDBu-stimulated contractile force, force transient, protein expression, LC20 phosphorylation levels, and smooth muscle wall thickness between wild-type and SMB(−/−) tissues, data were examined for significant differences using a two-tailed Student's t-test with equal variance. Data were considered significant at P < 0.05. For comparison of tonic contractile force with or without inhibitors in SMB(−/−) tissues, one-way ANOVA was used. Significance was also set at a P < 0.05. Statistical analyses and curve fitting were performed using Excel 2007 (Microsoft).
RESULTS
Expression of SMB, SMA, NM, and total MHC.
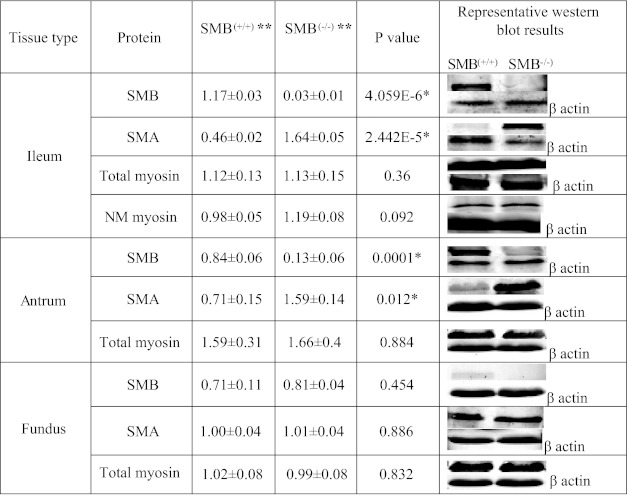
Deletion of exon 5B results in the absence of SMB MHC protein expression in the ileum and antrum while the SMA MHC protein expression is significantly increased in the SMB(−/−) ileum and antrum tissues compared with SMB(+/+) (Fig. 1). Because the SMB MHC is expressed at very low/undetectable levels in the SMB(+/+) fundus, the loss of SMB MHC in the SMB(−/−) fundus did not show a significant decrease in SMB MHC or increase in SMA MHC expression compared with SMB(+/+) fundus tissues. The results of Western blots using a SM + NM MHC antibody indicated that the expression of total MHC (including both the SMA/B SM MHC and NM MHC) was unaltered in all three tissues. The expression of NM MHC was not altered in SMB(−/−) ileum (Fig. 1). The loss of SMB MHC protein resulted in a compensatory switching from the SMB MHC isoform to the SMA MHC isoform such that neither NM MHC nor total MHC expression was altered.
Fig. 1.
Quantitative Western blot results of the expression of smooth muscle SMB and SMA myosin heavy chain (MHC) and total myosin in SMB(+/+) and SMB(−/−) ileum [including nonmuscle (NM) myosin], antrum, and fundus with β-actin as loading control. **Arbitrary densitometric units. Protein expression of SMB MHC is switched to SMA MHC in SMB(−/−) ileum, antrum. Expression of total myosin and NM myosin (ileum) remains the same in these tissues. Values are mean of percent protein [from SMB(+/+) or SMB(−/−) sample] of total protein [from SMB(+/+) and SMB(−/−) samples] ± SE. *P < 0.05, difference determined by t-test to be significant; n = 3 for all groups.
Histochemistry.
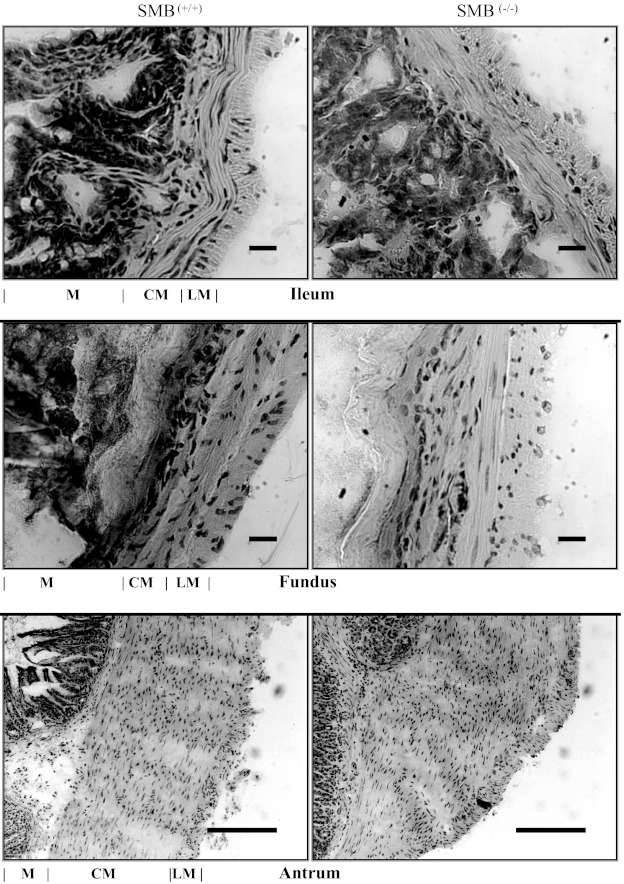
Figure 2 shows representative histologic mirographs of hematoxylin and eosin-stained cross sections of SMB(+/+) and SMB(−/−) ileum, antrum, and fundus. The width (μm) of the smooth muscle layer of SMB(−/−) tissues was similar to their matched WT tissues [ileum: 36.9 ± 3.51 (WT), 42.13 ± 7.44 (knockout); antrum: 549.02 ± 42.68 (WT), 524.73 ± 49.23 (knockout); fundus: 82.38 ± 20.75 (WT), 69.89 ± 11.72 (knockout); n = 3–4]. No gross histological differences were observed in these tissues between SMB(−/−) and SMB(+/+) animals.
Fig. 2.
Transverse hematoxylin and eosin section through SMB(+/+) (left) and SMB(−/−) (right) ileum (top), fundus (middle), and antrum (bottom). There are no obvious gross differences between the SMB(+/+) and SMB(−/−) tissues. M, mucosa, muscularis mucosa, and submucosa; CM, circular smooth muscle layer; LM, longitudinal smooth muscle layer. Scale bar = 20 μm (ileum and fundus) or 200 μm (antrum).
KPSS induced sustained (tonic phase) contractions.
