Abstract
Functional magnetic resonance imaging (fMRI) has become the dominant means of measuring behavior-related neural activity in the human brain. Yet the relation between the blood oxygen-level dependent (BOLD) signal and underlying neural activity remains an open and actively researched question. A widely accepted model, established for sensory neo-cortex, suggests that the BOLD signal reflects peri-synaptic activity in the form of the local field potential rather than the spiking rate of individual neurons. Several recent experimental results, however, suggest situations in which BOLD, spiking, and the local field potential dissociate. Two different models are discussed, based on the literature reviewed to account for this dissociation, a circuitry-based and vascular-based explanation. Both models are found to account for existing data under some testing situations and in certain brain regions. Because both the vascular and local circuitry-based explanations challenge the BOLD-LFP coupling model, these models provide guidance in predicting when BOLD can be expected to reflect neural processing and when the underlying relation with BOLD may be more complex than a direct correspondence.
Keywords: fMRI, electrophysiology, single neurons, local field potential, EEG, memory hippocampus, vasculature, perception, neocortex
Introduction: fMRI and Cognitive Neuroscience
Functional magnetic resonance imaging (fMRI) has become a mainstay of research in both clinical and cognitive neuroscience and is currently the dominant paradigm for assessing behavior-related brain physiological changes in humans. Yet there still remains much for us to understand about this relatively new methodology. Perhaps most importantly, we are still learning what aspect of neural processing the blood oxygen level-dependent (BOLD) response, the signal that forms the basis of fMRI, measures. A better understanding of the relationship of the BOLD signal to underlying neurophysiology is critical to how we interpret fMRI because this information tells us in which situations the BOLD signal serves as a proxy for neural activity. Based on a series of studies conducted in sensory cortices of lower mammals, evidence overwhelming supports the idea that the BOLD signal correlates strongly, in many cases, with the underlying local field potential (LFP), a measure, in part of peri-synaptic activity (Logothetis, 2008). Based on this widely accepted model, referred to here as the BOLD-LFP coupling model, it is generally assumed that neural activity indirectly drives the BOLD signal. Further, while typically not explicitly stated, it is often assumed in cognitive neuroscience that the relation between BOLD and peri-synaptic activity is both region and behavior independent (e.g., Huettel et al., 2004).
Several studies, however, note exceptions to the idea that the BOLD signal typically reflects LFPs. In some cases, the BOLD signal also correlates with the activity of single neurons. Some recent studies also highlight situations in which the BOLD signal decouples with LFPs as well with single neuron spiking activity. In this review, I will first provide a summary of the BOLD-LFP coupling model, detailing the evidence for this model. I will then describe situations in which the BOLD signal decouples from LFPs and describe how some of these situations might arise. I discuss two different models to account for situations in which the BOLD signal decouples with neural activity: a local circuitry-based model and vascular-based model. These two models present a challenge to the BOLD-LFP coupling model, suggesting areas for further investigation as well areas for caution with interpretation of fMRI results.
What the BOLD Signal Means
Early positron emission tomography (PET) studies, which employed radiolabeled compounds to track the presence of cerebral blood flow (CBF) and oxygen metabolism (CMRO2) in the brain, demonstrated that cerebral blood flow (CBF) significantly overshot oxygen metabolism (Fox and Raichle, 1986; Fox et al., 1988). The CBF/ CMRO2 overshoot provided a mechanism whereby MRI could detect neural activity via field inhomogeneities produced when deoxygenated hemoglobin exited an active brain area (Ogawa et al., 1992). Subsequent calibrated fMRI experiments, which independently measure CMR02 and CBF (discussed in more detail later), suggested a more modest coupling CBF/ CMR02 coupling ration in neocortex in the range of 2–4.5, with CBF at least doubling the rate of CMRO2 (Davis et al., 1998; Hoge et al., 1999; Leontiev et al., 2007; Pasley et al., 2007) but supported the idea that CBF typically outpaced CMRO2. Because fMRI is based on signal changes present largely in the venules and not the capillaries (Frahm et al., 1994), at least at lower (<= 3 T) field strengths, both changes in metabolism and changes in blood flow therefore contribute to the BOLD signal.
Because BOLD measures a combination of CBF and CMRO2 (and cerebral blood volume ([CBV] because the amount of deoxy-hemoglobin is relevant to BOLD), an additional issue concerns what aspects of brain metabolism these subcomponents of the BOLD signal, and thus the BOLD signal as a whole, reflect. One popular model suggested the possibility of a compartmentalization of lower energy-producing glycolisis in glia and higher energy-producing oxidative phosphorylation in neurons, involving shuttling of lactate from glia to neurons to power oxidative metabolism (Magistretti and Pellerin, 1999; Huettel et al., 2004; Raichle and Mintun, 2006). While glia play a role in recycling of glutamate released into the synaptic cleft (Eriksson et al., 1995; Sibson et al., 1997) and CMRO2 often strongly correlates with neural activity (Smith et al., 2002; Hyder, 2004), the story with regard to compartmentalization of glycolysis and oxidative phosphorylation is likely more complex (Hyder, 2004; Oz et al., 2004). C13 magnetic resonance spectroscopy (MRS) studies, which track the uptake of radiolabelled carbon into various metabolic products through C13-glucose (Henry et al., 2006), suggest that oxidative phosphorylation may also occur in the glia, thus providing critical ATP for fast conversion glutamate to glutamine during high-levels of synaptic signaling (Gruetter et al., 2001; Oz et al., 2004). Also, neurons utilize anaerobic glycolysis during periods of intense activity, presumably in part due to lower O2 supplies because of the uncoupling of CMRO2 and CBF (Fox et al., 1988; Malonek and Grinvald, 1996; Shulman et al., 2001). Thus, while debate remains regarding how Cglu/CMRO2/CBF/CMRO2 interrelate in the brain, it is clear that both neurons and glia utilize anaerobic glycolysis and oxidative phosphylation to supply needed energy for their processing.
