Abstract
Context
Ozone has been associated with various adverse health effects, including increased rates of hospital admissions and exacerbation of respiratory illnesses. Although numerous time-series studies have estimated associations between day-to-day variation in ozone levels and mortality counts, results have been inconclusive.
Objective
To investigate whether short-term (daily and weekly) exposure to ambient ozone is associated with mortality in the United States.
Design and Setting
Using analytical methods and databases developed for the National Morbidity, Mortality, and Air Pollution Study, we estimated a national average relative rate of mortality associated with short-term exposure to ambient ozone for 95 large US urban communities from 1987-2000. We used distributed-lag models for estimating community-specific relative rates of mortality adjusted for time-varying confounders (particulate matter, weather, seasonality, and long-term trends) and hierarchical models for combining relative rates across communities to estimate a national average relative rate, taking into account spatial heterogeneity.
Main Outcome Measure
Daily counts of total non–injury-related mortality and cardiovascular and respiratory mortality in 95 large US communities during a 14-year period.
Results
A 10-ppb increase in the previous week’s ozone was associated with a 0.52% increase in daily mortality (95% posterior interval [PI], 0.27%-0.77%) and a 0.64% increase in cardiovascular and respiratory mortality (95% PI, 0.31%-0.98%). Effect estimates for aggregate ozone during the previous week were larger than for models considering only a single day’s exposure. Results were robust to adjustment for particulate matter, weather, seasonality, and long-term trends.
Conclusions
These results indicate a statistically significant association between short-term changes in ozone and mortality on average for 95 large US urban communities, which include about 40% of the total US population. The findings indicate that this widespread pollutant adversely affects public health.
Exposure to Tropospheric Ozone is widespread in the United States,1,2 occurring also outside southern California, where ozone formation was first recognized.3 Short-term exposure to ozone has been linked to adverse health effects, including increased rates of hospital admissions and emergency department visits, exacerbation of chronic respiratory conditions (eg, asthma), and decreased lung function.4-8 Numerous time-series studies have addressed the relationship between ozone levels and mortality counts on short-term intervals of 1 or a few days, including some studies involving multiple locations; however, their findings have been inconsistent.9-17 Interpretation of this evidence is constrained by the limited range of locations included in these reports, the variability of methods used, and the imprecision of estimates from some of the studies. The study of ozone and health is complicated by the complex, nonlinear chemical formation of tropospheric ozone, which is temperature driven, with higher ozone levels at higher temperatures.18
In 1997, the US Environmental Protection Agency (EPA) proposed revisions to the National Ambient Air Quality Standard (NAAQS) for ozone, adding a daily maximum 8-hour standard of 80 ppb (parts per billion by volume) while phasing out the daily hourly maximum standard of 120 ppb. These changes were prompted by evidence from epidemiologic, controlled human exposure, and toxicologic studies that identified adverse health effects at ozone concentrations below the existing 1-hour NAAQS.19 Because of the relevance of epidemiologic evidence to the NAAQS for ozone and other pollutants, updated and expanded time-series studies of ozone are informative to the regulatory process.
With the National Morbidity, Mortality, and Air Pollution Study (NMMAPS), we have developed national approaches for multisite time-series analyses of particulate matter with an aerodynamic diameter less than 10 μm (PM10) and mortality and hospitalization data that provided evidence for decision making.20-26 As part of the NMMAPS, we developed 2-stage statistical models20,21,27 for estimating the percentage increase in mortality associated with exposure to PM10 or other pollutants. In our 2-stage approach, a time-series analysis is first performed within each community, and in the second stage of analysis, the results are combined across communities to produce a national average estimate that accounts for the within-community statistical uncertainty and the heterogeneity of the effects across the country.20,21
We use an updated NMMAPS database, including 95 large US urban areas for 1987-2000, to perform a multisite time-series study of ozone and mortality. Because ozone concentrations are typically available daily, at the first stage of our analysis we extend previous approaches to develop constrained and unconstrained distributed-lag models.26,28-34 Distributed-lag models are appropriate for estimating relative rates of mortality associated with exposure to pollution levels during several previous days, thus allowing more flexibility for exploring the lag between exposure and death than single-lag models. At the second stage, we use hierarchical models35-38 to combine the relative rate estimates obtained from the community-specific distributed-lag models to produce a national average estimate. With this 2-stage model, variation across communities in the short-term effects of ozone can be explored and an effect estimated for the nation.
METHODS
Mortality, Weather, and Pollution Data
This analysis is based on daily cause specific mortality counts for 1987-2000 obtained from the National Center for Health Statistics on 95 large urbanized areas. The mortality counts for each urban community are at the county level (either a single county or multiple adjacent counties representing the metropolitan area). The outcome measure was the daily number of deaths in each community, excluding those of nonresidents and those caused by injuries and other external causes (International Classification of Diseases, Ninth Revision [ICD-9] codes 800 and above, International Statistical Classification of Diseases, 10th Revision [ICD-10] codes S and above). International Classification of Diseases, Ninth Revision codes were used for 1987-1998 and the ICD-10 for 1999 and 2000. Mortality was further categorized by cardiovascular causes (ICD-9 codes 390-448, ICD-10 chapter I with codes <800) and respiratory causes, including chronic obstructive pulmonary disease and related disorders (ICD-9 codes 480-486, 490-497, or 507, ICD-10 chapter J with codes 100-118, 120-189, 209-499, or 690-700). The average daily death rate ranged from 2.2 deaths per day (Arlington, Va) to 190 deaths per day (New York, NY) and averaged 20 deaths per day across all communities. Deaths for people aged 75 years and older comprised approximately half of total deaths in these 95 communities.