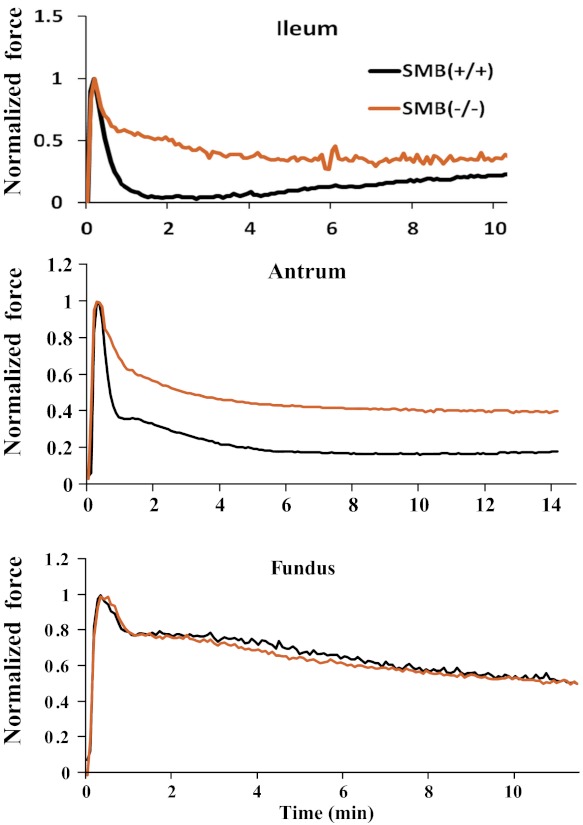
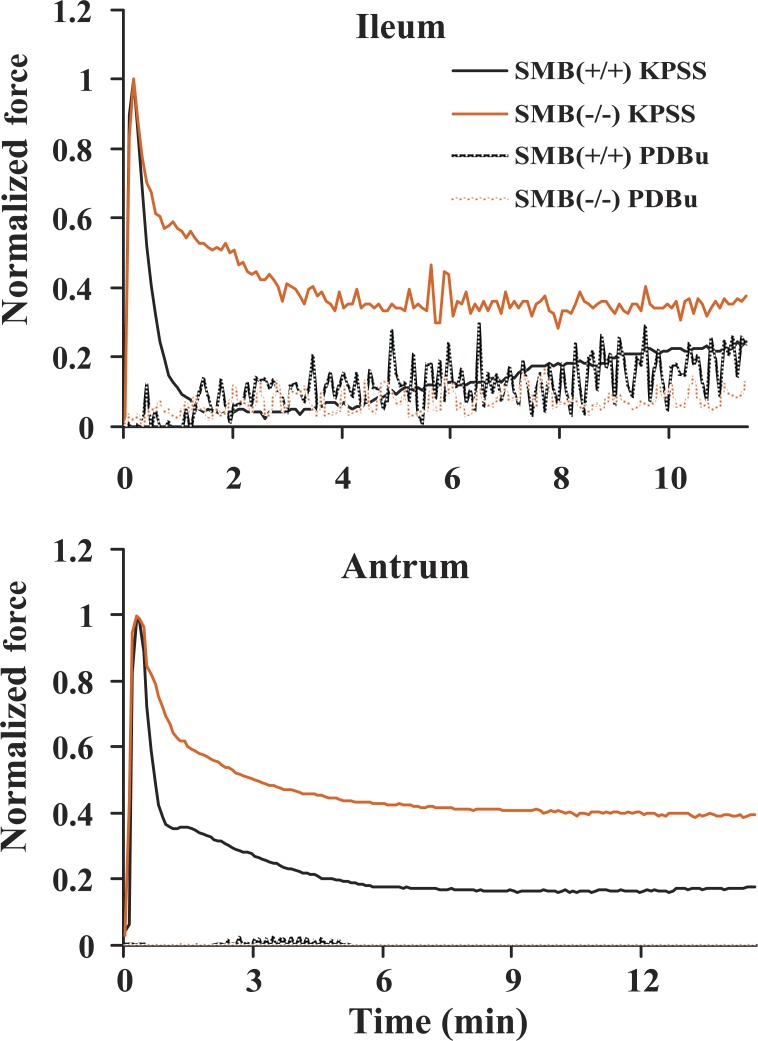
The tonic component of isometric force traces for SMB(+/+) and SMB(−/−) ileum, antrum, and fundus was compared. Figure 3 shows the superimposed SMB(+/+) and SMB(−/−) isometric force traces from KPSS stimulation with the peak force normalized to 1. No significant difference was observed in maximal stress between SMB(+/+) and SMB(−/−) ileum, antrum, or fundus (Table 1). Tonic force in SMB(−/−) ileum and antrum remained significantly greater than that recorded from the SMB(+/+) tissues (Fig. 3 and Table 2). In the fundus, tonic forces from SMB(+/+) and SMB(−/−) animals were not significantly different. Because the time course for the contractile response varied between the SMB(+/+) and SMB(−/−) animals, the data were analyzed in two ways. Area under the active tonic force curve (from peak force to 10 min after peak force) was measured as well as the 10-min tonic force (steady state) to quantify the tonic component of contraction induced by KPSS. The area under the active force curve (peak force to 10 min after peak force) was significantly larger in SMB(−/−) ileum and antrum compared with SMB(+/+). The 10-min tonic force measurement in KPSS force was also greater in SMB(−/−) ileum and antrum compared with SMB(+/+). For tonic fundus, the sustained tonic force was not significantly different between the SMB(+/+) and SMB(−/−) animals (Table 2).
Fig. 3.
Representative high potassium containing physiological saline solution (KPSS) isometric force traces for SMB(+/+) and SMB(−/−) ileum (top), SMB(+/+) and SMB(−/−) antrum (middle), and SMB(+/+) and SMB(−/−) fundus (bottom). To compare the contraction patterns between SMB(+/+) and SMB(−/−) smooth muscle tissues, active peak forces were normalized to 1. In both the ileum and antrum the tonic force component is significantly increased in the SMB(−/−) animals relative to the SMB(+/+).
Table 1.
Maximal stress and 10-min stress induced by KPSS, 5 μM CCh, or 1 μM PDBu
| Tissue | KPSS |
5 μM CCh |
1 μM PDBu |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Maximal stress | 10-min stress | n | Maximal stress | 10-min stress | n | Maximal stress | 30-min stress | |
| Ileum | |||||||||
| SMB(+/+) | 12 | 20.5 ± 2.2 | 7.8 ± 1.2 | 7 | 17.4 ± 2.4 | 5.6 ± 0.9 | 6 | 7.6 ± 1.1 | 2.3 ± 0.5 |
| SMB(−/−) | 12 | 27.0 ± 3.9 | 14.7 ± 2.8 | 7 | 22.9 ± 3.6 | 9.5 ± 1.6 | 7 | 8.2 ± 1.9 | 3.3 ± 0.8 |
| P value | 0.16 | 0.03* | 0.23 | 0.06 | 0.79 | 0.34 | |||
| Antrum | |||||||||
| SMB(+/+) | 10 | 14.6 ± 1.5 | 3.0 ± 0.2 | 6 | 7.5 ± 2.2 | 1.6 ± 0.3 | 5 | 2.1 ± 0.6 | 0.3 ± 0.1 |
| SMB(−/−) | 10 | 17.3 ± 3.0 | 5.4 ± 1.1 | 5 | 13.2 ± 4.3 | 2.9 ± 1.5 | 5 | 1.4 ± 0.3 | 0.2 ± 0.1 |
| P value | 0.42 | 0.04* | 0.24 | 0.37 | 0.31 | 0.51 | |||
| Fundus | |||||||||
| SMB(+/+) | 11 | 22.0 ± 2.0 | 10.3 ± 1.3 | 6 | 20.2 ± 3.3 | 7.9 ± 1.6 | 6 | N/A† | 4.3 ± 0.6 |
| SMB(−/−) | 11 | 21.8 ± 2.6 | 9.3 ± 1.5 | 5 | 20.3 ± 4.0 | 8.8 ± 2.0 | 5 | N/A† | 3.7 ± 1 |
| P value | 0.95 | 0.64 | 0.98 | 0.72 | N/A† | 0.61 | |||
Values are mean ± SE. Stress unit is mN/cm2. KPSS, physiological saline solution; CCh, carbachol; PDBu, phorbol dibutyrate; SM, smooth muscle; N/A, not applicable,
Significant at P < 0.05.