A final question then regards the types of processing that form the basis of neural and glia metabolic energy utilization. Based on the energetic demands of maintaining neural and glial resting potentials, signaling via glutamate, and energy required to propagate an action potential, mathematical analytic derivations and simulations suggest that post-synaptic potentials and action potentials demand the bulk of metabolic demands (Attwell and Laughlin, 2001; Attwell and Iadecola, 2002). Attwell and Iadecola estimated that approximately 74% of the energy budget of the brain would be devoted to post-synaptic potentials, 10% to action potentials, 8% for the resting potential, and 7% for activity at presynaptic terminals. Attwell and Laughlin (2001) further calculated that the majority of metabolic energy exhausted during synaptic signaling is spent restoring resting ion gradients with the ATP powered Na+/K+ pump (Attwell and Laughlin, 2001). These theoretical predictions then suggested the glucose utilization primarily reflects in the work involved in synaptic signaling and that metabolic measures of brain activity should correlate most strongly with measures that reflect synaptic processing rather than spike rate alone.
BOLD reflects LFPs and not spike rate
While the exact composition of the LFP remains under investigation, the LFP is generally thought to consistent of excitatory/inhibitory postsynaptic potentials (EPSPs/IPSPs) as well as dendritic afterhyperpolarizations and intrinsic membrane oscillations (Logothetis, 2003). Thus, LFPs represent a combination of post-synaptic and pre-synatpic activity at multiple neurons, and thus is most accurately described as “peri” synaptic activity. In attempt to empirically determine whether spike rate or LFPs correlated with the BOLD signal, Logothetis et al. constructed a recording device that allowed simultaneous acquisition of fMRI, LFPs, and the spiking activity of neurons in monkey visual cortex. Anesthetized monkeys viewed contrast gratings while BOLD and electrophysiological activity was recorded in primary visual cortex. Electrophysiological activity was filtered into two different components, high-frequency multi-unit (MUA) activity and lower-frequency LFPs (40–130 Hz). By correlating the simultaneously recorded LFPs and neural firing rate activity with the BOLD signal, Logothetis et al. reported a strong correlation between BOLD and LFPs and robust but slightly weaker correlation between BOLD and MUA (Logothetis et al., 2001). The fact that the LFP accounted for significantly greater amounts of variance across recording sites suggested that the LFP correlated better with the BOLD signal than spike rate (Logothetis and Wandell, 2004). Indeed, when individual recording sites were subsequently inspected, dissociations between spiking and the LFP always resulted in a strong correlation between the BOLD signal and the LFP and not spike rate. These data suggested that correlations between spike rate and LFPs contributed to the correlation between BOLD and spiking activity.
Thus, the LFP was taken to be a better representation of the BOLD signal compared to spiking activity (Logothetis, 2003). Consistent with this idea, subsequent studies demonstrated that abolition of spiking in visual cortex, either through manipulation of pharmacology (Rauch et al., 2008) or stimulus characteristics (Viswanathan and Freeman, 2007), could still lead to a robust BOLD/CMRO2 and LFP correlation. These data bolstered the idea that spiking was not necessary for a robust BOLD response and that in fact, spiking occurred secondarily as a result of drive by the LFP. Later studies also suggested that significant (but weaker) correlations between BOLD and LFPs could be obtained in the visual cortex of awake behaving monkeys as well (Goense and Logothetis, 2008). Furthermore, subsequent studies also showed that decreases in LFPs and MUA correlated with decreases in the BOLD signal in visual cortex (Shmuel et al., 2006) and decreases in LFPs (but not MUAs) correlated with decreases in blood oxygenation in sensory cortex (Devor et al., 2007)
A series of elegant experiments using simultaneous CBF/LFP/MUA measurements further supported the idea that aspects of the BOLD signal strongly correlate with LFPs and not neural firing rate. Mathiesen 1998; 2000 simultaneously recorded CBF, LFP, and neural firing in rat cerebellum, taking advantage of circuitry present there. Stimulation of parallel fibers caused monosynaptic excitation of Purkinje cells and concomitant disynaptic inhibition of the same neurons via inhibitory basket cells. This mechanism allowed simultaneous activation of synaptic activity via parallel fibers yet inhibition of spikes via basket cells. The net result was an increase in CBF linked to increases in parallel fiber-induced synaptic activity despite the presence of any spiking activity (Mathiesen et al., 1998; Mathiesen et al., 2000; Thomsen et al., 2004). Other groups have also demonstrated strong correlations between CBF (a critical part of the BOLD signal) and evoked-potential/LFPs and related glutamatergic activity in sensory and visual cortex (Brinker et al., 1999; Kayser et al., 2004; Ureshi et al., 2004; Hewson-Stoate et al., 2005; Niessing et al., 2005; Gsell et al., 2006; Martin et al., 2006; Huttunen et al., 2008; Masamoto et al., 2008).