Air-pollution data for ozone and PM10 were supplied by the US EPA’s Aerometric Information Retrieval Service (now called the Air Quality System database). To protect against outliers, a 10% trimmed mean was used to average across monitors after correction for yearly averages for each monitor.23 For ozone, the 24-hour average, maximum 8-hour average, and maximum hourly concentrations were calculated for each day. In several locations, ozone values were measured only during the peak ozone season, often April to October. (Descriptive statistics on each community are provided at http://www.ihapss.jhsph.edu/data/NMMAPS/descriptives/frame.htm.)
Daily average values for dew point and temperature were calculated from hourly values obtained from the National Climatic Data Center on the Earth-Info CD database.39 Daily averages were chosen according to extensive analyses conducted for related work.23,24 Measurements from multiple weather stations were averaged to provide weather variables representing each community.40
Statistical Approach
A 2-stage statistical model35-38 was used to estimate a national average association between short-term ambient ozone levels and mortality risk, accounting for other factors such as weather, seasonality, long-term trends, and PM10. In the first stage, distributed-lag over dispersed Poisson regression models26,28,41,42 were used for estimating community-specific relative rates of mortality associated with exposure to ozone in the last week. First-stage community-specific models included indicator variables for the day of the week to allow for different baseline mortality rates for each day of the week. Smooth functions of calendar time (natural cubic splines) were used to adjust for seasonality and long-term trends, such as influenza epidemics. We also added interaction terms between smooth functions of time and age-specific indicators (<65, 65-74, ≥75 years) to further adjust for seasonal mortality patterns that could vary by age group. We controlled for the potential confounding effect of weather by including smooth functions of temperature, the average of the 3 previous days’ temperature, dew point, and the average of the 3 previous days’ dew points.
At the second stage, we combined the community-specific relative rates to generate a national average estimate of the association between ozone and mortality that accounts for within-community and across-community variability (also called heterogeneity).27,43 The second-stage model also provides community-specific Bayesian estimates that are approximately equal to a weighted average of the maximum likelihood estimate (from the first stage) for that community and the national average with shrinkage weights equal to w and 1–w, respectively. The degree of shrinkage w of each Bayesian estimate to the national average is inversely proportional to the heterogeneity of the community-specific relative rates. The higher the heterogeneity, the less the Bayesian estimates shrink toward the overall mean.
We assessed the relationship between ambient ozone concentrations and the risk of mortality on subsequent days at various single-day lags. For instance, a lag of 0 days corresponds to the association between ozone concentrations on a given day and the risk of mortality on that same day. A lag of 2 days refers to the association between ozone levels on a given day and the risk of mortality 2 days later. In the single-lag models, we considered pollution for the same day and up to 3 days before.
We also investigated cumulative exposure during several days. Our distributed-lag model estimates the association between the risk of mortality and the cumulative exposure to ambient ozone levels during the previous week, allowing each day to have an effect. We used an unconstrained distributed-lag model that simultaneously included variables for the same day and up to 6 days before to estimate the effect of the previous week’s ozone levels on current-day mortality. We also used a constrained distributed-lag model to estimate how the previous week’s pollution levels affected mortality. This model constrains lag-specific regression coefficients to be a step function by including variables for the average of the same day’s concentration and that of up to 6 days before, the average of the same day’s and the previous 3 days’ concentrations minus the average of the same day’s and previous 6 days’ ozone levels, and the current day’s concentration minus the average of the same day’s and previous 3 days’ concentrations.
We examined the sensitivity of key findings with respect to (1) the specification of the statistical model: constrained and unconstrained distributed-lag models26,28 and single-day lag for ozone exposure; (2) inclusion of PM10 in the statistical model as a potential confounder; (3) exclusion of days with temperatures above specified thresholds to control for the potential confounding effect of temperature and heat waves; (4) specification of the degrees of freedom (df) in the smooth functions of time to control for seasonality and long-term trends; and (5) use of different ozone exposure metrics: daily average, 8-hour maximum, and 1-hour maximum.
Calculations were implemented using the statistical software S-Plus44 and with strict convergence parameters.45 The data and statistical models used have been made available on the Internet-based Health and Air Pollution Surveillance System (http://www.ihapss.jhsph.edu/index.htm), maintained by the Johns Hopkins Bloomberg School of Public Health and sponsored by the Health Effects Institute.
RESULTS
Mortality, Pollution, and Weather Variables
The daily ozone concentrations varied by community, averaging approximately 26 ppb across the 95 urbanized communities. Daily PM10 concentrations were generally not highly correlated with ozone concentrations(correlations with in communities ranged from −0.38 to 0.63, averaging 0.30) or with temperature (correlations within communities ranged from 0.34 to 0.61, averaging 0.33). The correlation between daily ozone and temperature ranged from −0.41 for Honolulu, Hawaii, which also had the lowest ozone concentrations, to 0.87 for Louisville, Ky, and averaged 0.52.
Total Mortality
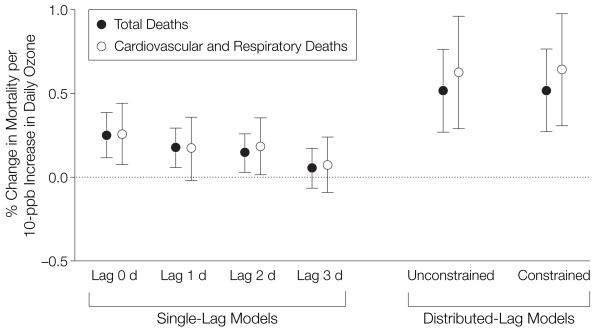
Figure 1 provides the national average estimates for total mortality obtained under several model specifications. Results from the constrained distributed-lag model indicated that a 10-ppb increase in daily ozone levels of the previous week corresponded to a 0.52% (95% posterior interval [PI], 0.27%-0.77%) increase in daily mortality. The 95% PI is the Bayesian formulation of the 95% confidence interval. The unconstrained distributed-lag model provided similar estimates. This result was statistically significant, with a posterior probability of 1 that this overall effect is larger than zero. Results from single-lag models indicated that ozone concentrations from the same day and a few preceding days affected mortality. The relative rate estimates decreased as the lag increased, indicating that exposure to ozone on more recent days, such as the same or previous day, was associated with a larger risk of mortality than exposure on less recent days, such as 2 or 3 days ago. However, single-day lag models underestimate the cumulative effect of ozone on mortality because they take into account only 1 day’s ozone exposure. Distributed-lag models estimate cumulative relative rates of mortality associated with ozone concentrations in the few preceding days.
Figure 1.
Percentage Change in Daily Mortality for a 10-ppb Increase in Ozone for Total and Cardiovascular Mortality, for Single-Lag and Distributed-Lag Models
The single-lag model reflects the percentage increase in mortality for a 10-ppb increase in ozone on a single day. The distributed-lag model reflects the percentage change in mortality for a 10-ppb increase in ozone during the previous week. Error bars indicate 95% posterior intervals.
Effects of ozone on mortality for 3 age groups (<65, 65-74, and ≥75 years) were estimated separately by using the constrained distributed-lag model to investigate whether the elderly are particularly susceptible to the effects of ozone on mortality. Effect estimates for the 65- to 74-year age category were 0.70% (95% PI, 0.28%-1.12%) for a 10-ppb increase in daily ozone, whereas estimates for the youngest and eldest groups were similar to the overall estimate for the total population, at 0.50% (95% PI, 0.10%-0.92%) and 0.52% (95% PI, 0.18%-0.87%), respectively, for a 10-ppb increase.
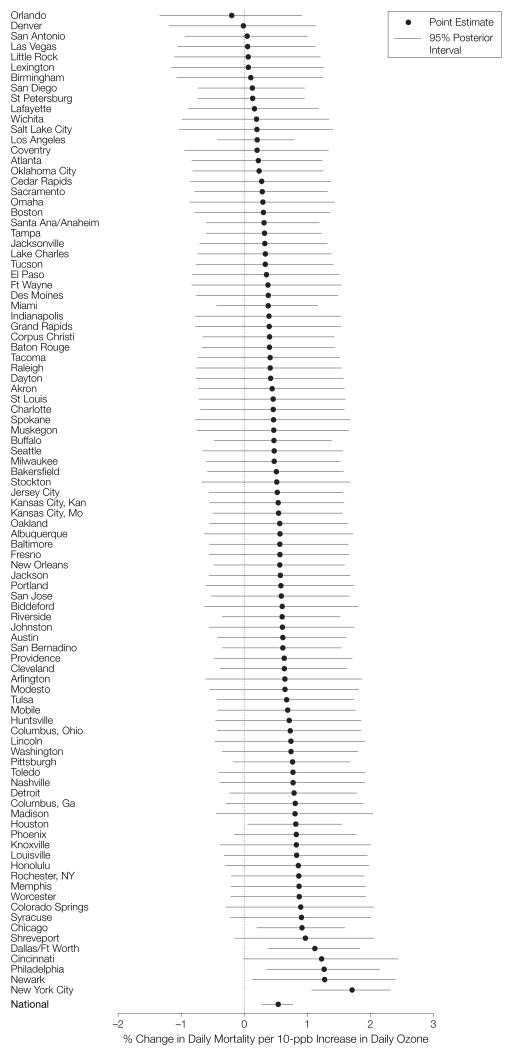
Figure 2 shows the community specific Bayesian estimates and their 95% PIs. The heterogeneity, which denotes the between-location SD of the community-specific relative rates in relation to their mean, was 0.64. The mean effect of 0.52% indicated that 95% of the true community-specific relative rates were within the interval −0.73% to 1.77%.
Figure 2.
Community-Specific Bayesian Estimates, Constrained Distributed-Lag Model
We evaluated whether the estimated national average effect was due entirely to days with ozone levels higher than the current regulatory standard of 80 ppb for the daily 8-hour maximum, which is roughly equivalent to a 60-ppb daily average. With this restriction, we repeated the statistical analyses using only days with ozone levels less than 60 ppb and using a single day of exposure at a lag of 1 day. We found that the national average effect was equal to a 0.15% increase in daily mortality (95% PI, 0.04%-0.27%) for a 10-ppb increase in ozone of the previous day. The effect estimate using all days was 0.18% (95% PI, 0.06%-0.30%).
Relative rate estimates for cardiovascular and respiratory mortality are shown in Figure 1. The national estimate for cardiovascular and respiratory mortality was slightly larger than the one for total mortality: 0.64% (95% PI, 0.31%-0.98%) increase in mortality for a 10-ppb increase in the preceding week’s ozone levels.
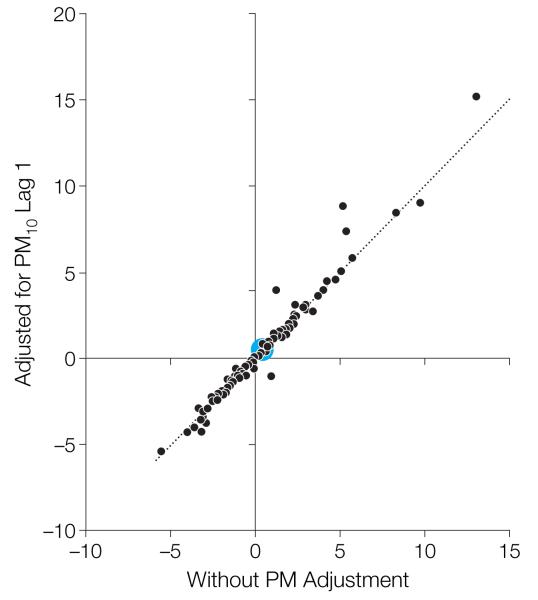
We found the key results for ozone to be robust to adjustment for PM10. We adjusted for 1-day lag PM10 because this lag yielded the largest effect estimate in previous time-series analysis of PM and mortality for 90 US urban communities.22-24 Because monitoring for PM10 is required for only 1 of every 6 days, we limited this sensitivity analysis to those days for which PM10 and ozone were available. In single-lag models, the relative rates estimated for mortality and ozone at various lags (0, 1, and 2 days) were affected little by inclusion of PM10 at lags 0, 1, or 2 days. Figure 3 compares the maximum likelihood estimates for the constrained distributed-lag model for ozone, with and without adjustment for PM10, and shows that the community-specific estimates for ozone were also robust to the adjustment for PM10.
Figure 3.
Community-Specific Maximum Likelihood Estimates of the Short-term Effects of Ozone on Mortality, With and Without Adjustment for PM10
Results are obtained with a 2-stage constrained distributed-lag model applied to the same data set (days with data for ozone and particulate matter <10 μm [PM10]). The distributed-lag model reflects the percentage increase in mortality for a 10-ppb increase in ozone during the previous week. The large blue circle indicates the national average effect.
We explored whether the association between ozone and mortality was modified by the long-term average of PM2.5 (PM with an aerodynamic diameter less than 2.5 μm) by performing a weighted second-stage linear regression with the community-specific estimate of ozone’s effect on mortality as the dependent variable and the long-term PM2.5 average as the independent variable. No association was observed.
Sensitivity Analyses
The national average effect of ozone on mortality was similar for the following data sets: all 95 urban communities using all data, all communities for days from April to October, and 55 communities that have yearly data for ozone. Estimates for these 3 sets of analyses using the constrained distributed-lag model were 0.52% (95% PI, 0.27%-0.77%), 0.39% (95% PI, 0.13%-0.65%), and 0.48% (95% PI, 0.16%-0.78%) increase in mortality for a 10-ppb increase in the previous week’s ozone level. Further, the national average estimate of short-term effects of ozone (using all days) on mortality was robust to the exclusion of days with high temperatures with a cutoff as low as 29°C (85°F); the range of effects for these analyses was 0.50% (95% PI, 0.25%-0.75%) to 0.55% (95% PI, 0.30%-0.80%) for a 10-ppb increase in the previous week’s daily ozone concentration. Effect estimates were slightly higher at lower temperatures.
The national average estimate of the constrained distributed-lag model was robust to the degrees of freedom for smoothing of calendar time (ie, long-term trends). The central estimate of the mortality increase associated with a 10-ppb increase in the previous week’s ozone concentrations ranged from 0.41% to 0.54%, with smoothing of calendar time varying from 7 to 21 df per year.
Statistically significant relationships were found for multiple ozone concentration metrics by using the constrained distributed-lag model. The concentrations of the different metrics were highly correlated, although relationships among them varied because of differences in weather and the nature of sources. The increase in mortality was 0.52% (95%PI, 0.27%-0.77%) for a 10-ppb increase in the daily average, 0.64% (95%PI, 0.41%-0.86%) for a 15-ppb increase in the daily 8-hour maximum, and 0.67% (95% PI, 0.42%-0.92%) for a 20-ppb increase in the daily hourly maximum.
COMMENT
This multisite time-series study of 95 large US urban communities through-out a 14-year period provides strong evidence of an association between mortality and short-term exposure to ozone. On average across the 95 communities, we estimated a 0.52% (95% PI, 0.27%-0.77%) increase in daily mortality for a 10-ppb increase in the previous week’s ozone concentration. We found that the community-specific estimates were heterogeneous. Air pollution effect estimates may be heterogeneous because of many factors, including city-specific differences in pollution characteristics, the use of air conditioning, time spent indoors vs out-doors, and socioeconomic factors.
The estimated effect was relatively robust to estimation with several statistical models and to the degree of confounding adjustment for seasonality, long-term trends, and temperature. The results indicate a substantial health burden from ozone pollution. For example, according to our national average estimate from the constrained distributed-lag model, a 10-ppb increase in daily ozone would correspond to an additional 319 (95% PI, 168-472) annual premature deaths for New York City and 3767 (95% PI, 1976-5562) premature deaths annually for the 95 urban communities, based on mortality data from 2000. This value is probably an underestimate of the total mortality burden from such an increase in ozone because it accounts for only the short-term effects. Further, we found a relationship between mortality and ozone at pollution levels below the current regulatory standard. Our analysis focused on 95 large urbanized areas, although rural communities may also experience elevated ozone levels, especially because of large biogenic emissions of volatile organic compounds and the movement of ozone and ozone precursors from other regions.
Our study resolves inconsistencies in the findings of previous time-series studies of ozone and mortality. The national average estimate was comparable to those from other pooled analyses, including meta-analyses and other, smaller multicity studies. To compare results across these studies, which have used diverse metrics for ozone exposure, we converted the estimates from all of the studies to a common metric, the daily average. Although the relationship between different ozone concentration metrics can vary by location, we used ratios of 2.5 and 1.33 to convert estimates according to the daily 1-hour maximum and 8-hour maximum, respectively, to the daily average, as has been done by others.46,47
Earlier multicity time-series studies of ozone and mortality have estimated a broad range of effects. A 10-ppb increase in daily ozone was associated with estimated increases in daily mortality of 2.84% (95% PI, 0.95%-4.77%) for 4 European cities,48 0.61% (95% PI, −0.38% to 1.60%) for 7 Spanish cities,49 1.40% (95% PI, 0.68%-2.12%) for 6 French cities,50 and 0.43% (95% PI, 0.23%-0.63%) for 80 US urban centers from 1987 to 199422; however, a negative, non-statistically significant association was reported for 7 major Korean cities for 1991-1997.51
Recent meta-analyses were reported by Thurston and Ito,47 who combined results of 16 studies and considered differences in their approaches to the modeling of weather; Levy et al,46 who used 4 US studies based in Cook County, Illinois, and Philadelphia; Stieb et al,52,53 who extracted results from 109 single- and multicity studies for random effects pooling; and Anderson et al,54 who conducted a meta-analysis of ozone and PM as part of a World Health Organization project. The overall estimates from the meta-analysis studies, expressed as the percentage increase in daily mortality for a 10-ppb increase in daily ozone, are 0.89% (95% CI, 0.56%-1.22%)47; 1.37% (95% CI, 0.78%-1.96%),47 considering only studies that allow nonlinear associations between temperature and mortality; 0.98% (95% CI, 0.59%-1.38%)46; 1.12% (95% CI, 0.32%-1.92%)52,53; and 0.78% (95% CI, 0.39%-1.18%).54 Our distributed-lag model’s estimate was 0.52% (95% PI, 0.27%-0.77%) for a 10-ppb increase in the previous week’s ozone levels, whereas our estimates for a single day’s lag was 0.25% (95% PI, 0.12%-0.39%) and 0.18% (95% PI, 0.06%-0.30%) for a 10-ppb increase in the same day’s and previous day’s ozone concentrations, respectively. The lower value estimated by our model could be due to publication bias in the single-city studies that are incorporated into the meta-analyses. Because the same statistical approach was applied to time-series data from the 95 large US urban communities, our results are not subject to publication bias.
A key advance in our study is the use of distributed-lag models, rather than models that estimate the effect of a single day or several days at a particular lag. Using single-day lagged models and the distributed-lag approach, we found statistically significant associations between ozone levels on the preceding days (primarily the current day and 2 previous days) and daily mortality. This temporal pattern of effect would be anticipated for ozone, which produces acute inflammatory responses in the lung; adaptation of this inflammatory response with several days of repeated exposure has been demonstrated.55,56 Although the temporal dynamics of the underlying processes linking ozone exposure to increased mortality may differ from those of the inflammatory response, inflammation has been postulated as having a central role in the increased mortality and morbidity associated with ozone.
Several groups within the population have been considered at increased risk from ozone exposure, including older persons and those with underlying chronic heart and lung diseases. We did not find evidence of significantly greater risk for these 2 groups; the estimated increments in risk were similar across age groups and for total mortality and cardiorespiratory mortality. However, this lack of evidence for increased susceptibility should be interpreted in the context of effect modification on the relative risk scale in the statistical models that were used. In these models, higher underlying mortality rates are increased multiplicatively by the effect of ozone, implying a substantially greater absolute effect of ozone in older persons or those with cardiac or pulmonary diseases. Because the older population has a larger baseline mortality rate than the general population, the same relative rate estimate for the older and the general populations leads to a larger number of extra deaths for the elderly.
One critical concern is the extent to which effect estimates may be confounded by either temperature or other pollutants. In the communities included in the present analysis, the concentration of ozone was not correlated with concentrations of PM10. This lack of correlation and the stability of the ozone estimate with inclusion of PM10, and vice versa, in the models provide strong evidence against confounding of the effects of these 2 pollutants. The ozone and mortality results do not appear to be confounded by temperature, as evidenced by analyses using subsets of the data at various temperature levels and periods.
However, the estimated effect of ozone, although robust to the adjustment for PM10, may still reflect the risk from the photochemical pollution mixture more generally. Atmospheric photochemistry produces several hazardous pollutants, in addition to ozone, such as peroxyacyl nitrates.18 Ozone may act as a surrogate indicator for this highly complex and geographically variable mixture and is likely to be an imperfect measure of potential toxicity. The degree to which ozone functions as a surrogate for other pollutants or the pollutant mixture in general, and thereby misclassifies toxicity, may vary across locations and depend on the mix of sources and meteorologic factors. Although statistically significant relationships were identified for all ozone concentration metrics considered, the analysis did not identify a particular metric as the optimum predictor of mortality.
Ozone pollution is now widespread in urban areas in the United States and many other countries. Its rise reflects primarily increased numbers of motor vehicles and miles traveled; vehicle emissions are a major source of precursor hydro carbons and nitrogen oxides. In the United States, more than a hundred areas are not in compliance with the 8-hour NAAQS for ozone, with the most extreme violations in California.2 Our findings, interpreted in the context of the already extensive epidemiologic and toxicologic evidence on ozone toxicity, indicate that this widespread pollutant adversely affects mortality, in addition to other health effects that have been associated with ozone.4-6 The consequences of control strategies for public health can be tracked with the methods and databases described in this report.
Acknowledgment
We thank Roger Peng, PhD, for his assistance.
Funding/Support: Funding for Drs Bell, Dominici, and McDermott was provided by the US Environmental Protection Agency (EPA 3D-6867-NAEX). Funding for Drs Dominici, Samet, and Zeger was also provided by the National Institute for Environmental Health Sciences (NIEHS; ES012054-01) and by the NIEHS Center in Urban Environmental Health (P30 ES 03819).
Role of the Sponsors: None of the funding agencies played any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Bell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bell, Zeger, Samet, Dominici.
Acquisition of data: McDermott, Zeger.
Analysis and interpretation of data: Bell, McDermott, Zeger, Dominici.
Drafting of the manuscript: Bell, Dominici.
Critical revision of the manuscript for important intellectual content: Bell, McDermott, Zeger, Samet, Dominici.
Statistical analysis: Bell, McDermott, Zeger, Dominici.
Obtained funding: Zeger, Dominici.
Study supervision: Zeger, Samet, Dominici.
REFERENCES
- 1.US Environmental Protection Agency . Latest Findings on National Air Quality: 2002 Status and Trends. EPA Office of Air Quality Planning and Standards, Emissions, Monitoring, and Analysis Division; Research Triangle Park, NC: 2003. EPA 454/K-03-001. [Google Scholar]
- 2.US Environmental Protection Agency [Accessed October 22, 2004];Green book nonattainment areas for criteria pollutants. Available at: http://www.epa.gov/oar/oaqps/greenbk/
- 3.Haagen-Smit AJ. Chemistry and physiology of Los Angeles smog. Indust Eng Chem. 1952;44:1342–1346. [Google Scholar]
- 4.Dockery DW. Pope CA III. Acute respiratory effects of particulate air pollution. Annu Rev Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- 5.Lippmann M. Health effects of tropospheric ozone: review of recent research findings and their implications to ambient air quality standards. J Expo Anal Environ Epidemiol. 1993;3:103–129. [PubMed] [Google Scholar]
- 6.Thurston GD, Ito K. Epidemiological studies of ozone exposure effects. In: Holgate ST, Samet JM, Koren HS, Maynard RL, editors. Air Pollution and Health. Academic Press; San Diego, Calif: 1999. pp. 485–510. [Google Scholar]
- 7.Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society Health effects of outdoor air pollution. Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 8.Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society Health effects of outdoor air pollution: part 2. Am J Respir Crit Care Med. 1996;153:477–498. doi: 10.1164/ajrccm.153.2.8564086. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg MS, Burnett RT, Brook J, et al. Associations between daily cause-specific mortality and concentrations of ground-level ozone in Montreal, Quebec. J Epidemiol. 2001;154:817–826. doi: 10.1093/aje/154.9.817. [DOI] [PubMed] [Google Scholar]
- 10.Hoek G, Schwartz JD, Groot B, Eilers P. Effects of ambient particulate matter and ozone on daily mortality in Rotterdam, the Netherlands. Arch Environ Health. 1997;52:455–463. doi: 10.1080/00039899709602224. [DOI] [PubMed] [Google Scholar]
- 11.Hong Y-C, Lee J-T, Kim H, Kwon H-J. Air pollution: a new risk factor in ischemic stroke mortality. Stroke. 2002;33:2165–2169. doi: 10.1161/01.str.0000026865.52610.5b. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Thurston GD. Daily PM10/mortality associations: an investigation of at-risk subpopulations. J Expo Anal Environ Epidemiol. 1996;6:79–95. [PubMed] [Google Scholar]
- 13.Anderson HR, Bremner SA, Atkinson RW, Harrison RM, Walters S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom. Occup Environ Med. 2001;58:504–510. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borja-Aburto VH, Loomis DP, Bangdiwala SI, Shy CM, Rascon-Pacheco RA. Ozone, suspended particulates, and daily mortality in Mexico City. Am J Epidemiol. 1997;145:258–268. doi: 10.1093/oxfordjournals.aje.a009099. [DOI] [PubMed] [Google Scholar]
- 15.Hong Y-C, Leem J-H, Ha E-H, Christiani DC. PM10 exposure, gaseous pollutants, and daily mortality in Ichon, South Korea. Environ Health Perspect. 1999;107:873–878. doi: 10.1289/ehp.99107873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH. Air pollution and infant mortality in Mexico City. Epidemiology. 1999;10:118–123. [PubMed] [Google Scholar]
- 17.Prescott GJ, Cohen GR, Elton RA, Fowkes FG, Agius RM. Urban air pollution and cardiopulmonary ill health. Occup Environ Med. 1998;55:697–704. doi: 10.1136/oem.55.10.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. John Wiley & Sons; New York, NY: 1998. [Google Scholar]
- 19.US Environmental Protection Agency National Ambient Air Quality Standards for ozone, final rule. Federal Register. 1997;62:38855–38896. [Google Scholar]
- 20.Dominici F, Daniels M, Zeger SL, Samet JM. Air pollution and mortality: estimating regional and national dose-response relationships. J Am Stat Assoc. 2002;97:100–111. [Google Scholar]
- 21.Dominici F, McDermott A, Zeger SL, Samet JM. National maps of the effects of particulate matter on mortality. Environ Health Perspect. 2003;111:39–43. doi: 10.1289/ehp.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health Effects Institute (HEI) Revised Analyses of Time-Series Studies of Air Pollution and Health. HEI; Cambridge, Mass: 2003. [Google Scholar]
- 23.Samet JM, Dominici F, Zeger SL, Schwartz J, Dockery DW. The National Morbidity, Mortality, and Air Pollution Study Part I: Methods and Methodologic Issues. HEI; Cambridge, Mass: 2000. [PubMed] [Google Scholar]
- 24.Samet JM, Zeger SL, Dominici F, et al. The National Morbidity, Mortality, and Air Pollution Study Part II: Morbidity and Mortality From Air Pollution in the United States. HEI; Cambridge, Mass: 2000. [PubMed] [Google Scholar]
- 25.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz J, Zanobetti A, Bateson T. Revised Analyses of Time-Series Studies of Air Pollution and Health. Cambridge, Mass; HEI: 2003. Morbidity and mortality among elderly residents of cities with daily PM measurements; pp. 25–58. [Google Scholar]
- 27.Dominici F, Samet JM, Zeger SL. Combining evidence on air pollution and daily mortality from the 20 largest US cities: a hierarchical modeling strategy. J R Stat Soc [Ser A] 2000;163:263–302. [Google Scholar]
- 28.Zanobetti A, Wand MP, Schwartz J, Ryan LM. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics. 2000;1:279–292. doi: 10.1093/biostatistics/1.3.279. [DOI] [PubMed] [Google Scholar]
- 29.Braga AL, Zanobetti A, Schwartz J. The time course of weather-related deaths. Epidemiology. 2001;12:662–667. doi: 10.1097/00001648-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Braga AL, Zanobetti A, Schwartz J. The lag structure between particulate air pollution and respiratory and cardiovascular deaths in 10 US cities. J Occup Environ Med. 2001;43:927–933. doi: 10.1097/00043764-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Goodman PG, Dockery DW, Clancy L. Cause-specific mortality and the extended effects of particulate pollution and temperature exposure. Environ Health Perspect. 2004;112:179–185. doi: 10.1289/ehp.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11:320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Zanobetti A, Schwartz J, Samoli E, et al. The temporal pattern of mortality responses to air pollution. Epidemiology. 2002;13:87–93. doi: 10.1097/00001648-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Zanobetti A, Schwartz J, Samoli E, et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ Health Perspect. 2003;111:1188–1193. doi: 10.1289/ehp.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlin BP, Louis TA. Bayes and Empirical Bayes Methods for Data Analysis. 2nd ed. Chapman & Hall/CRC; New York, NY: 2000. [Google Scholar]
- 36.DuMouchel WH, Harris JE. Bayes methods for combining the results of cancer studies in humans and other species. J Am Stat Assoc. 1983;78:293–308. [Google Scholar]
- 37.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. 2nd ed. Chapman & Hall/CRC; New York, NY: 2003. [Google Scholar]
- 38.Lindley DV, Smith AFM. Bayes estimates for the linear model. J R Stat Soc Series B. 1972;34:1–41. [Google Scholar]
- 39.National Climatic Data Center . NCDC Surface Airways. EarthInfo Inc.; Boulder, Colo: CD database. [Google Scholar]
- 40.National Morbidity Mortality and Air Pollution Study (NMMAPS) database [Accessed October 28, 2004]; Available at: http://www.ihapss.jhsph.edu/
- 41.Kelsall JE, Samet JM, Zeger SL, Xu J. Air pollution and mortality in Philadelphia, 1974-1988. Am J Epidemiol. 1997;146:750–762. doi: 10.1093/oxfordjournals.aje.a009351. [DOI] [PubMed] [Google Scholar]
- 42.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. Chapman & Hall/CRC; New York, NY: 1989. [Google Scholar]
- 43.Everson P. Two-Level Normal Independent Sampling Estimation (TLNise) Swarthmore College; Swarthmore, Pa: 2000. [Google Scholar]
- 44.Insightful Corporation [Accessed October 28, 2004]; Available at: http://www.insightful.com/products/splus/default.asp.
- 45.Dominici F, McDermott A, Zeger SL, Samet JM. On the use of generalized additive models in time-series of air pollution and health. Am J Epidemiol. 2002;156:193–203. doi: 10.1093/aje/kwf062. [DOI] [PubMed] [Google Scholar]
- 46.Levy JI, Carrothers TJ, Tuomisto JT, Hammitt JK, Evans JS. Assessing the public health benefits of reduced ozone concentrations. Environ Health Perspect. 2001;109:1215–1226. doi: 10.1289/ehp.011091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thurston GD, Ito K. Epidemiological studies of acute ozone exposures and mortality. J Expo Anal Environ Epidemiol. 2001;11:286–294. doi: 10.1038/sj.jea.7500169. [DOI] [PubMed] [Google Scholar]
- 48.Touloumi G, Katsouyanni K, Zmirou D, et al. Short-term effects of ambient oxidant exposure on mortality: a combined analysis within the APHEA Project. Am J Epidemiol. 1997;146:177–185. doi: 10.1093/oxfordjournals.aje.a009249. [DOI] [PubMed] [Google Scholar]
- 49.Saez M, Ballester F, Barceló MA, et al. A combined analysis of the short-term effects of photochemical air pollutants on mortality within the EM-ECAM project. Environ Health Perspect. 2002;110:221–228. doi: 10.1289/ehp.02110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Tertre A, Quénel P, Eilstein D, et al. Short-term effects of air pollution on mortality in nine French cities. Arch Environ Health. 2002;57:311–319. doi: 10.1080/00039890209601414. [DOI] [PubMed] [Google Scholar]
- 51.Lee JT, Kim H, Hong YC, et al. Air pollution and daily mortality in seven major cities of Korea, 1991-1997. Environ Res. 2000;84:247–254. doi: 10.1006/enrs.2000.4096. [DOI] [PubMed] [Google Scholar]
- 52.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc. 2002;52:470–484. doi: 10.1080/10473289.2002.10470794. [DOI] [PubMed] [Google Scholar]
- 53.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: update in relation to the use of generalized additive models. J Air Waste Manag Assoc. 2003;53:258–261. doi: 10.1080/10473289.2003.10466149. [DOI] [PubMed] [Google Scholar]
- 54.Anderson HR, Atkinson RW, Peacock JL, Marston L, Konstantinou K. Meta-analysis of Time-Series Studies and Panel Studies of Particulate Matter (PM) and Ozone (O3) World Health Organization; Copenhagen, Denmark: 2004. [Google Scholar]
- 55.Folinsbee LJ, Horstman DH, Kehrl HR, Harder S, Abdul-Salaam S, Ives PJ. Respiratory responses to repeated prolonged exposure to 0.12 ppm ozone. Am J Respir Crit Care Med. 1994;149:98–105. doi: 10.1164/ajrccm.149.1.8111607. [DOI] [PubMed] [Google Scholar]
- 56.Frank R, Liu MC, Spannhake EW, et al. Repetitive ozone exposure of young adults: evidence of persistent small airway dysfunction. Am J Respir Crit Care Med. 2001;164:1253–1260. doi: 10.1164/ajrccm.164.7.2010043. [DOI] [PubMed] [Google Scholar]