No initial rapidly developed maximal stress for fundus induced by 1 μM PDBu.
Table 2.
Quantification of tonic phase activated by KPSS
| Tissue | n | Area Under the Curve† | 10-min Force† |
|---|---|---|---|
| Ileum | |||
| SMB(+/+) | 13 | 4.03 ± 0.36 | 0.37 ± 0.03 |
| SMB(−/−) | 13 | 5.47 ± 0.35 | 0.48 ± 0.04 |
| P value | 0.017* | 0.044* | |
| Antrum | |||
| SMB(+/+) | 7 | 2.73 ± 0.24 | 0.19 ± 0.02 |
| SMB(−/−) | 7 | 3.81 ± 0.21 | 0.27 ± 0.03 |
| P value | 0.005* | 0.027* | |
| Fundus | |||
| SMB(+/+) | 11 | 5.85 ± 0.54 | 0.47 ± 0.06 |
| SMB(−/−) | 11 | 5.84 ± 0.47 | 0.49 ± 0.06 |
| P value | 0.991 | 0.818 |
Values are mean ± SE.
Significant at P < 0.05.
Area under the curve was measured from peak force to 10 min after peak force. 10-min force is the 10-min tonic force normalized to peak force.
Effect of PKC and ROCK inhibitors.
To determine whether the PKC/CPI-17 or Rho/ROCK signaling pathways (24, 28, 47, 48, 53, 63) are involved in the increased tonic contraction in SMB(−/−) ileum and antrum, intact tissue strips were contracted with KPSS in the presence of the PKC inhibitors Gö 6976 or GF 109203X or the ROCK inhibitors Y27632 or H1152. The area under the active force curve (from peak force to 10 min after the peak force) and 10-min tonic force were measured with or without these inhibitors. Use of the PKC and ROCK inhibitors failed to alter the increased sustained tonic force induced by KPSS in SMB(−/−) ileum and antrum, suggesting that neither the PKC/CPI-17 nor the Rho/ROCK pathway is responsible for the increased tonic contraction in these SMB(−/−) tissues (Table 3).
Table 3.
Effects of PKC inhibitors (Gö6976, GF109203X), and ROCK inhibitors (Y27632 and H1152)
| Tissue/ Measurements | n | Control | Gö6976, 1 μM | GF109203X, 1 μM | n | Control | Y27632, 10 μM | H1152, 0.1 μM |
|---|---|---|---|---|---|---|---|---|
| Ileum SMB(−/−) | ||||||||
| Area under the curve† | 13 | 5.32 ± 0.35 | 5.55 ± 0.33 | 5.0573 ± 0.56 | 8 | 5.32 ± 0.35 | 4.66 ± 0.58 | 4.66 ± 0.58 |
| 10-min force, g | 8 | 0.73 ± 0.11 | 0.59 ± 0.11 | 0.51 ± 0.12 | 5 | 0.83 ± 0.21 | 0.69 ± 0.12 | 0.74 ± 0.15 |
| Antrum SMB(−/−) | ||||||||
| Area under the curve† | 8 | 3.87 ± 0.21 | 4.31 ± 0.46 | 4.59 ± 0.26 | 7 | 3.92 ± 0.21 | 5.51 ± 0.54 | 4.99 ± 0.87 |
| 10-min force, g | 8 | 1.28 ± 0.39 | 1.73 ± 0.48 | 1.78 ± 0.38 | 5 | 2.05 ± 0.72 | 2.24 ± 1.1 | 2.5 ± 1.1 |
Values are mean ± SE.
Difference determined by ANOVA to be significant at P < 0.05.
Area under the curve was measured from peak force to 10 min after peak force.
LC20 phosphorylation.
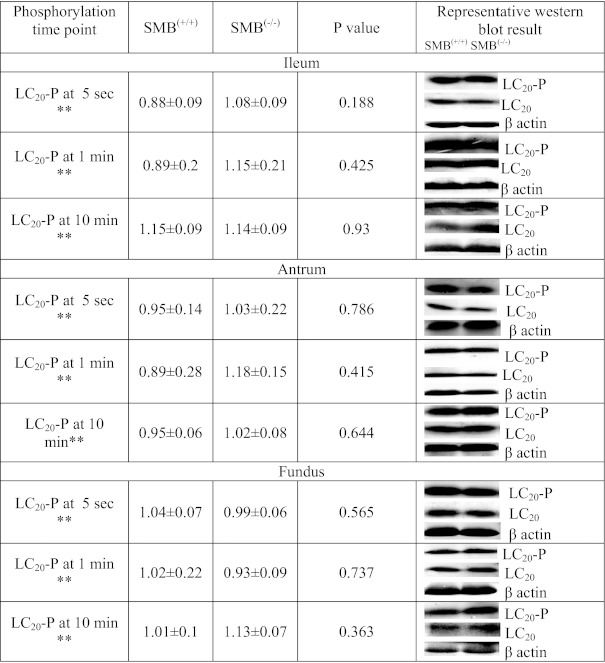
The phosphorylation status of LC20 in ileum and antrum strips were determined at 5 s (rapid phasic force transient), 1 min [the time in the force trace showing the largest change in tonic force between SMB(+/+) and SMB(−/−)], and 10 min (steady state) following KPSS activation. The phosphorylation status of LC20 was not significantly different between SMB(+/+) and SMB(−/−) in ileum and antrum at either the 5 s, 1 min, or 10 min KPSS time points (Fig. 4). LC20 phosphorylation level (5 s) was not different during the force transient to the maximal force between SMB(+/+) and SMB(−/−), or during the increased tonic contraction (Table 2) in SMB(−/−) ileum and antrum; thus the difference in mechanical response is not due to the differences in LC20 phosphorylation levels during force generation or during force maintenance. Similarly, no significant difference of LC20 phosphorylation was observed in SMB(+/+) and SMB(−/−) fundus at these time points (Fig. 4).
Fig. 4.