It is important to note that in the original Logothetis et al. experiments, spike rate accounted for significant amounts of the variance in the BOLD signal across electrode sites. Spiking activity and LFPs in many cases are strongly correlated in sensory cortices, leading to correlations between BOLD and spike rate. This likely arose due to linear summation of peri-synaptic activity resulting in increases in spike rate. In support of this idea, intracellular recordings from V1 showed that synaptic activity tended to summate linearly in visual cortex (Jagadeesh et al., 1993). This predicted a strong correlation between spiking and LFPs in visual cortex, a finding confirmed in extracellular recording studies as well (Nase et al., 2003; Henrie and Shapley, 2005; Spinks et al., 2008) and also demonstrated in primary auditory cortex (Mukamel et al., 2005). Consistent with the occasional strong correlations observed between spike rate and LFPs in sensory cortex, several studies in fact have shown strong correlations between BOLD and spike rate (Rees et al., 2000; Smith et al., 2002; Hyder, 2004; Kim et al., 2004; Mukamel et al., 2005; Kida et al., 2006; Nir et al., 2007). These data suggest that in some cases, BOLD also provides a measure of spike rate due to correlations between LFPs and spike rate. Correlations between spike rate and LFPs, though, are likely particularly dependent on the input into a region and specific circuitry stimulated due to the heterogeneous nature of the LFP (Mitzdorf, 1985) and thus BOLD and spike rate correlations cannot typically be assumed.
Given that LFPs and spikes may correlate under some conditions, this suggests that the BOLD signal may actually represent spike rate in some situations (Heeger and Ress, 2002). An important question then is to what extent the LFP can serve as a proxy for spike rate and to what extent the two signals are independent. Spike rate and LFPs may correlate in many cases in sensory cortex but may dissociate in some cases outside of this region due to differences in local circuitry. Two studies investigated the relation between spike rate and LFPs in the human hippocampus using intracranial recordings in patients with pharmacologically intractable epilepsy. During encoding and retrieval of spatial landmarks (Ekstrom et al., 2007) and coding of specific items (Kraskov et al., 2007), the LFP and spiking are dissociated, showing little consistent relationship across recording electrodes (but see: Manning et al., 2009). Kreiman et al. have also reported dissociations between LFPs and spikes in infero-temporal cortex of monkeys (Kreiman et al., 2006).
One reason why one might not expect a correlation between spike rate and LFPs in a region such as the hippocampus is that the hippocampus tends to use sparse, compared to distributed coding, particularly during coding of specific stimuli (Marr, 1971; Waydo et al., 2006). Also, neural responses in the hippocampus show little anatomical topography (Redish et al., 2001), in contrast to sensory cortex (Mountcastle, 1997), suggesting that spiking activity would be evenly distributed through the hippocampus and lack the focal arrangement to show a clear summation in the LFP. But what about a situation in which sufficiently large numbers of hippocampal neurons are active during a task? Disentangling aspects of peri-synaptic activity in the hippocampal LFP may be particularly difficult due the high degree of inhibitory and recurrent circuitry present there (Amaral and Lavenex, 2007; Buzsaki et al., 2007; Angenstein et al., 2009). This “circuitry” based argument suggests that even if principle cell firing rate is sufficiently high and distributed, the LFP is unlikely to reflect neural firing rate alone because of the additional contributions of extra-hippocampal input and inhibitory local circuitry.
What happens when three signals dissociate and why: Dissociations between the BOLD signal, spiking activity, and local field potentials
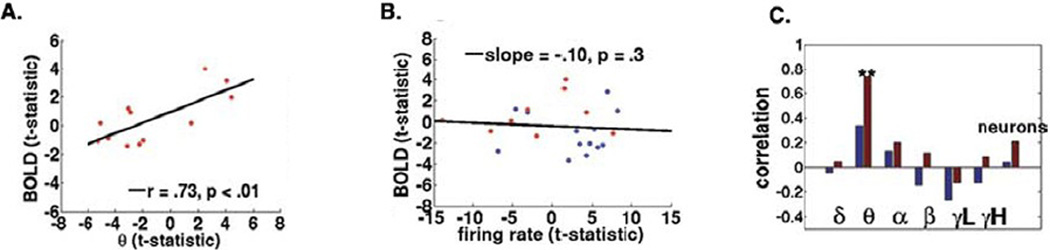
As argued above, situations arise in which spike rate and LFPs dissociate, due to coding properties and/or aspects of the anatomical layout of a brain region. But could some of these properties also contribute to dissociations between neural activity and BOLD? To address this issue, we performed fMRI on patients with pharmacologically intractable epilepsy prior to implantation with depth electrodes during a spatial navigation task at high-resolution (1.6 X 1.6 X 3mm). Patients repeated the task (with some variations to control for signal habituation) following implantation with depth electrodes (Ekstrom et al., 2009). The firing rate of a significant number of neurons and the LFPs of a large number of sites were modulated by the task and the BOLD signal showed significant clusters of activation in both hippocampus and the parahippocampal region (PHR). Across 27 electrode recording sites and 6 patients, the BOLD signal strongly correlated (r2=.49) with theta-band LFPs in the PHR; the BOLD signal and spiking activity, though, showed no correlation there (Figure 1). However, the BOLD signal in the hippocampus showed only a weak correlation with LFPs and no significant correlation with spiking activity. The finding of a positive correlation between the BOLD signal and LFPs in the PHR is perhaps unsurprising and was also reported recently by Ojemann et al. in human temporal cortex (Ojemann et al., 2009), consistent with the BOLD-LFP coupling model. But why then should the hippocampal BOLD signal show no correlation with LFPs while parahippocampal BOLD correlated well with LFPs?
Figure 1. Correlations Between Parahippocampal BOLD Activity and LFPs but not between Hippocampal BOLD Activity and LFPs. Neither Parahippocampal nor Hippocampal BOLD Signal Correlated with Spike Rate.
A) Maximal BOLD t-statistic vs. maximal theta-band t-statistic for the parahippocampal region (PHR) for electrode ROI in navigation vs. control comparison in 27 electrode recordings from 6 patients with implanted depth electrodes; the correlation coefficient was .73 (r2 = .49). B) Maximal BOLD t-statistic vs. maximal spike rate t-static; the correlation was not significant for spike rate in PHR (red) and hippocampus (blue). C) While there was a weak correlation between hippocampal BOLD and LFPs, this correlation was only significant for parahippocampal BOLD and LFPs. The only LFP-band showing a significant correlation with the BOLD signal was the theta-band LFP.
A study by Angenstein et al. in the rat hippocampus also reported dissociations between spike rate, BOLD, and synaptic input and provides some additional clues into why the three signals might decouple (Angenstein et al., 2009). Angenstein et al. stimulated rat hippocampus at different levels via the perforant path and measured spiking activity (via the population spike) in the dentate gyrus and subiculum. Because the perforant path provides the major input into the hippocampus, stimulating its fibers thus provided an experimentally manipulatable measure of synaptic input. The same levels of perforant path stimulation were also delivered during fMRI of the rat hippocampus. Intriguingly, different levels of input often resulted in different BOLD and population spike responses. Stimulation on early trials resulted in an increased population spike in dentate gyrus yet a decrease in BOLD there. In contrast, stimulation on later trials often resulted in decreased population spikes but increased BOLD responses. Different patterns of synaptic input also mapped inconsistently onto different levels of BOLD responses in different subregions. The authors note, as discussed above, the extensive recurrent, intrinsic connections in the hippocampus. Specifically, perforant path stimulation, although resulting in stimulation of granule cells in dentate gyrus, also stimulates principle cells and inhibitory interneurons in CA3 and CA1. The authors argue that the LFP, which can be thought of a measure of synaptic input into a region, and spiking activity, which can be thought of as the output of a region (Logothetis, 2003), may thus dissociate under conditions of extensive local circuitry, as is present in the hippocampus.
The “local circuitry” model thus provides a possible explanation for why BOLD, LFPs, and spiking activity might dissociate in the hippocampus. In an area such as the hippocampus, with both extensive input from other brain regions as well as extensive inhibitory and excitatory reciprocal connections, disentangling synaptic based input and neural firing rate may be difficult (Buzsaki et al., 2007). This is particularly true when considering inhibitory connections because inhibitory interneurons will demand metabolic energy yet their activity may inhibit observable electrophysiological activity (Ackermann et al., 1984; Buzsaki et al., 2007). Given that the theta-rhythm, a prominent oscillation in the hippocampus, is generated in part by interneuron activity but also by cholinergic modulation (Bland, 1986), it does not seem surprising that the BOLD signal may show a more complex relationship with underlying electrophysiological activity in the hippocampus than often observed in other brain regions (see also: Sanchez-Arroyos et al., 1993; Uecker et al., 1997 for early examples of a dissociation between theta activity and metabolism).
While the local circuitry-based explanation for the dissociation of BOLD, LFPs, and spike rate in the hippocampus is appealing, there may be other possible explanations for why the BOLD signal, LFPs, and spike rate dissociate in the hippocampus. Both our and the Angenstein et al. study noted negative changes in the hippocamal BOLD signal were often accompanied by increased spike rate or synaptic input (in the Ekstrom et al study, the LFP; in Angenstein et al. study, stimulation of the perforant path). A recent study by Schridde et al in rats, discussed in more detail shortly, also noted hippocampal negative BOLD changes uncorrelated with underlying increases in neural activity (Schridde et al., 2008). Negative BOLD changes, though, seem difficult to account for based solely on the local circuitry model as it does not provide a direct explanation for this phenomenon. For example, the local circuitry model does not explain how increases in interneuron activity result in decreases in BOLD.
Both positive and negative fMRI activations are derived through comparison of an experimental condition against a control condition. When activity during a control condition exceeds that of the experimental condition, “negative” activations result. Thus, the baseline condition is important for determining the types of activations observed. Hippocampal negative activations are frequently reported during memory encoding and retrieval tasks (Rekkas et al., 2005; Axmacher et al., 2007; Shipman and Astur, 2008; Axmacher et al., 2009). Furthermore, some have argued that positive hippocampal activations are difficult to obtain, as a comparison of a memory task against resting baseline often results in negative, not positive, hippocampal activitions (Stark and Squire, 2001; Shipman and Astur, 2008). The difficulty in finding positive hippocampal activations during memory tasks in humans (when compared with a rest baseline) has led to both neurovascular and behavioral explanations. The hippocampus might be constitutively active due to its role in the default network (Raichle et al., 2001) or it may be particularly susceptible to rumination and behavioral distraction (Fletcher et al., 1995). The neurovascular explanation would appear to be a better explanation of existing data in the hippocampus. Comparable baseline tasks in human hippocampal single neuron studies and LFP studies (looking at a blank screen between stimuli) lead to little increases in neural firing rate relative to viewing stimuli (Quiroga et al., 2005; Kraskov et al., 2007), suggesting a decoupling of BOLD with at least single neuron firing during rest. Also, BOLD signal decreases in the visual cortex correlate with reductions in neural activity in visual cortex (Shmuel et al., 2006), although the studies discussed above do not support the same findings in the hippocampal area. Finally, under some conditions, positive hippocampal BOLD signal changes appear to correlate with increases in LFPs, suggesting the decoupling with neural activity could be unique to hippocampal negative activations (Canals et al., 2008; Englot et al., 2008; Ekstrom et al., 2009; Englot et al., 2009).
The organization of vasculature of the hippocampus offers additional preliminary support for a “vascular-based” explanation of hippocampal processing and the conundrum of both negative activations and BOLD signal-neural activity decouplings. Borowsky and Collins, in a study of the vascularization of the rat brain, found that the capillary density for dentate gyrus was about 50% of neo-cortex. Additional studies suggest some subregions of the hippocampus show particularly low capillary vascularization compared to other subregions (hilus compared to CA3), providing a possible explanation for the enhanced sensitivity to ischemia in this subregion (Lokkegaard et al., 2001; Grivas et al., 2003). The selective vulnerability of the hippocampus generally to anoxia compared to neocortex may also have a vascular explanation (Scharrer, 1940; Bartsch et al., 2007), although the sensitivity of hippocampal cells to glutamatergic toxicity is also a factor (DeReuck et al., 1979; McBain et al., 1990; Duvernoy, 1998). Furthermore, phenomena such as transient global ischemia (Bartsch et al., 2007), the shorter capillary length of hippocampal vasculature compared to neocortex (Borowsky and Collins, 1989), and its more limited blood supply compared to surrounding entorhinal cortex (Van Hoesen, 2002) argue for a sparser and less evolved blood supply to the hippocampus compared to neo-cortex.
The consequences of a sparser blood supply may have implications for the BOLD signal. Periods of high-metabolic activity in the hippocampus could result in oxygen metabolism transiently outmatching the typically observed cerebral blood flow overshoot, leading to a net decrease in the BOLD signal (Buxton et al., 2004). Precisely this situation was recently described by in a study by Schridde et al. in the rat hippocampus. The study looked at both the rat hippocampus and neo-cortex during seizures and recorded CBF, CMRO2, LFPs, and spiking activity. Large increases in hippocampal neural activity were often accompanied by decreases in the BOLD signal. In contrast, comparably large increases in neural activity in neo-cortex were typically accompanied by the expected increases in the BOLD signal. Recordings of hippocampal CBF in the Schridde et al. study suggested that in many cases, CMRO2 often exceeded fresh supplies of blood, as measured with CBF. Note that this situation is the opposite of what is typically observed in sensory cortex, where blood flow typically overshoots metabolism (Fox and Raichle, 1986; Malonek and Grinvald, 1996).
While the BOLD-neural decouplings in the Schridde et al. study arose during abnormal seizure events in the anaesthetized epileptic hippocampus of the rat, memory-encoding studies in normal human volunteers suggest weaker couplings between CBF and metabolism may also arise in the hippocampus compared to neocortex. In a calibrated fMRI study, Restom et al. had subjects perform a scene encoding task and subsequently calculated CMRO2 and CBF rates during the task in the medial temporal lobes (Restom et al., 2008). Restom et al. found that the mean coupling ratio between CBF and CMR02 was about 1.6 – 1.7 in the medial temporal lobes, lower than the mean coupling values reported in sensory cortex, which typically ranged between 2 – 4.5. Thus, even during memory tasks, hippocampal oxygen metabolism may show a weaker coupling with blood flow than in other regions. This would lead to the possibility of a weaker BOLD signal during high-demanding memory tasks. The fact that rest periods result in greater BOLD signal than active memory tasks could be explained by the fact that resting tasks still result in blood flow to the hippocampus (yet little active metabolism), possibly as a result of its role in “default” processes (Raichle et al., 2001). In contrast, demanding memory tasks will result in increased oxygen metabolism, and due to the sparse nature of the hippocampal vasculature, blood flow may match metabolism more closely than typically observed in the neocortex (Figure 2).
Figure 2. Schematic representation of hippocampal BOLD response in frequently studied behavioral tasks.
Three different scenarios are described along with the predicted BOLD signal. All comparisons are made relative to a “true” hippocampal baseline task, or tasks that do not directly require the hippocampus (such as making odd-even number judgments). In the first situation, “rest” results in increased blood flow due to the involvement of the hippocampus in the default network, resulting in increased activity relative to an “inactive” baseline. In the second situation, tasks not involving the hippocampus (such as familiarity judgments) result in no net BOLD change because two inactive tasks result in no net change. In the final situation, a weakly positive BOLD change arises because hippocampal metabolism may outpace blood flow. Note that if situation #1 were used as baseline for situation #3, as applied in many cases previously, hippocampal negative BOLD changes result.
Yet why should recollection, a process well established to involve the hippocampus, result in a comparatively stronger hippocampal BOLD signal than familiarity (Ranganath et al., 2004)? This could be because tasks not directly involving the hippocampus disrupt the default network, resulting in blood flow away from the hippocampus to other brain regions (examples would include making familiarity judgments, thought to involve the perirhinal/parahippocampal cortex). Recollection, though, a hippocampally-based task, recruits neurons and circuitry in the hippocampus, causing increased blood flow to this region. However, due to the vasculature and circuitry of the hippocampus, metabolic demands may outpace the typically-observed cerebral blood flow overshoot (Fox and Raichle, 1986), resulting in greater deo-oxygenated blood and a net decrease in BOLD relative to baseline. However, the net signal appears as an increase relative to no activity during a familiarity task (Figure 2).
While the above-mentioned reasoning would suggest that the vascular-based model is broadly consistent with previous findings on the hippocampus, it will be important to compare the vascular-based model with the local-circuitry model. Comparing predictions of the two models is valuable in determining which model provides the best explanation for the data. If hippocampal vasculature, rather than local circuitry, is responsible for dissociations between BOLD and LFPs, the CBF/CMRO coupling ratio should be weaker in neocortex than hippocampus. This finding has been largely confirmed by Restom et al. but more calibrated fMRI and PET studies are needed to bolster this finding in the hippocampus. Another possible way to test the vascular vs. local circuitry idea involves comparisons within neocortex. Parts of association cortex, which reportedly have a sparser vasculatization than sensory cortex (Harrison et al., 2002), provide an opportunity for testing the vascular vs. local circuitry hypothesis. Areas with sparser vasculature should yield weaker BOLD signals due to blood flow failing to match oxygen metabolism to the extent typically observed in sensory neocortex. Indeed, lower metabolism/blood flow couplings have also been observed in other brain regions (Ances et al., 2008). Another area for comparison involves the presence or absence of negative BOLD changes as the local circuitry model does not provide a direct explanation for the presence of negative activations. Brain regions showing negative activations during a task could be further investigated with fMRI and a comparison made with vascularization and neural activity of this region.
The danger in dissociation: Where to go from here
Dissociations between the BOLD signal and underlying neural activity lead to the question of how one might proceed with imaging and reaching conclusions about brain regions, given many of the concerns cited above. I have attempted to provide two possible explanations for why three-signals may dissociate in a brain region such as the hippocampus. The explanations, however, are perhaps secondary as the basic phenomenon poses a problem for the interpretations of the BOLD signal, at least when considering the BOLD signal as a proxy for neural activity. In Table 1, I have listed studies discussed in detail here as well as other studies not directly addressed and whether they demonstrate dissociations between BOLD/metabolic measures and electrophysiological measures. As can be seen, a significant number of studies show both correlations between the metabolic measures and spike rate (total number of studies, [N]=12) and metabolic measures and LFPs (N=30) under at least some testing conditions. Other studies, though, show dissociations between metabolic measures and spike rate (N=15) and metabolic measures and LFPs (N=13). Several studies (N=6) though demonstrate a three-way dissociation, i.e., dissociations between metabolic measures, LFPs, and spike rate. Note, however, that no studies to date have shown a double dissociation in the same study, e.g., a correlation between metabolic measures and spike rate and a dissociation between metabolic measures and LFPs (the greater number of studies showing dissociations between metabolic measures and LFPs compared to metabolic measures, LFPs, and spike rate is because some studies did not record spike rate in addition to the LFP in comparisons with metabolic measures). Thus, it seems reasonable to assume that dissociations between the BOLD signal and LFPs will generally mean that the BOLD signal and spike rate will similarly be dissociated. This would suggest that the actual number of studies showing a three-way dissociation in Table 1 is actually closer to ten.
Table 1.
Studies investigating BOLD signal/CBF/CBV/CMRO2 (“metabolic signal”) and electrophysiological inter-relations as well as studies investigating intra-relations for electrophysiological signals (e.g., LFPs vs. spike rate). Studies are grouped according to region and concluded effect. Correlations with r2 values less than .2 generally considered to show little or no correlation between two listed measures (hence, these studies are included in the dissociation column). Studies listed more than once show more than one effect or correlation, typically because an intervention (behavioral or pharmacological) was applied to look for dissociations. Results from studies shown in bold demonstrate a three-way dissociation, i.e., a dissociation between BOLD, LFPs, and spike rate. Note, however, that no studies to date have shown a correlation between BOLD and spike rate and a dissociation between BOLD and LFPs in the experimental testing conditions (i.e., a double dissociation).
The data presented thus suggest a mixture of hope and caution regarding how effectively the BOLD signal can be used as a proxy for underlying neural activity. Under some situations, particularly in neo-cortex, BOLD, spike rate, and LFP measures are often well correlated. More frequently, though, BOLD and LFPs correlate more strongly than BOLD and spike rate in neocortex, consistent with the BOLD-LFP coupling model. Outside of neo-cortex, no studies yet show a correlation between BOLD and spike rate although several show correlations between BOLD and LFP measures, also consistent with the BOLD-LFP coupling model. Five out of nine studies from the hippocampus, however, show dissociations between metabolic measures and LFPs. Clearly, tabulating the situations in which one might expect fMRI to provide a good measure of underlying activity is a useful endeavor as this knowledge may help constrain conclusions about BOLD and electrophysiological activity, especially in regions outside of sensory neocortex.
One safeguard, if one’s intention is to use fMRI to reach conclusions about underlying electrophysiology, might be to simultaneously record BOLD and neural activity, such as can be obtained using simultaneous BOLD-scalp EEG recordings. Simultaneous BOLD-scalp EEG studies have provided significant information regarding correlations between alpha activity and negative BOLD changes in neo-cortex (Goldman et al., 2002; Laufs et al., 2003b; Laufs et al., 2003a; Moosmann et al., 2003; Scheeringa et al., 2009) and remain a promising venue for characterizing when EEG and the BOLD signal correlate during behavior in the brain (see also: Debener et al., 2005; Koch et al., 2006; Meltzer et al., 2008). EEG recordings, however, largely provide information about superficial areas of neo-cortex (Nunez and Silberstein, 2000) and do not provide direct information about spiking activity in the brain.
Imaging, though, is clearly of exigent and immediate value in clinical situations, such as identifying language areas of cortex during planning of tumor surgery (Bookheimer, 2007). A clinical researcher should not have to spend significant amounts of time second-guessing whether the BOLD response actually provides information about neural activity or not. The BOLD-LFP coupling model, which is firmly established under many testing conditions in neocortex, suggests that fMRI does in fact reflect underlying neural activity in many instances. When fMRI dissociates with underlying LFPs under some testing situations (see also: Caesar et al., 2003; Maier et al., 2008; Sirotin and Das, 2009 for descriptions outside of the hippocampus of this phenomenon), the lack of a correlation with the LFP may come about for a variety of reasons. One possibility is of course no neural activity is present at that recording site. It may also be the case that peri-synaptic events do not summate due to an arrangement in the geometry of neurons unfavorable to an LFP (Mitzdorf, 1985). Finally, it may also be the case that neuromodulatory signals from downstream neural events modulate upstream vascular events, resulting in LFP changes and vasoconstriction/vasodilation in distant yet related brain areas (Sirotin and Das, 2009). Thus, while a dissociation between the BOLD signal and LFPs in a specific brain region certainly should give cognitive neuroscientists pause for thought and lead to careful consideration of the circuitry stimulated in the experiment, a zero-sum LFP may not always indicate no neural activity either.
For basic research projects the value of multi-modal and comparative imaging methods is obvious as there is much information to be gained by combining imaging modalities. In addition to simultaneous scalp EEG/fMRI, promising areas of development for multimodal imaging involve optical probes that can be inserted directly with microelectrode recording devices. These probes are designed to permit simultaneous observation of metabolic, LFP, and spiking activity, even during human intracranial recordings (Strick et al., 2008; Keller et al., 2009). These promising new methodologies are likely to reveal much additional information about the neural basis of the BOLD signal in the human and monkey brain, particularly in deeper structures than neo-cortex.
BOLD and neural signal decouplings also suggest the value, whenever possible, of calibrated fMRI and PET studies of brain regions of interest. Calibrated fMRI, as discussed earlier, allows independent measurements of CBF and CMRO2, thus providing the potential to identify brain regions where the BOLD signal may show a more complex interplay with these measures. Knowledge of when CBF and CMRO2 show different couplings than those classically reported in neocortex is important in determining how well the BOLD signal alone may reflect oxidative processes performed by neurons during information processing. PET, although lacking the spatial and temporal resolution of fMRI, is also valuable here as it additionally allows determination of glucose utilization and can now be measured simultaneously in some cases with fMRI (Schlemmer et al., 2008). More information is needed specifically on how CBF, CMRO2, and Cglu vary across different brain regions as this information will be critical in determining how reliable a measure BOLD alone is of underlying events.
In conclusion, while fMRI is a potentially extremely useful and valuable tool for understanding brain regions involved in cognition, caution is necessary in interpreting its results, particularly with regard to making direct inferences about underlying neural activity. More studies need to be conducted, particularly in brain regions outside of visual cortex, to provide a firmer understanding for situations when fMRI and neural signals may be coupled and when they dissociate.
Acknowledgements
Thanks to Eve Isham, Charan Ranganath, Andy Yonelinas, Yevgeniy Sirotin, Michael Kahana, and Andrew Watrous for constructive comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann RF, Finch DM, Babb TL, Engel J., Jr Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. Hippocampal Neuroanatomy. In: Andersen P, Morris M, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. New York: Oxford University Press; 2007. [Google Scholar]
- Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI. Neuroimage. 2008;39:1510–1521. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenstein F, Kammerer E, Scheich H. The BOLD response in the rat hippocampus depends rather on local processing of signals than on the input or output activity. A combined functional MRI and electrophysiological study. J Neurosci. 2009;29:2428–2439. doi: 10.1523/JNEUROSCI.5015-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. Working memory-related hippocampal deactivation interferes with long-term memory formation. J Neurosci. 2009;29:1052–1960. doi: 10.1523/JNEUROSCI.5277-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Alfke K, Deuschl G, Jansen O. Evolution of hippocampal CA-1 diffusion lesions in transient global amnesia. Ann Neurol. 2007;62:475–480. doi: 10.1002/ana.21189. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Pre-surgical language mapping with functional magnetic resonance imaging. Neuropsychol Rev. 2007;17:145–155. doi: 10.1007/s11065-007-9026-x. [DOI] [PubMed] [Google Scholar]
- Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989;288:401–413. doi: 10.1002/cne.902880304. [DOI] [PubMed] [Google Scholar]
- Brinker G, Bock C, Busch E, Krep H, Hossmann KA, Hoehn-Berlage M. Simultaneous recording of evoked potentials and T2*-weighted MR images during somatosensory stimulation of rat. Magn Reson Med. 1999;41:469–473. doi: 10.1002/(sici)1522-2594(199903)41:3<469::aid-mrm7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar K, Thomsen K, Lauritzen M. Dissociation of spikes, synaptic activity, and activity-dependent increments in rat cerebellar blood flow by tonic synaptic inhibition. Proc Natl Acad Sci U S A. 2003;100:16000–16005. doi: 10.1073/pnas.2635195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals S, Beyerlein M, Murayama Y, Logothetis NK. Electric stimulation fMRI of the perforant pathway to the rat hippocampus. Magn Reson Imaging. 2008 doi: 10.1016/j.mri.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeReuck J, Van Kerckvoorde L, De Coster W, vander Eecken H. Ischemic lesions of the hippocampus and their relation to Ammon's horn sclerosis. A neuropathological study of two cases and a comparison to the vascular anatomy. J Neurol. 1979;220:157–168. doi: 10.1007/BF00705534. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization, and Serial Sections with MRI. Berlin: Springer; 1998. [Google Scholar]
- Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S. Correlation Between BOLD fMRI and Theta-Band Local Field Potentials in the Human Hippocampal Area. J Neurophysiol. 2009;101:2668–2678. doi: 10.1152/jn.91252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Viskontas I, Kahana MJ, Jacobs J, Upchurch K, Bookheimer S, Fried I. Contrasting Roles of Neural Firing Rate and Local Field Potentials in Human Memory. Hippocampus. 2007;17:606–607. doi: 10.1002/hipo.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29:13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson G, Peterson A, Iverfeldt K, Walum E. Sodium-dependent glutamate uptake as an activator of oxidative metabolism in primary astrocyte cultures from newborn rat. Glia. 1995;15:152–156. doi: 10.1002/glia.440150207. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain. 1995;118(Pt 2):401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frahm J, Merboldt KD, Hanicke W, Kleinschmidt A, Boecker H. Brain or vein--oxygenation or flow? On signal physiology in functional MRI of human brain activation. NMR Biomed. 1994;7:45–53. doi: 10.1002/nbm.1940070108. [DOI] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI Signal in Awake Monkeys. Curr Biol. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Jr, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivas I, Michaloudi H, Batzios C, Chiotelli M, Papatheodoropoulos C, Kostopoulos G, Papadopoulos GC. Vascular network of the rat hippocampus is not homogeneous along the septotemporal axis. Brain Res. 2003;971:245–249. doi: 10.1016/s0006-8993(03)02475-2. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281:E100–E112. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- Gsell W, Burke M, Wiedermann D, Bonvento G, Silva AC, Dauphin F, Buhrle C, Hoehn M, Schwindt W. Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat. J Neurosci. 2006;26:8409–8416. doi: 10.1523/JNEUROSCI.4615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RV, Harel N, Panesar J, Mount RJ. Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb Cortex. 2002;12:225–233. doi: 10.1093/cercor/12.3.225. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3:142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J Neurophysiol. 2005;94:479–490. doi: 10.1152/jn.00919.2004. [DOI] [PubMed] [Google Scholar]
- Henry PG, Adriany G, Deelchand D, Gruetter R, Marjanska M, Oz G, Seaquist ER, Shestov A, Ugurbil K. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn Reson Imaging. 2006;24:527–539. doi: 10.1016/j.mri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hewson-Stoate N, Jones M, Martindale J, Berwick J, Mayhew J. Further nonlinearities in neurovascular coupling in rodent barrel cortex. Neuroimage. 2005;24:565–574. doi: 10.1016/j.neuroimage.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Huttunen JK, Grohn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage. 2008;39:775–785. doi: 10.1016/j.neuroimage.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Hyder F. Neuroimaging with calibrated FMRI. Stroke. 2004;35:2635–2641. doi: 10.1161/01.STR.0000143324.31408.db. [DOI] [PubMed] [Google Scholar]
- Jagadeesh B, Wheat HS, Ferster D. Linearity of summation of synaptic potentials underlying direction selectivity in simple cells of the cat visual cortex. Science. 1993;262:1901–1904. doi: 10.1126/science.8266083. [DOI] [PubMed] [Google Scholar]
- Kayser C, Kim M, Ugurbil K, Kim DS, Konig P. A comparison of hemodynamic and neural responses in cat visual cortex using complex stimuli. Cereb Cortex. 2004;14:881–891. doi: 10.1093/cercor/bhh047. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Cash SS, Narayanan S, Wang C, Kuzniecky R, Carlson C, Devinsky O, Thesen T, Doyle W, Sassaroli A, Boas DA, Ulbert I, Halgren E. Intracranial microprobe for evaluating neuro-hemodynamic coupling in unanesthetized human neocortex. J Neurosci Methods. 2009;179:208–218. doi: 10.1016/j.jneumeth.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida I, Smith AJ, Blumenfeld H, Behar KL, Hyder F. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. Neuroimage. 2006;29:216–224. doi: 10.1016/j.neuroimage.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kim DS, Ronen I, Olman C, Kim SG, Ugurbil K, Toth LJ. Spatial relationship between neuronal activity and BOLD functional MRI. Neuroimage. 2004;21:876–885. doi: 10.1016/j.neuroimage.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Koch SP, Steinbrink J, Villringer A, Obrig H. Synchronization between background activity and visually evoked potential is not mirrored by focal hyperoxygenation: implications for the interpretation of vascular brain imaging. J Neurosci. 2006;26:4940–4948. doi: 10.1523/JNEUROSCI.3989-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraskov A, Quiroga RQ, Reddy L, