Abstract
Studies indicate that the mortality effects of temperature may vary by population and region, although little is known about the vulnerability of subgroups to these risks in Korea. This study examined the relationship between temperature and cause-specific mortality for Seoul, Korea, for the period 2000–7, including whether some subgroups are particularly vulnerable with respect to sex, age, education and place of death. The authors applied time-series models allowing nonlinear relationships for heat- and cold-related mortality, and generated exposure–response curves. Both high and low ambient temperatures were associated with increased risk for daily mortality. Mortality risk was 10.2% (95% confidence interval 7.43, 13.0%) higher at the 90th percentile of daily mean temperatures (25 °C) compared to the 50th percentile (15 °C). Mortality risk was 12.2% (3.69, 21.3%) comparing the 10th (−1 °C) and 50th percentiles of temperature. Cardiovascular deaths showed a higher risk to cold, whereas respiratory deaths showed a higher risk to heat effect, although the differences were not statistically significant. Susceptible populations were identified such as females, the elderly, those with no education, and deaths occurring outside of a hospital for heat- and cold-related total mortality. Our findings provide supportive evidence of a temperature–mortality relationship in Korea and indicate that some subpopulations are particularly vulnerable.
Keywords: climate change, cold, extreme temperature, heat, mortality, vulnerable populations, weather
1. Introduction
Numerous epidemiologic studies have demonstrated that low or high ambient temperature is associated with increased risk of daily mortality in many parts of the world [1–6]. These health risks could be exacerbated by climate change including more frequent and extreme episodes of particularly hot or cold temperatures [7]. A recent National Institutes of Health (NIH) report noted that the identification of populations vulnerable to climate change health impacts, such as heat-related mortality, is a crucial research need [8].
The effect of temperature on mortality may differ by population characteristics including socioeconomic factors. There is a need to characterize this effect modification and identify susceptible populations in temperature-related mortality studies. Better knowledge of these modifiers would be useful for public policy making to avert temperature-related health burdens and in risk assessments of how weather conditions impact health. Further, research on which populations are most vulnerable to temperature-related health consequences is vital to research on climate change and health, especially as the distribution of these characteristics may be changing, such as an increase in older populations. Several studies have reported variation in the temperature–mortality association by race, sex, age, housing characteristics, air conditioning, and several socioeconomic factors such as income, education, and unemployment in the United States, Italy, France, England and Wales, Mexico, Brazil and Chile [3, 9–14]. However, the temperature–mortality relationship and vulnerability to temperature effects may vary by population and region, and the influence of some characteristics still remain unclear. For example, some studies identified women to be at higher risk, while others showed no difference by gender or observed men to be at higher risk [10, 15–17]. Bell et al [13] examined heat-related mortality for three Latin American cities and reported that vulnerability by sex and education differed by city. Thus, more studies in other locations are needed to evaluate susceptibility to temperature’s effect on mortality.
In Korea, studies have been conducted to examine how mortality is affected by winter temperatures [18], heat [19], and previous-winter’s mortality [20]. However, relatively few epidemiologic studies have focused on identifying vulnerable subgroups. This study examines the relationship between temperature and total, cardiovascular and respiratory mortality for Seoul, Korea, from 2000 to 2007, focusing on whether some subgroups are particularly vulnerable to temperature-related mortality with respect to sex, age, education and place of death.
2. Methods
2.1. Data
Daily counts of deaths in Seoul between 1 January 2000 and 31 December 2007 were obtained from the National Statistical Office, Republic of Korea. Mortality data included date of death, cause of death, sex, age, educational level and place of death (in or out of hospital). We considered total mortality as all causes of death except external causes (International Classification of Diseases, ICD-10, A00–R99). Cardiovascular causes (ICD-10, I00–I99) and respiratory causes (ICD-10, J00–J99) were analyzed separately. Age was categorized as 0–14, 15–64, 65–74 and ≥75 yr. Educational level was assessed for those ≥20 yr as ≤6, 7–12 and >12 yr of educational attainment.
We investigated whether the relationship between temperature and mortality is confounded by air pollution levels. Hourly particulate matter with aerodynamic diameter ≤10 μm (PM10) and ozone (O3) levels were obtained for 27 monitoring stations in Seoul and operated by the Department of Environment, Republic of Korea during the whole study period. We used 24 h averages as the exposure index for PM10, by first averaging hourly values across all monitors for each day and then calculating 24 h values. For O3, we calculated the maximum daily 8 h moving average as the exposure index. The National Meteorological Administration, Republic of Korea, provided hourly measurements of ambient temperature and relative humidity for Seoul during the study period. We converted weather data into 24 h values.
2.2. Statistical analysis
To estimate the relationship between daily mortality and temperature, we used an over-dispersed Poisson generalized linear model with natural cubic splines for time and meteorology:
| (1) |
where E(Yt) = expected number of deaths on day t; β0 = model intercept; DOWt = categorical variable for day of the week; ns(timet) = natural cubic spline of a variable representing time to adjust for long-term trends, with 7 degrees of freedom (df) per year; ns(Tt–lag) = natural cubic spline of temperature for a specific lag from day t, with 3 df and equally spaced knots; ns(humidity) = natural cubic spline of humidity on day t, with 4 df.
We estimated the temperature–mortality response curve and also estimated heat- and cold-related temperature effects for specific portions of the curve. For the heat effect, we present results comparing the mortality risk at mean daily temperatures of 25–15 °C (90th–50th percentile of temperatures in Seoul) and 29–25 °C (99th–90th percentile). For the cold effect, we present results comparing mortality risk at −1 to 15 °C (10th–50th percentile) and −4 to −1 °C (1st–10th percentile).
Previous work has shown that low temperature–mortality effects persist for longer lag times whereas high temperature effects act on shorter lag times [14]. We considered lag structures of temperature on the same day and up to 28 days earlier. A similar approach was applied in a previous study [14].
As a sensitivity analysis, we included variables for PM10 and ozone in the model to assess potential confounding. Lag structures for each pollutant were selected as the single-day lag with the highest point estimate.
We applied stratified models to investigate the susceptibility by cause of death (total, cardiovascular and respiratory mortality), sex, age, educational level, and place of death. All analyses were conducted using R 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Table 1 shows summary statistics of the study population, weather, and pollution variables. A total of 272 040 deaths for all causes were included in this analysis. More deaths were attributed to cardiovascular than respiratory causes. The distributions of population characteristics are provided for men and women separately in supplemental table 1 (available at stacks.iop.org/ERL/6/034027/mmedia) and for age groups separately in supplemental table 2 (available at stacks.iop.org/ERL/6/034027/mmedia). The pattern of each cause of death was similar in males and females. A higher proportion of female cases received no education and were in the ≥75 age group. The older persons had less education.
Table 1.
Summary statistics of the study population, weather, and pollution variables in Seoul, Korea, 2000–7 (N = 272 040).
| Variables | |
|---|---|
| Weather and pollution (mean (SD)) | |
| Daily mean temperature (°C) | 12.87 (10.10) |
| 24 h PM10 (μg m−3) | 66.08 (46.26) |
| Daily maximum 8 h O3 (ppb) | 27.73 (15.47) |
| Population characteristics (N (%)) | |
| Sex | |
| Male | 145 902 (53.7%) |
| Female | 126 003 (46.3%) |
| Age (yr) | |
| 0–14 yr | 4349 (1.6%) |
| 15–64 | 90 954 (33.4%) |
| 65–74 | 59 967 (22.0%) |
| ≥75 | 116 770 (42.9%) |
| Education (for those ≥20 yr) | |
| None | 65 213 (24.4%) |
| ≤12 yr | 167 204 (62.6%) |
| >12 yr | 32 557 (12.2%) |
| Unknown | 2104 (0.8%) |
| Place of death | |
| Out of hospital | 122 407 (45.1%) |
| In hospital | 149 168 (54.9%) |
| Daily mortality (mean (SD)) | |
| Total | 93.1 (11.6) |
| Cardiovascular | 25.5 (5.5) |
| Respiratory | 5.4 (2.6) |
We investigated multiple lag structures of the temperature effect by calculating the heat effect (99th versus 90th percentile) and cold effect (1st versus 10th percentile) estimates for the same day and for multiple days of exposure including the same day and previous days up to 28 days earlier (supplemental figure 1 available at stacks.iop.org/ERL/6/034027/mmedia). For heat-related mortality, the effect estimates declined when longer exposure timeframes were considered, while the cold effect persisted for longer lag times. We chose lag 0 for heat effect and lag 0–25 for cold effect for subsequent analysis.
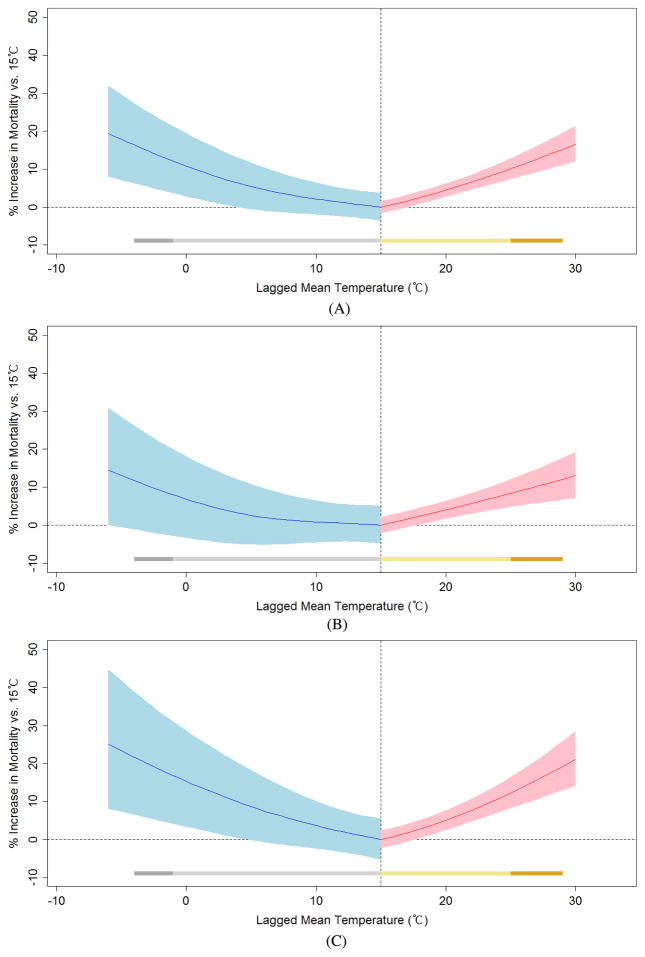
We generated temperature–mortality response curves based on the selected temperature lag structure (lag 0 for heat effect; lag 0–25 for cold effect). Figure 1 shows the increase in mortality risk for a given temperature compared with a reference temperature (15 °C, approximately the 50th percentile). Both cold and heat effects are presented simultaneously, using different lag structures, with the red portion of the curve showing the heat effect and the blue portion showing the cold effect. This method better captures the temperature–mortality relationship than approaches using equal lag structures for heat and cold effects. Separate figures are provided for men and women. Higher responses were noted for women, as indicated by the higher slopes of the heat and cold portions of the curve.
Figure 1.
Relationship between temperature and risk of mortality, comparing various temperature levels with a reference temperature of 15 °C for (A) total, (B) male and (C) female. (Note: lag 0 day for heat effect; lag 0–25 days for cold effect. The shaded portions of the curves represent 95% confidence intervals. The bars represent the ranges of the curves measured as heat effects (dark yellow: 99th–90th percentile; light yellow: 90th–50th percentile) and cold effects (dark gray: 1st–10th percentile; light gray: 10th–50th percentile).)
We calculated the increase in risk of all-cause mortality comparing various points on this temperature–mortality curve. The heat effect was a 10.2% (95% confidence interval 7.43, 13.0%) increase in mortality risk comparing the 90th and 50th percentiles of temperatures and 3.89% (−0.29, 8.25%) comparing the 99th and 90th percentiles. The cold effect increase in mortality was 12.2% (3.69, 21.3%) comparing the 10th and 50th percentiles of temperature and 4.45% (−6.78, 17.0%) comparing the 1st and 10th percentiles.
We compared the association between temperature and daily mortality with and without pollution adjustment (table 2). Pollutant lag structures for PM10 and O3 were selected as the single-day lag with the highest point estimate (results not shown). We used previous-day PM10 and same-day O3. We performed analysis including PM10 and ozone exposures separately or simultaneously. PM10 and ozone exposure were not correlated (Pearson correlation coefficients = 0.08). Heat and cold effects remained after inclusion of pollution variables. For the heat effect, estimates for temperature and mortality were slightly lower when pollution was included in the model, for either PM10 and/or ozone, while cold effect estimates were slightly higher with pollution adjustment. For the heat effect, the increase in mortality risk comparing lag 0 temperatures of 25 to 15 °C was 10.2% (7.4, 13.0%), which lowered to 9.3% (6.47, 12.2%) with ozone and PM10 adjustment. For the cold effect, the increase in mortality risk was 12.2% (3.7, 21.3%) comparing −1 to 15 °C for lag 0–25, which increased to 12.9% (4.40, 22.1%) with adjustment by PM10 and ozone.
Table 2.
Percentage change in mortality risk for heat and cold effects, with and without pollution adjustment.
| Pollution adjustment | Heat effect
|
Cold effect
|
||||||
|---|---|---|---|---|---|---|---|---|
| 90th percentile (25 °C) to 50th percentile (15 °C)
|
99th percentile (29 °C) to 90th percentile (25 °C)
|
10th percentile (−1 °C) to 50th percentile (15 °C)
|
1st percentile (−4 °C) to 10th percentile (−1 °C)
|
|||||
| Estimate (%) | 95% CI | Estimate (%) | 95% CI | Estimate (%) | 95% CI | Estimate (%) | 95% CI | |
| No pollution adjustment | 10.16 | 7.43, 12.96 | 3.89 | −0.29, 8.25 | 12.17 | 3.69, 21.34 | 4.45 | −6.78, 17.03 |
| Adjusted by PM10 | 10.01 | 7.26, 12.82 | 3.85 | −0.36, 8.24 | 12.38 | 3.89, 21.56 | 4.50 | −6.73, 17.07 |
| Adjusted by O3 | 9.39 | 6.59, 12.28 | 3.57 | −0.80, 8.13 | 12.77 | 4.25, 21.98 | 4.62 | −6.62, 17.20 |
| Adjusted by PM10 and O3 | 9.30 | 6.47, 12.19 | 3.55 | −0.84, 8.13 | 12.92 | 4.40, 22.14 | 4.65 | −6.58, 17.23 |
We evaluated the effect modification of the temperature–mortality relationship by several characteristics for total mortality. Table 3 shows the heat effect (comparison of 25 and 15 °C) and cold effect (comparison of −1 and 15 °C) stratified by sex, age, education, and place of death. Heat effects were larger for respiratory mortality than cardiovascular, whereas for cold effect, the reverse was observed. Heat and cold effects for females were higher than for males. Older persons were consistently more susceptible to both heat and cold effects, with trends of increasing risk with older age categories and the highest risk for the oldest age group (≥75 yr). For both heat- and cold-related mortality, the highest effect estimates were observed for those with no education. Heat and cold effects for out-of-hospital deaths were significantly higher than in-hospital deaths.
Table 3.
Percentage increase in total mortality risk for heat effects (comparison of 25 to 15 °C) and cold effect (comparison of −1 to 15 °C) by cause, sex, age, education and place of death.
| Heat effect
|
Cold effect
|
|||
|---|---|---|---|---|
| Estimate (%) | 95% CI | Estimate (%) | 95% CI | |
| All observations | 10.16 | 7.43, 12.96 | 12.17 | 3.69, 21.34 |
| Cause of death | ||||
| Cardiovascular | 6.07 | 1.24, 11.13 | 21.17 | 4.87, 40 |
| Respiratory | 12.01 | 1.25, 23.92 | 8.61 | −20.59, 48.54 |
| Sex | ||||
| Male | 8.40 | 4.82, 12.11 | 7.99 | −2.83, 20.01 |
| Female | 12.33 | 8.27, 16.55 | 16.88 | 4.2, 31.11 |
| Age | ||||
| 0–14 yr | 1.77 | −12.26, 18.06 | −13.48 | −46.19, 39.11 |
| 15–64 | 5.24 | 0.95, 9.71 | 8.36 | −4.98, 23.57 |
| 65–74 | 6.82 | 1.42, 12.51 | 14.43 | −2.68, 34.54 |
| ≥75 | 16.16 | 11.79, 20.69 | 16.44 | 3.4, 31.14 |
| Education (for those ≥20 yr) | ||||
| None | 16.11 | 10.37, 22.16 | 28.68 | 9.96, 50.59 |
| ≤12 yr | 8.73 | 5.44, 12.13 | 6.7 | −3.12, 17.52 |
| >12 yr | 8.61 | 1.19, 16.58 | 14.49 | −8.22, 42.83 |
| Place of death | ||||
| Out of hospital | 18.39 | 13.93, 23.03 | 30.56 | 15.8, 47.2 |
| In hospital | 4.2 | 0.75, 7.78 | −1.24 | −11.13, 9.76 |
Table 4 shows heat and cold effects for cause-specific mortality by sex, age, education, and place of death. For cardiovascular mortality, susceptibility by sex differed for the heat and cold effects. The association was higher for males for the cold effect, and higher for females for the heat effect. For respiratory mortality, the effect estimates for males were higher than for females for both heat and cold effects. For all cause-specific mortality results, the 95% confidence intervals for men and women overlapped. The highest effect estimates by age were observed for the oldest group (≥75 yr) for cardiovascular mortality for the heat effect and for respiratory mortality for the cold effect, but not for respiratory-related heat or cardiovascular-related cold effects. Effect estimates for out-of-hospital deaths were higher than those of in-hospital deaths for both cardiovascular and respiratory mortality, heat and cold effects, although the 95% intervals in all groups overlapped.
Table 4.
Percentage increase in cause-specific mortality risk for the heat effect (comparison of 25 to 15 °C) and cold effect (comparison of −1 to 15 °C) by cause, sex, age, education and place of death.
| Cardiovascular mortality
|
Respiratory mortality
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Heat
|
Cold
|
Heat
|
Cold
|
|||||
| Estimate (%) | 95% CI | Estimate (%) | 95% CI | Estimate (%) | 95% CI | Estimate (%) | 95% CI | |
| All observations | 6.07 | 1.24, 11.13 | 21.17 | 4.87, 40 | 12.01 | 1.25, 23.92 | 8.61 | −20.59, 48.54 |
| Sex | ||||||||
| Male | 0.46 | −5.88, 7.23 | 26.13 | 3.05, 54.38 | 14.16 | 0.9, 29.16 | 24.55 | −15.1, 82.71 |
| Female | 12.02 | 4.9, 19.62 | 16.49 | −5.0, 42.84 | 4.21 | −9.42, 19.88 | −13.63 | −43.07, 31.02 |
| Age | ||||||||
| 0–14 yr | 2.29 | −19.6, 30.14 | 13.77 | −37.85, 108.26 | 6.3 | −18.45, 38.57 | −44.81 | −75.13, 22.47 |
| 15–64 | −2.17 | −10.43, 6.84 | 68.6 | 28.1, 121.91 | 4.59 | −11.15, 23.12 | −3.56 | −42.74, 62.44 |
| 65–74 | 8.55 | −1.01, 19.02 | 14.42 | −13.92, 52.1 | 3.53 | −11.21, 20.71 | −21.23 | −50.05, 24.22 |
| ≥75 | 9.41 | 2.18, 17.15 | 5.01 | −15, 29.74 | 6.12 | −6.08, 19.91 | 3.46 | −28.71, 50.15 |
| Education (for those ≥20 yr) | ||||||||
| None | 9.0 | −0.42, 19.3 | 16.22 | −12.08, 53.64 | −1.45 | −15.06, 14.33 | 5.47 | −32.08, 63.8 |
| ≤12 yr | 6.16 | 0.07, 12.63 | 20.34 | 0.19, 44.55 | 16.96 | 2.46, 33.5 | −7.54 | −38.75, 39.58 |
| >12 yr | 1.14 | −10.78, 14.64 | 74.85 | 25.58, 143.47 | −3.06 | −18.34, 15.1 | −17.21 | −47.86, 31.45 |
| Place of death | ||||||||
| Out of hospital | 14.71 | 7.34, 22.59 | 39.21 | 13.15, 71.29 | 26.0 | 9.8, 44.6 | 27.38 | −16.48, 94.25 |
| In hospital | −1.83 | −7.99, 4.73 | 5.78 | −13.37, 29.16 | −2.42 | −13.62, 10.25 | −9.08 | −37.2, 31.63 |
We further examined the association between temperature and mortality risk with multiple susceptibilities. We investigated effect modification by sex with other potential susceptibilities by age, education, and place of death. For both men and women, the highest effect was for the oldest age group, except for females for the cold effect (table 5). For both men and women, the education group with the highest cold and heat effects was the lowest education level, and associations with out-of-hospital deaths were higher than for in-hospital deaths (table 5). Table 6 provides results by age group further stratified by education. The highest association remains for the no education group compared to other education categories for those in the 20–64 and 65–74 yr age groups. In the oldest age category, the effect estimates for the most educated group were higher than for other education groups.
Table 5.
Percentage increase in total mortality risk for the heat effect (comparison of 25 to 15 °C) and cold effect (comparison of −1 to 15 °C) for men and women separately, by age, education and place of death.
| Heat effect
|
Cold effect
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Male
|
Female
|
Male
|
Female
|
|||||
| Effect (%) | 95% CI | Effect (%) | 95% CI | Effect (%) | 95% CI | Effect (%) | 95% CI | |
| Total | 8.40 | 4.82, 12.11 | 12.33 | 8.27, 16.55 | 7.99 | −2.83, 20.01 | 16.88 | 4.2, 31.11 |
| Age | ||||||||
| 0–14 yr | 5.76 | −10, 24.27 | 1.19 | −15.28, 20.88 | −2.31 | −41.93, 64.33 | −37.85 | −63.16, 4.87 |
| 15–64 | 3.53 | −1.45, 8.77 | 9.41 | 1.24, 18.23 | 6.6 | −8.78, 24.58 | 12.48 | −11.98, 43.72 |
| 65–74 | 6.09 | −0.74, 13.38 | 8.61 | −0.04, 18.0 | 7.26 | −12.81, 31.95 | 27.88 | −1.34, 65.75 |
| ≥75 | 18.16 | 10.98, 25.8 | 15.17 | 9.78, 20.83 | 14.89 | −5.44, 39.6 | 16.93 | 0.85, 35.57 |
| Education (for those ≥20 yr) | ||||||||
| None | 26.61 | 13.33, 41.44 | 13.75 | 7.51, 20.36 | 50.65 | 6.99, 112.13 | 23.56 | 3.77, 47.12 |
| ≤12 yr | 6.61 | 2.5, 10.87 | 12.05 | 6.62, 17.75 | 2.57 | −9.31, 16.0 | 13.39 | −2.88, 32.38 |
| >12 yr | 8.08 | 0.05, 16.77 | 7.97 | −6.05, 24.08 | 1.06 | −17.12, 23.23 | 13.14 | −18.04, 56.18 |
| Place of death | ||||||||
| Out of hospital | 17.1 | 10.93, 23.62 | 19.73 | 13.58, 26.21 | 27.27 | 7.45, 50.76 | 32.97 | 12.86, 56.65 |
| In hospital | 2.99 | −1.38, 7.55 | 5.97 | 0.68, 11.53 | −4.15 | −16.35, 9.83 | 2.96 | −12.17, 20.69 |
Table 6.
Percentage increase in total mortality risk for the heat effect (comparison of 25 to 15 °C) and cold effect (comparison of −1 to 15 °C) by age and education.
| Age (yr)
|
||||||
|---|---|---|---|---|---|---|
| 20–64 yr
|
65–74
|
≥75
|
||||
| Effect (%) | 95% CI | Effect (%) | 95% CI | Effect (%) | 95% CI | |
| Heat effect | ||||||
| Education (for those ≥20 yr) | ||||||
| None | 23.08 | 5.89, 43.06 | 8.23 | −4.06, 22.1 | 16.75 | 10.25, 23.63 |
| ≤12 yr | 5.21 | 0.3, 10.35 | 6.42 | 0.04, 13.2 | 15.01 | 8.99, 21.37 |
| >12 yr | 4.88 | −4.71, 15.44 | 2.08 | −10.1, 15.91 | 18.55 | 4.47, 34.53 |
| Cold effect | ||||||
| Education (for those ≥20 yr) | ||||||
| None | 59.12 | −1.02, 155.79 | 82.51 | 24.81, 166.88 | 18.45 | −0.71, 41.31 |
| ≤12 yr | 2.27 | −12.04, 18.91 | 5.57 | −12.91, 27.98 | 13.25 | −4.13, 33.79 |
| >12 yr | 16.75 | −13.73, 58.0 | −1.49 | −33.64, 46.24 | 32.75 | −9.36, 94.43 |
4. Discussion
In this study, we found that both high and low ambient temperatures were associated with daily mortality in Seoul, Korea. Regarding cause-specific mortality, cardiovascular deaths showed a higher risk to cold, whereas respiratory deaths showed a higher risk to heat effect. We identified susceptible populations such as females, the elderly, those with no education, and deaths occurring outside of a hospital for heat- and cold-related total mortality. Also, we found some differences in susceptibility by cause of death. For instance, susceptibility according to sex differed by heat- and cold-related cardiovascular mortality. For respiratory mortality, the effect estimate for males was higher than for females for both heat and cold effect.
Our findings suggest that a short lag is appropriate to capture the effect of heat on mortality, and longer lags are required to capture cold’s impact on mortality. A number of previous studies on heat-related mortality have identified risk from recent exposure such as same day and a few days earlier [6, 21]. For cold-related mortality, many studies have applied one or more weeks’ lag time [12, 22–24]. Cold temperatures more indirectly affect mortality, whereas heat effects result from a rapid physical response. Much of the cold-related effect may be explained by other factors that strongly influence mortality in winter such as influenza epidemics and infectious disease [25, 26].
We found that the associations for heat and cold were adjusted slightly but remained positive and significant when ozone and/or PM10 were included in the model. The previous findings for confounding and/or effect modification by air pollutants remain mixed, and results vary by location. Recent studies suggest that PM and ozone may be confounders of weather–mortality relationships, and some studies found ozone to be a confounder especially on hot days or in the warm months [16, 21, 27]. However, other studies reported no significant confounding by pollution on the association between temperature and mortality [10, 28]. A study by O’Neill et al [3] found a small decrease in the association between temperature and mortality when adding ozone and PM10 individually or jointly. Another study in nine US cities [7] found no confounding or effect modification due to air pollution.
In this study, the effects of temperature on mortality varied with cause of death. We observed that respiratory mortality was strongly associated with heat-related mortality. Cold effects were higher for cardiovascular mortality than for respiratory mortality. This finding is consistent with previous studies. Hajat et al [12] found that deaths from respiratory and external causes were more strongly associated with heat mortality than deaths from cardiovascular and other causes. Cold weather has a strong association with cardiovascular disease deaths, both in the United States [4] and elsewhere [5]. A study in Dublin [29] found no effect of heat events on cardiovascular deaths but an immediate effect of cold on cardiovascular mortality that decreased over the subsequent three weeks. Several biological mechanisms have been postulated for heat-related mortality. Increased blood viscosity, elevated cholesterol levels associated with higher temperature, higher sweating threshold, and increased stress on heart and lungs due to overload of the body’s ability to regulate rising temperature may trigger heat-related mortality [30]. The biological mechanisms that may explain increases in cardiovascular death with cold temperature may relate to blood pressure [4]. Blood pressure is higher during the winter, and exposure to cold temperatures causes a decrease in blood irrigation to the skin to prevent heat loss, which implies an increase in blood volume in the central organs, with a subsequent cardiac overload and an increased blood concentration with higher blood viscosity [31]. Results from a recent study suggest that inflammation may have a role in the intermediate processes leading to cold-related cardiovascular deaths [24, 32].
A number of studies have examined potential susceptibility to temperature-related mortality for a particular study population and location. We observed that elderly people are most susceptible to temperature-related mortality for both heat and cold effects in Seoul, Korea. Our findings confirm the trend of increasing risk with age for men and women. Most previous studies consistently found a higher effect of both cold and hot temperatures on mortality in the elderly compared to younger persons [6, 33, 34]. In addition, while some studies have identified children and infants to be at increased risk for mortality [15, 21, 23, 35], we found children to be the least susceptible to temperature-related total mortality.
We examined modification by sex and found women to be at higher risk for heat- and cold-related mortality. Previous findings reported that women in various locations had higher risk than men [10, 16, 36], while others observed men to be at higher risk [13]. Some investigators reported no difference by sex [3, 15]. A previous study suggests that clothing is an important modifier in sex differences [37]. More and better clothes in cold weather may act as a protective measure. Another study also suggests that biologic differences between the ability to thermoregulate may play a role [4]. Body temperature is regulated by the hypothalamus neurons and these are directly influenced by estrogen through the estrogen receptor.
We also found that those with no education, which may be a predictor of low socioeconomic status, were most vulnerable to heat- and cold-related mortality. Previous findings suggest that those with low education and socioeconomic status have a greater susceptibility to temperature-related mortality [3, 38], which could be related to poor baseline health status, limited access to health care, and housing conditions such as the lack of air conditioning and electric fans [39]. However, a study by Bell et al [13] reported that the highest effect estimates were observed for the most educated group in Mexico City, which corresponds to some findings in our study (e.g. the most educated group for cold-related cardiovascular mortality). The association between socioeconomic indicators and health outcomes can differ by location because education is one of many factors relating to overall socioeconomic status and this association can interact with other factors affecting susceptibility. Further, the influence of education and socioeconomic status on health may vary by community and country.
We found that deaths outside a hospital were more strongly associated with hot and cold temperatures regardless of cause of death or sex. This finding is consistent with previous studies and supports the hypothesis that exposure to ambient temperature affects mortality. People who die in a hospital are more likely to be in an air filtrated, air conditioned or heated environment and thus less likely to experience extreme ambient temperature conditions. A study by O’Neill et al [3] examined effect modification of heat- and cold-related mortality by age, race, gender, education, and place of death in seven US cities and reported that place of death was the strongest effect modifier. Another study also found that those dying outside a hospital were more susceptible to extreme temperatures, especially to heat, compared to those dying inside a hospital [5].
An important finding of our study is that susceptibility to temperature varies according to the cause of death. Few studies have investigated the susceptibility to temperature by cause of death. A US study [3] found that cold-associated respiratory mortality more strongly affected blacks, those who died outside of hospital, and young persons, but observed no differences in susceptibility for cardiovascular mortality. In this study, we observed that females were more susceptible to heat-related cardiovascular mortality than respiratory mortality, whereas males had higher risk of heat-related respiratory mortality than cardiovascular mortality. These findings on differences in susceptibilities according to the cause of death are relevant to research on biological mechanisms of weather-related health responses and can help interventions target the most susceptible populations. However, the nature of susceptibility in temperature–mortality relationships is complex, and many other factors affect susceptibility such as population demographics, housing characteristics and adaptation to local climate. Thus, more research using various study populations and different locations is needed.
To our knowledge, this is the first study to investigate effect modification and identify susceptible populations in temperature-related mortality associations using more detailed and multiple indicators (e.g., the effects of education level stratified by age) in Asia. In conclusion, our findings provide evidence that subpopulations such as females, the elderly, and those with no education, an indicator of low socioeconomic conditions, are especially vulnerable to heat- and cold-related total mortality. Our results also suggest that deaths occurring outside of a hospital evidenced a stronger temperature dependence than deaths inside a hospital. Susceptibility in the temperature–mortality relationship may vary by cause of death. This research indicates that programs to reduce the public health burden of weather-related mortality should emphasize particular populations, as some people are more susceptible than others. Additional work is needed to examine susceptibility in other locations.
Supplementary Material
Acknowledgments
Funding sources: this work was supported by the US EPA STAR Graduate Fellowship (91689201-0), a US National Science Foundation Graduate Fellowship, and the US National Institute of Environmental Health Sciences (RO1 ES015028).
Footnotes
Online supplementary data available from stacks.iop.org/ERL/6/034027/mmedia
Conflict of interest: the authors declare they have no conflicts of interest.
References
- 1.Iñiguez C, Ballester F, Ferrandiz J, Pérez-Hoyos S, Sáez M, López ATEMPRO-EMECAS. Relation between temperature and mortality in thirteen Spanish cities. Int J Environ Res Public Health. 2010;7:3196–210. doi: 10.3390/ijerph7083196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocklöv J, Forsberg B. The effect of high ambient temperature on the elderly population in three regions of Sweden. Int J Environ Res Public Health. 2010;7:2607–19. doi: 10.3390/ijerph7062607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neill MS, Zanobetti A, Schwartz J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol. 2003;157:1074–82. doi: 10.1093/aje/kwg096. [DOI] [PubMed] [Google Scholar]
- 4.Barnett AG. Temperature and cardiovascular deaths in the US elderly: changes over time. Epidemiology. 2007;18:369–72. doi: 10.1097/01.ede.0000257515.34445.a0. [DOI] [PubMed] [Google Scholar]
- 5.Medina-Ramon M, Zanobetti A, Cavanagh DP, Schwartz J. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect. 2006;114:1331–6. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L, Patz JA. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155:80–7. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Zanobetti A, Schwartz J. Temperature and mortality in nine US cities. Epidemiology. 2008;19:563–70. doi: 10.1097/EDE.0b013e31816d652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portier CJ, et al. A Human Health Perspective on Climate Change: A Report Outlining the Research Needs on the Human Health Effects of Climate Change. Research Triangle Park, NC: Environmental Health Perspectives/National Institute of Environmental Health Sciences; 2010. available at www.niehs.nih.gov/climatereport. [DOI] [Google Scholar]
- 9.Schwartz J. Who is sensitive to extremes of temperature? A case-only analysis. Epidemiology. 2005;16:67–72. doi: 10.1097/01.ede.0000147114.25957.71. [DOI] [PubMed] [Google Scholar]
- 10.Stafoggia M, et al. Vulnerability to heat-related mortality: a multicity, population-based, case-crossover analysis. Epidemiology. 2006;17:315–23. doi: 10.1097/01.ede.0000208477.36665.34. [DOI] [PubMed] [Google Scholar]
- 11.Vandentorren S, Bretin P, Zeghnoun A, Mandereau-Bruno L, Croisier A, Cochet C, Ribéron J, Siberan I, Declercq B, Ledrans M. August 2003 heat wave in France: risk factors for death of elderly people living at home. Eur J Public Health. 2006;16:583–91. doi: 10.1093/eurpub/ckl063. [DOI] [PubMed] [Google Scholar]
- 12.Hajat S, Kovats RS, Lachowycz K. Heat-related and cold-related deaths in England and Wales: who is at risk? Occup Environ Med. 2007;64:93–100. doi: 10.1136/oem.2006.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell ML, O’Neill MS, Ranjit N, Borja-Aburto VH, Cifuentes LA, Gouveia NC. Vulnerability to heat-related mortality in Latin America: a case-crossover study in Sao Paulo, Brazil, Santiago, Chile and Mexico City, Mexico. Int J Epidemiol. 2008;37:796–804. doi: 10.1093/ije/dyn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiology. 2009;20:205–13. doi: 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu R, Ostro BD. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. Am J Epidemiol. 2008;168:632–7. doi: 10.1093/aje/kwn170. [DOI] [PubMed] [Google Scholar]
- 16.Vaneckova P, Beggs PJ, de Dear RJ, McCracken KW. Effect of temperature on mortality during the six warmer months in Sydney, Australia between 1993 and 2004. Environ Res. 2008;108:361–9. doi: 10.1016/j.envres.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Díaz J, Linares C, García-Herrera R, López C, Trigo R. Impact of temperature and air pollution on the mortality of children in Madrid. J Occup Environ Med. 2004;46:768–74. doi: 10.1097/01.jom.0000135542.12974.49. [DOI] [PubMed] [Google Scholar]
- 18.Ha J, Yoon J, Kim H. Relationship between winter temperature and mortality in Seoul, South Korea, from 1994 to 2006. Sci Total Environ. 2009;407:2158–64. doi: 10.1016/j.scitotenv.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Ha J, Park J. High temperature, heat index, and mortality in 6 major cities in South Korea. Arch Environ Occup Health. 2006;61:265–70. doi: 10.3200/AEOH.61.6.265-270. [DOI] [PubMed] [Google Scholar]
- 20.Ha J, Kim H, Hajat S. Effect of previous-winter mortality on the association between summer temperature and mortality in South Korea. Environ Health Perspect. 2011;119:542–6. doi: 10.1289/ehp.1002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill MS, Hajat S, Zanobetti A, Ramirez-Aguilar M, Schwartz J. Impact of control for air pollution and respiratory epidemics on the estimated associations of temperature and daily mortality. Int J Biometeorol. 2005;50:121–9. doi: 10.1007/s00484-005-0269-z. [DOI] [PubMed] [Google Scholar]
- 22.Analitis A, et al. Effects of cold weather on mortality: results from 15 European cities within the PHEWE project. Am J Epidemiol. 2008;168:1397–408. doi: 10.1093/aje/kwn266. [DOI] [PubMed] [Google Scholar]
- 23.Gouveia N, Hajat S, Armstrong B. Socioeconomic differentials in the temperature–mortality relationship in Sao Paulo, Brazil. Int J Epidemiol. 2003;32:390–7. doi: 10.1093/ije/dyg077. [DOI] [PubMed] [Google Scholar]
- 24.Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Associations between outdoor temperature and markers of inflammation: a cohort study. Environ Health. 2010;9:42. doi: 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kysely J, Pokorna L, Kyncl J, Kriz B. Excess cardiovascular mortality associated with cold spells in the Czech Republic. BMC Public Health. 2009;9:19. doi: 10.1186/1471-2458-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mourtzoukou EG, Falagas ME. Exposure to cold and respiratory tract infection. Int J Tuberc Lung Dis. 2007;11:938–43. [PubMed] [Google Scholar]
- 27.Ren C, Williams GM, Morawska L, Mengersen K, Tong S. Ozone modifies associations between temperature and cardiovascular mortality: analysis of the NMMAPS data. Occup Environ Med. 2008;65:255–60. doi: 10.1136/oem.2007.033878. [DOI] [PubMed] [Google Scholar]
- 28.Basu R, Feng WY, Ostro BD. Characterizing temperature and mortality in nine California counties. Epidemiology. 2008;19:138–45. doi: 10.1097/EDE.0b013e31815c1da7. [DOI] [PubMed] [Google Scholar]
- 29.Goodman PG, Dockery DW, Clancy L. Cause-specific mortality and the extended effects of particulate pollution and temperature exposure. Environ Health Perspect. 2004;112:179–85. doi: 10.1289/ehp.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8:40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keatinge WR. Winter mortality and its causes. Int J Circumpolar Health. 2002;61:292–9. doi: 10.3402/ijch.v61i4.17477. [DOI] [PubMed] [Google Scholar]
- 32.Schneider A, et al. Air temperature and inflammatory responses in myocardial infarction survivors. Epidemiology. 2008;19:391–400. doi: 10.1097/EDE.0b013e31816a4325. [DOI] [PubMed] [Google Scholar]
- 33.Baccini M, et al. Heat effects on mortality in 15 European cities. Epidemiology. 2008;19:711–9. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- 34.El-Zein A, Tewtel-Salem M, Nehme G. A time-series analysis of mortality and air temperature in Greater Beirut. Sci Total Environ. 2004;330:71–80. doi: 10.1016/j.scitotenv.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Hajat S, Armstrong BG, Gouveia N, Wilkinson P. Mortality displacement of heat-related deaths: a comparison of Delhi, São Paulo, and London. Epidemiology. 2005;16:613–20. doi: 10.1097/01.ede.0000164559.41092.2a. [DOI] [PubMed] [Google Scholar]
- 36.Yu W, Vaneckova P, Mengersen K, Pan X, Tong S. Is the association between temperature and mortality modified by age, gender, and socio-economic status? Sci Total Environ. 2010;408:3513–8. doi: 10.1016/j.scitotenv.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 37.The Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet. 1997;349:1341–6. [PubMed] [Google Scholar]
- 38.Borrell C, Mari-Dell’Olmo M, Rodriguez-Sanz M, Garcia-Olalla P, Caylà JA, Benach J, Muntaner C. Socioeconomic position and excess mortality during the heat wave of 2003 in Barcelona. Eur J Epidemiol. 2006;21:633–40. doi: 10.1007/s10654-006-9047-4. [DOI] [PubMed] [Google Scholar]
- 39.McGeehin MA, Mirabelli M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect. 2001;109(Suppl 2):185–9. doi: 10.1289/ehp.109-1240665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.