LC20 phosphorylation status at 5-s, 1-min, and 10-min time points in KPSS stimulation. There are no significant difference in LC20 Phosphorylation status at 5-s, 1-min, and 10-min time points in KPSS stimulation between SMB(+/+) and SMB(−/−) tissues. **Arbitrary densitometric units. Values are mean of percent phosphorylated LC20 [of total SMB(+/+) and SMB(−/−) samples] divided by percent total LC20 [of total SMB(+/+) and SMB(−/−) samples] after each was corrected for loading (β-actin) ± SE. *P < 0.05, difference determined by t-test to be significant; n = 3 for all groups.
Expression of other contractile regulatory proteins.
Western blotting was carried out to quantitatively compare the expression of various contractile regulatory proteins such as MLCK, MLCP, PKCδ, PKCα, and CPI-17 in SMB(−/−) and SMB(+/+) ileum, antrum, and fundus that could also be altered as a result of compensatory changes in the SMB(−/−) mice. Results (Fig. 5) show that for all of the SM tissues studied, the levels of the above-mentioned proteins except PKCα in the antrum were not significantly altered between the SMB(−/−) and SMB(+/+) animals (Fig. 5). PKCα protein expression was significantly reduced in the antrum but not in the ileum or fundus of SMB(−/−) mice.
Fig. 5.
Quantitative Western blot results of the expression of various contraction regulatory proteins in SMB(+/+) and SMB(−/−) ileum, antrum and fundus with β actin as loading control. The expression of protein tested is not altered in SMB(−/−) ileum, antrum and fundus compared with SMB(+/+) with exception for the expression of PKCα is downregulated in SMB(−/−) antrum. **Arbitrary densitometric units. Values are mean of percent protein [from SMB(+/+) or SMB(−/−) sample] of total protein [from SMB(+/+) and SMB(−/−) samples] after each was corrected for loading (β-actin) ± SE. *P < 0.05, difference determined by t-test to be significant; n = 3 for all groups.
Effect of PDBu.
To determine if the decrease in PKCα protein expression affects the enhanced sustained tonic contraction in the antrum of SMB(−/−) animals, tissue strips were activated using 1 μM PDBu and tonic force was studied. If KPSS-induced tonic contraction in SMB(−/−) ileum and antrum is mediated by the PKC signaling pathway, one would predict that directly activating PKC with PDBu would have the same result. Figure 6 shows representative superimposed force traces for SMB(+/+) and SMB(−/−) ileum and antrum contractions induced by 1 μM PDBu compared with the KPSS responses. The stresses induced by PDBu in both the SMB(+/+) and SMB(−/−) tissues were very small (Table 1) and barely above zero for the antrum. In addition, they were not significantly different from each other (Table 4). Thus the downregulation of PKCα in SMB(−/−) antrum appears to not be responsible for the increased tonic contraction with KPSS stimulation in SMB(−/−) animals.
Fig. 6.
Representative comparison of 1 μM phorbol dibutyrate (PDBu) and KPSS isometric force traces of SMB(+/+) and SMB(−/−) ileum and antrum. KPSS active peak force is normalized to 1. KPSS tonic force is significantly greater in the KPSS tonic phase of the SMB(−/−) tissues vs. the SMB(+/+). There is no significant difference between the force responses of the SMB(+/+) and SMB(−/−) tissues in response to PDBu.
Table 4.
Quantification of tonic phase activated by 1 μM PDBu
| Tissue | n | Area Under the Curve† | 30-min Force† |
|---|---|---|---|
| Ileum | |||
| SMB(+/+) | 6 | 15.01 ± 2.83 | 0.28 ± 0.09 |
| SMB(−/−) | 8 | 15.79 ± 1.49 | 0.49 ± 0.07 |
| P value | 0.799 | 0.074 | |
| Antrum | |||
| SMB(+/+) | 4 | 5.63 ± 1.76 | 0.14 ± 0.02 |
| SMB(−/−) | 5 | 7.7 ± 5.99 | 0.16 ± 0.1 |
| P value | 0.774 | 0.817 |
Values are means ± SE.
Significant at P < 0.05. †Area under the curve was measured from peak force to 30 min after peak force. 30-min force is the 30-min tonic force normalized to peak force.
Carbachol-induced steady-state (tonic phase) contractions.
Acetylcholine has been reported as the major excitatory neurotransmitter released from the enteric nervous system to cause gut motility (5, 17, 18, 43). The maximum stress values were not different between CCh-activated SMB(+/+) and SMB(−/−) ileum, antrum, and fundus (Table 1). To determine whether switching from SMB MHC expression to SMA MHC expression could also result in increased tonic force via altered neurotransmitter stimulation, SMB(+/+) and SMB(−/−) antrum, ileum, and fundus strips were activated with 5 μM CCh. Comparison between SMB(+/+) and SMB(−/−) antrum and fundus shows that neither the area under the curve (peak force to 10 min after peak force; Fig. 7) nor the 10-min force (steady state) changed significantly (Table 5) except for the ileum. The area under the curve measurement is significantly increased while the 10-min force measurement is not in the SMB(−/−) ileum (Table 5). Unlike KPSS stimulation, CCh activation includes spontaneous contractions of gastrointestinal smooth muscle as shown in Fig. 7 (14, 52, 62), which may confound the area under the curve measurement. To test this, the spontaneous contractions induced by CCh were compared between SMB(+/+) and SMB(−/−) by comparing spike number and average amplitude from the time of peak force to 10 min after. No significant differences were observed (Table 6).
Fig. 7.
Representative 5 μM carbachol (CCh) isometric force traces for SMB(+/+) and SMB(−/−) ileum (top), SMB(+/+) and SMB(−/−) antrum (middle), and SMB(+/+) and SMB(−/−) fundus (bottom). To compare the contraction patterns between SMB(+/+) and SMB(−/−) smooth muscle tissues, active peak forces were normalized to 1. There are no significant differences between the CCh response for the SMB(+/+) and SMB(−/−) tissues shown except for the area under the curve for ileum (see Table 5).
Table 5.
Quantification of tonic phase activated by 5 μM CCh
| Tissue | Area Under the Curve† | 10-min Force† |
|---|---|---|
| Ileum | ||
| SMB(+/+) | 4.15 ± 0.47 | 0.58 ± 0.06 |
| SMB(−/−) | 5.84 ± 0.18 | 0.57 ± 0.04 |
| P value | 0.007* | 0.91 |
| Antrum | ||
| SMB(+/+) | 3.72 ± 1.12 | 0.21 ± 0.09 |
| SMB(−/−) | 2.29 ± 0.31 | 0.072 ± 0.01 |
| P value | 0.179 | 0.092 |
| Fundus | ||
| SMB(+/+) | 5.49 ± 0.27 | 0.52 ± 0.03 |
| SMB(−/−) | 6.21 ± 0.36 | 0.53 ± 0.04 |
| P value | 0.160 | 0.844 |
Values are means ± SE; n = 6 for all groups.
Significant at P < 0.05.
Area under the curve was measured from peak force to 10 min after peak force. 10-min force is the 10 min tonic force normalized to peak force.
Table 6.
Comparison of spontaneous contraction induced by 5 μM CCh between SMB (+/+) and SMB(−/−) ileum and antrum
| 5 μM CCh/Measurement | SMB(+/+) | n | SMB(−/−) | n | P Value |
|---|---|---|---|---|---|
| Ileum | |||||
| Amplitude, g | 0.29 ± 0.06 | 5 | 0.49 ± 0.12 | 4 | 0.145 |
| Spike number | 22.4 ± 8.74 | 5 | 42.75 ± 9.46 | 4 | 0.16 |
| Antrum | |||||
| Amplitude, g | 0.87 ± 0.21 | 6 | 1.35 ± 0.45 | 6 | 0.361 |
| Spike number | 15.67 ± 3.16 | 6 | 15.57 ± 2.36 | 6 | 0.98 |
Values are means ± SE.
Difference determined by t-test to be significant at P < 0.05.
Measurement of rate of force transient.
To estimate maximal shortening velocity (Vmax), the force transient following activation was measured. Three measurements were made to estimate Vmax (see methods). Results show that the estimates of Vmax for the SMB(−/−) ileum and antrum were significantly slower than the SMB(+/+) tissues, while there was no difference for the fundus tissue (Table 7).
Table 7.
Force transient comparison for SMB (+/+) and SMB(−/−) ileum, antrum, and fundus with KPSS or 5-μM CCh stimulation
| Treatment/Tissue | n | Slope of Linear Ascending Force, g/s | n | Time to Peak Tension, min | n | Area Under the Curve (Force Initiation to Peak Force) |
|---|---|---|---|---|---|---|
| KPSS | ||||||
| Ileum | ||||||
| SMB(+/+) | 11 | 0.8 ± 0.14 | 11 | 0.19 ± 0.03 | 11 | 0.13 ± 0.02 |
| SMB(−/−) | 12 | 0.31 ± 0.04 | 11 | 0.32 ± 0.04 | 11 | 0.21 ± 0.03 |
| P value | 0.002* | 0.012* | 0.009* | |||
| Antrum | ||||||
| SMB(+/+) | 7 | 1.16 ± 0.23 | 12 | 0.09 ± 0.01 | 12 | 0.05 ± 0.01 |
| SMB(−/−) | 7 | 0.54 ± 0.1 | 12 | 0.24 ± 0.04 | 12 | 0.1 ± 0.02 |
| P value | 0.029* | 0.001* | 0.02* | |||
| Fundus | ||||||
| SMB(+/+) | 12 | 1.07 ± 0.23 | 12 | 0.29 ± 0.02 | 12 | 0.2 ± 0.06 |
| SMB(−/−) | 14 | 0.66 ± 0.14 | 13 | 0.41 ± 0.06 | 13 | 0.2 ± 0.02 |
| P value | 0.123 | 0.091 | 0.996 | |||
| CCh | ||||||
| Ileum | ||||||
| SMB(+/+) | 5 | 0.35 ± 0.13 | 13 | 0.44 ± 0.01 | 13 | 0.21 ± 0.04 |
| SMB(−/−) | 8 | 0.09 ± 0.02 | 13 | 0.51 ± 0.01 | 13 | 0.43 ± 0.03 |
| P value | 0.029* | 0.04* | 0.02* | |||
| Antrum | ||||||
| SMB(+/+) | 6 | 0.61 ± 0.07 | 12 | 0.47 ± 0.02 | 12 | 0.32 ± 0,02 |
| SMB(−/−) | 5 | 0.24 ± 0.06 | 12 | 0.74 ± 0.05 | 12 | 0.48 ± 0.01 |
| P value | 0.006* | 0.01* | 0.02* | |||
| Fundus | ||||||
| SMB(+/+) | 6 | 0.47 ± 0.08 | 7 | 0.48 ± 0.07 | 7 | 0.42 ± 0.1 |
| SMB(−/−) | 7 | 0.35 ± 0.09 | 7 | 0.56 ± 0.12 | 7 | 0.45±.05 |
| P value | 0.3 | 0.581 | 0.732 |
Values are means ± SE.
Difference determined by t-test to be significant at P < 0.05.
DISCUSSION
The major findings of this study indicate that the protein expression levels of SMA and SMB MHC isoforms determine the kinetics of tension development and the extent of KPSS-induced tonic and phasic components of smooth muscle contraction. The level of SMA and SMB MHC protein expression is preferential to tonic and phasic tissues, respectively, and are the predominantly expressed MHC in adult smooth muscle tissues. The replacement of SMB by SMA MHC and increased tonic force in phasic smooth muscle tissues of SMB(−/−) mice suggest that SMB MHC isoform is the major contributor of phasic contraction in phasic tissues like antrum and ileum.
The SMB(−/−) mice appear physically and anatomically normal. Histological analysis of SMB(−/−) ileum, antrum, and fundus smooth muscle layers reveals no gross differences relative to wild-type mice (Fig. 2), which is consistent with previous reports (3) suggesting that the loss of SMB MHC isoform does not cause any muscle pathology or affect survival. This could be due to the compensatory upregulation of the SMA MHC isoform, which appears to maintain smooth muscle structure and function in SMB(−/−) mice.
Phasic smooth muscle tissues such as ileum and stomach antrum predominantly express the SMB MHC isoform. The loss of the SMB MHC isoform in these tissues is compensated for by the upregulation of the SMA isoform. Fundus, which primarily expresses the SMA MHC isoform (11), does not show upregulation of SMA MHC protein in the SMB(−/−) mice. Our data (Fig. 1) also show that the total SM myosin (SM + NM) content is not altered, suggesting that loss of SMB MHC is compensated for by the upregulated SMA MHC isoform. Besides the SM MHC isoform, NM MHC may also affect the total MHC content. Further more, NM myosin has been reported to regulate tonic contraction (39, 42). Measurement of NM MHC protein expression in the ileum shows no significant difference between the SMB(−/−) and wild-type control (Fig. 1). Multiple studies (6, 12, 13, 15, 20, 40) have reported that the expression of NM MHC is developmentally regulated and not abundantly expressed in adult animal tissues. Babu et al. (3) also reported that the expression of NM MHC is unchanged in SMB(+/+) and SMB(−/−) mouse bladder. Taken together, these data show that the SM MHC isoform in SMB(−/−) ileum and antrum switches from SMB to SMA MHC, without a significant change in NM MHC expression. The low and unaltered NM MHC expression in SMB(−/−) animals is thus unlikely to participate in any changes of smooth muscle contractile patterns in SMB(−/−) tissues.
If the SMA MHC is responsible for tonic contraction, it is expected that the sustained tonic force would increase in SMB(−/−) phasic tissues where the SMB MHC isoform switches to the SMA MHC isoform. Our data show that with KPSS stimulation switching from SMB to SMA MHC significantly increases the sustained tonic force in SMB(−/−) ileum and antrum tissues (Fig. 3 and Table 2). Tonic fundus tissue does not show a significant change in tonic contraction as there is not a significant change in SMB MHC protein expression. These data suggest that the SMA MHC isoform is preferentially involved in tonic contraction and provide support for the hypothesis that the SMA MHC isoform is one of the major determinants of tonic contraction.
Second messenger pathway regulation of LC20 phosphorylation has been proposed to play an important role in tonic contraction. There have been numerous studies reporting the involvement of PKC/CPI-17 and Rho/ROCK second messenger pathways in regulating tonic contraction via control of LC20 phosphorylation (24, 28, 47–49, 53, 54, 63). We tested this hypothesis by using PKC or ROCK inhibitors to block the PKC/CPI-17 or Rho/ROCK signaling pathways, respectively. The results show both PKC inhibitors (Gö 6976: conventional PKC inhibitor; and GF109203X: conventional and novel PKC inhibitor) and ROCK inhibitors (Y27632 and H1152) fail to eliminate the increased sustained tonic force in these tissues (Table 3), suggesting that the PKC/CPI-17 and Rho/ROCK signaling pathways are not responsible for the increased tonic contraction in the SMB(−/−) animals. Consistent with this, when directly activating PKC with PDBu, tonic force generated by PDBu stimulation is not different between SMB(−/−) and SMB(+/+) tissues (Table 4). In addition, only minimal force is produced in both SMB(+/+) and SMB(−/−) tissues with PDBu activation, which is insignificant compared with the increased KPSS tonic force in SMB(−/−) ileum and antrum. Thus changes in the PKC/CPI-17 and Rho/ROCK signaling pathways are unlikely to be the cause of the increased tonic contraction observed in SMB(−/−) ileum and antrum (Table 2).
There are many other regulatory pathways that may also affect LC20 phosphorylation that we did not measure. To test for these possible effects, we tested for changes in LC20 phosphorylation of SMB(−/−) and SMB(+/+) ileum, antrum, and fundus at the 1-min [the most prominent point of enhanced tonic contraction in SMB(−/−) ileum and antrum] and 10-min (steady-state tonic force) time points. Phosphorylation of LC20 is not different between SMB(+/+) and SMB(−/−) tissues following KPSS activation, indicating that the increased tonic contraction in SMB(−/−) ileum and antrum is not a result of different levels of LC20 phosphorylation (Fig. 4). This suggests that changes in second messenger pathway mechanisms to alter LC20 phosphorylation are not responsible for the tonic contraction enhancement with KPSS activation in SMB(−/−) ileum and antrum.
Tonic force was also measured using agonist activation. CCh, an acetylcholine analog is a M3 receptor agonist, and the major activator of gastrointestinal smooth muscle contraction (17, 36, 43). Our results indicate no increase in the tonic contractile component of the tissues examined with the exception of the area under the curve measurement for the ileum (Fig. 7, Table 5). A reason why CCh-induced contractions did not maintain tonic contractions could be that the receptors become inactivated, that G proteins turn off, or that calcium channels turn off. Thus it may be that the SMA MHC is required for enhanced tonic force, but this only occurs when the proper regulatory pathways are also invoked to bring about this maintained force generation. In addition, it has been reported that CCh activation results in spontaneous contraction of gastrointestinal smooth muscle (52, 62), and our force trace data show spontaneous contractions caused by CCh (Fig. 7). The presence of spontaneous contractions superimposed on a tonic contraction may interfere with tonic force maintenance even though the frequency and average amplitude of the spontaneous contractions between SMB(+/+) and SMB(−/−) ileum and antrum were not found to be significantly different (Table 6). It is possible that unloading stress at the cross bridges during a tonic contraction (via the superimposed spontaneous contractions) allows cross bridges to dissociate and therefore reduces tonic force maintenance.
As reported, the loss of SMB MHC isoform in the SMB(−/−) tissues also results in downregulation of PKCα in the antrum and upregulation of SMA MHC in the antrum and ileum. While similar changes in expression could also happen for other proteins, we did not find this to be the case for any of the other proteins we measured (MLCK, MLCP, PKCδ, and CPI-17; Fig. 5). PKCα downregulation in SMB(−/−) antrum is inconsistent with the study from Hypolite et al. (27) reporting PKCα protein expression is upregulated in detrusor smooth muscle. The difference may be tissue specific regulation of PKCα in SMB(−/−) mice. The minimal total force generated and lack of change in this force induced by PDBu between SMB(+/+) and SMB(−/−) suggest the change in KPSS tonic force is not due to the downregulation of PKCα in antrum (Tables 1 and 4).
It has been reported that maximum stress is decreased in the SMB(−/−) bladder but increased in mesenteric vessels under the depolarized condition (3, 4). In addition, Hypolite et al. (27) reported that the maximum stress of SMB(−/−) bladder detrusor smooth muscle is significantly increased over SMB(+/+) tissue. Studies from Karagiannis et al. (30) using permeabilized bladder tissues show that the maximum stress is not altered in SMB(−/−) bladder, and Patzak et al. (44) also failed to find any difference of maximal stress induced by KCl between SMB(+/+) and SMB(−/−) afferent arterioles. Tuck et al. (60) also reported no significant difference in SMB(−/−) airway peak resistance in response to methacholine. We also tried to determine if there is any correlation between SMA/SMB MHC isoform expression and maximal force generation in the phasic ileum, antrum and tonic fundus. Our data show that replacing the SMB MHC with the SMA MHC isoform in the ileum, antrum, and fundus does not significantly alter the maximum stress these tissues generate when activated either by KPSS or CCh (Table 1), suggesting that the different kinetic properties of SMA/B MHC isoforms do not affect smooth muscle maximum force production. It remains unclear why the results of these studies are not consistent regarding the relationship of SMA/SMB MHC isoform expression and maximum stress generation.
To estimate whether SMA/SMB MHC isoforms affect Vmax in phasic and tonic gastrointestinal tissues, we used three measurements to compare the isometric force transient (as an estimate of Vmax) with either KPSS or CCh stimulation in SMB(+/+) and SMB(+/+) ileum antrum, and fundus. Our results show that the force transient is significantly slower in SMB(−/−) ileum and antrum (Table 7). This is consistent with the results from other studies reporting a decreased Vmax using this SMB(−/−) mouse model (2–4, 27, 29, 30, 60), and results that the SMB MHC isoform has a twofold greater ATPase activity than SMA and moves actin filament 2.5-fold faster than SMA in an in vitro motility assay (31, 58). Because the shortening velocity of smooth muscle is also correlated with LC20 phosphorylation, we also measured this. LC20 phosphorylation induced by KPSS at 5 s following tissue activation was not different between the SMB(+/+) and SMB(−/−) tissues suggesting the slower force transient in SMB(−/−) ileum and antrum is not caused by lower LC20 phosphorylation (Table 5). The decreased force transient appears to be a result of the slower kinetic properties of SMA MHC actomyosin ATPase and not a difference in LC20 phosphorylation. The isometric force transient of fundus did not change, which is consistent with no detectable SMB to SMA MHC protein expression switching in this tissue. The slow vs. fast actomyosin ATPase activity of SMA/SMB MHC isoform may be important for SMA MHC to maintain force in tonic tissues. The slow kinetics of actomyosin ATPase could result in high ADP affinity and a low rate of ADP release in the presence of actin, which enables SMA MHC to remain attached to actin longer. The actin-myosin-ADP state of SMA MHC has been reported to require mechanical strain to release ADP (37). With tonic force maintenance, the long attachment time of SMA to actin may explain the hypothesized latch cross bridge. The spontaneous contractions present with CCh activation may provide variable strain to dissociate the actin-myosin cross bridge and thus explain the lack of increased tonic force with this activation.
Interestingly, the seemingly unaltered spontaneous contractions mentioned above in SMB(−/−) ileum and antrum suggest that SMB MHC may not be required for the phasic visceral smooth muscle to produce the fast and transient rhythmic contractions which are critical for peristalsis and food digestion. The upregulated SMA MHC in the absence of SMB MHC is capable of generating these spontaneous contractions. Thus while the tonic force resulting from a CCh contraction is not enhanced in the SMB(−/−) mouse tissues, the spontaneous contractions continue to occur in the absence of the SMB MHC.
Our study based on SMB(−/−) mice shows SMB MHC expression is turned off and SMA MHC isoform expression is increased in SMB(−/−) phasic ileum and antrum tissues while there is no significant shift in the already tonic fundus tissue that expresses almost exclusively the SMA MHC isoform. The SMA MHC protein isoform cannot generate phasic force as quickly as the SMB MHC isoform resulting in a reduced rate of force generation in the ileum and antrum but is capable of generating rapid contractions like spontaneous contractions in visceral phasic smooth muscles. The increased SMA MHC expression enhances the sustained tonic contraction observed in K+ depolarized phasic tissues, suggesting SMA MHC isoform is involved in regulating tonic contraction. Further work is required to resolve additional factors that are at play when tissue is activated with CCh and whether other thin filament regulatory proteins and cytoskeletal remodeling mechanisms may also contribute to increased tonic contraction observed in SMB(−/−) phasic tissues.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-62237.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.J.B. and M.P. generated SMB(+/−) mice; Q.H. and T.J.E. conception and design of research; Q.H. performed experiments; Q.H. and T.J.E. analyzed data; Q.H. and T.J.E. interpreted results of experiments; Q.H. prepared figures; Q.H. and T.J.E. drafted manuscript; Q.H., G.J.B., M.P., and T.J.E. edited and revised manuscript; Q.H., G.J.B., M.P., and T.J.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Biological Sciences Department and the Graduate School, Marquette University for support.
REFERENCES
- 1.Babij P, Kelly C, Periasamy M. Characterization of a mammalian smooth muscle myosin heavy-chain gene: complete nucleotide and protein coding sequence and analysis of the 5′ end of the gene. Proc Natl Acad Sci USA 88: 10676–10680, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu GJ, Celia G, Rhee AY, Yamamura H, Takahashi K, Brozovich FV, Osol G, Periasamy M. Effects of h1-calponin ablation on the contractile properties of bladder versus vascular smooth muscle in mice lacking SM-B myosin. J Physiol 577: 1033–1042, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu GJ, Loukianov E, Loukianova T, Pyne GJ, Huke S, Osol G, Low RB, Paul RJ, Periasamy M. Loss of SM-B myosin affects muscle shortening velocity and maximal force development. Nat Cell Biol 3: 1025–1029, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Babu GJ, Pyne GJ, Zhou Y, Okwuchukuasanya C, Brayden JE, Osol G, Paul RJ, Low RB, Periasamy M. Isoform switching from SM-B to SM-A myosin results in decreased contractility and altered expression of thin filament regulatory proteins. Am J Physiol Cell Physiol 287: C723–C729, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil 16 Suppl 1: 34–38, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Borrione AC, Zanellato AM, Scannapieco G, Pauletto P, Sartore S. Myosin heavy-chain isoforms in adult and developing rabbit vascular smooth muscle. Eur J Biochem 183: 413–417, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science 211: 495–497, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Driska SP, Aksoy MO, Murphy RA. Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am J Physiol Cell Physiol 240: C222–C233, 1981 [DOI] [PubMed] [Google Scholar]
- 9.Eddinger TJ. Unique contractile and structural protein expression in dog ileal inner circular smooth muscle. J Smooth Muscle Res 45: 217–230, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Eddinger TJ, Meer DP. Myosin II isoforms in smooth muscle: heterogeneity and function. Am J Physiol Cell Physiol 293: C493–C508, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Eddinger TJ, Meer DP. Single rabbit stomach smooth muscle cell myosin heavy chain SMB expression and shortening velocity. Am J Physiol Cell Physiol 280: C309–C316, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Eddinger TJ, Murphy RA. Developmental changes in actin and myosin heavy chain isoform expression in smooth muscle. Arch Biochem Biophys 284: 232–237, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Eddinger TJ, Wolf JA. Expression of four myosin heavy chain isoforms with development in mouse uterus. Cell Motil Cytoskeleton 25: 358–368, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Ehlert FJ, Sawyer GW, Esqueda EE. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci 64: 387–394, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Frid MG, Printesva OY, Chiavegato A, Faggin E, Scatena M, Koteliansky VE, Pauletto P, Glukhova MA, Sartore S. Myosin heavy-chain isoform composition and distribution in developing and adult human aortic smooth muscle. J Vasc Res 30: 279–292, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Fuglsang A, Khromov A, Torok K, Somlyo AV, Somlyo AP. Flash photolysis studies of relaxation and cross-bridge detachment: higher sensitivity of tonic than phasic smooth muscle to MgADP. J Muscle Res Cell Motil 14: 666–677, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Furness JB, Young HM, Pompolo S, Bornstein JC, Kunze WA, McConalogue K. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology 108: 554–563, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol 91: 963–972, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Giuriato L, Scatena M, Chiavegato A, Tonello M, Scannapieco G, Pauletto P, Sartore S. Non-muscle myosin isoforms and cell heterogeneity in developing rabbit vascular smooth muscle. J Cell Sci 101: 233–246, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Hai CM, Murphy RA. Cross-bridge dephosphorylation and relaxation of vascular smooth muscle. Am J Physiol Cell Physiol 256: C282–C287, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Hamada Y, Yanagisawa M, Katsuragawa Y, Coleman JR, Nagata S, Matsuda G, Masaki T. Distinct vascular and intestinal smooth muscle myosin heavy chain mRNAs are encoded by a single-copy gene in the chicken. Biochem Biophys Res Commun 170: 53–58, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Han S, Speich JE, Eddinger TJ, Berg KM, Miner AS, Call C, Ratz PH. Evidence for absence of latch-bridge formation in muscular saphenous arteries. Am J Physiol Heart Circ Physiol 291: H138–H146, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Herlihy JT, Murphy RA. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res 33: 275–283, 1973 [DOI] [PubMed] [Google Scholar]
- 26.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science 315: 111–115, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Hypolite JA, Chang S, LaBelle E, Babu GJ, Periasamy M, Wein AJ, Chacko S. Deletion of SM-B, the high ATPase isoform of myosin, upregulates the PKC-mediated signal transduction pathway in murine urinary bladder smooth muscle. Am J Physiol Renal Physiol 296: F658–F665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang MJ, Morgan KG. Intracellular calcium levels in phorbol ester-induced contractions of vascular muscle. Am J Physiol Heart Circ Physiol 253: H1365–H1371, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Karagiannis P, Babu GJ, Periasamy M, Brozovich FV. Myosin heavy chain isoform expression regulates shortening velocity in smooth muscle: studies using an SMB KO mouse line. J Muscle Res Cell Motil 25: 149–158, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Karagiannis P, Babu GJ, Periasamy M, Brozovich FV. The smooth muscle myosin seven amino acid heavy chain insert's kinetic role in the crossbridge cycle for mouse bladder. J Physiol 547: 463–473, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem 268: 12848–12854, 1993 [PubMed] [Google Scholar]
- 32.Khromov A, Somlyo AV, Somlyo AP. MgADP promotes a catch-like state developed through force-calcium hysteresis in tonic smooth muscle. Biophys J 75: 1926–1934, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khromov A, Somlyo AV, Trentham DR, Zimmermann B, Somlyo AP. The role of MgADP in force maintenance by dephosphorylated cross-bridges in smooth muscle: a flash photolysis study. Biophys J 69: 2611–2622, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiernan JA. Histological and Histochemical Methods: Theory and Practice. Bloxham, UK: Scion, 2008, p. zvi 606 [Google Scholar]
- 35.Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol 295: C768–C778, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitazawa T, Hashiba K, Cao J, Unno T, Komori S, Yamada M, Wess J, Taneike T. Functional roles of muscarinic M2 and M3 receptors in mouse stomach motility: studies with muscarinic receptor knockout mice. Eur J Pharmacol 554: 212–222, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA 104: 9994–9999, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovacs M, Wang F, Hu A, Zhang Y, Sellers JR. Functional divergence of human cytoplasmic myosin II: kinetic characterization of the non-muscle IIA isoform. J Biol Chem 278: 38132–38140, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Lofgren M, Ekblad E, Morano I, Arner A. Nonmuscle Myosin motor of smooth muscle. J Gen Physiol 121: 301–310, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loukianov E, Loukianova T, Periasamy M. Myosin heavy chain isoforms in smooth muscle. Comp Biochem Physiol B Biochem Mol Biol 117: 13–18, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol 519: 829–840, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, Haase H, Bader M. Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol 2: 371–375, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Patzak A, Petzhold D, Wronski T, Martinka P, Babu GJ, Periasamy M, Haase H, Morano I. Constriction velocities of renal afferent and efferent arterioles of mice are not related to SMB expression. Kidney Int 68: 2726–2734, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Pavalko FM, Adam LP, Wu MF, Walker TL, Gunst SJ. Phosphorylation of dense-plaque proteins talin and paxillin during tracheal smooth muscle contraction. Am J Physiol Cell Physiol 268: C563–C571, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Phillips CL, Yamakawa K, Adelstein RS. Cloning of the cDNA encoding human nonmuscle myosin heavy chain-B and analysis of human tissues with isoform-specific antibodies. J Muscle Res Cell Motil 16: 379–389, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Poole DP, Furness JB. PKC delta-isoform translocation and enhancement of tonic contractions of gastrointestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 292: G887–G898, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Rattan S, De Godoy MA, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 288: C769–C783, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Rembold CM, Murphy RA. Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res 58: 803–815, 1986 [DOI] [PubMed] [Google Scholar]
- 51.Rosenfeld SS, Xing J, Chen LQ, Sweeney HL. Myosin IIb is unconventionally conventional. J Biol Chem 278: 27449–27455, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Sinn DH, Min BH, Ko EJ, Lee JY, Kim JJ, Rhee JC, Kim S, Ward SM, Rhee PL. Regional differences of the effects of acetylcholine in the human gastric circular muscle. Am J Physiol Gastrointest Liver Physiol 299: G1198–G1203, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Sohn UD, Cao W, Tang DC, Stull JT, Haeberle JR, Wang CL, Harnett KM, Behar J, Biancani P. Myosin light chain kinase- and PKC-dependent contraction of LES and esophageal smooth muscle. Am J Physiol Gastrointest Liver Physiol 281: G467–G478, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Somlyo AP, Somlyo AV. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev 20: 197–272, 1968 [PubMed] [Google Scholar]
- 56.Somlyo AV, Matthew JD, Wu X, Khromov AS, Somlyo AP. Regulation of the cross-bridge cycle: the effects of MgADP, LC17 isoforms and telokin. Acta Physiol Scand 164: 381–388, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Sutherland C, Walsh MP. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem 264: 578–583, 1989 [PubMed] [Google Scholar]
- 58.Sweeney HL, Rosenfeld SS, Brown F, Faust L, Smith J, Xing J, Stein LA, Sellers JR. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem 273: 6262–6270, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Szymanski PT, Tao T. Localization of protein regions involved in the interaction between calponin and myosin. J Biol Chem 272: 11142–11146, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Tuck SA, Maghni K, Poirier A, Babu GJ, Periasamy M, Bates JH, Leguillette R, Lauzon AM. Time course of airway mechanics of the (+)insert myosin isoform knockout mouse. Am J Respir Cell Mol Biol 30: 326–332, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci USA 94: 12407–12412, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unno T, Matsuyama H, Izumi Y, Yamada M, Wess J, Komori S. Roles of M2 and M3 muscarinic receptors in cholinergic nerve-induced contractions in mouse ileum studied with receptor knockout mice. Br J Pharmacol 149: 1022–1030, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urban NH, Berg KM, Ratz PH. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitization of arterial muscle. Am J Physiol Cell Physiol 285: C1377–C1385, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Wahl M, Eddinger TJ, Hai CM. Sinusoidal length oscillation- and receptor-mediated mRNA expression of myosin isoforms and α-SM actin in airway smooth muscle. Am J Physiol Cell Physiol 287: C1697–C1708, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J Biol Chem 278: 27439–27448, 2003 [DOI] [PubMed] [Google Scholar]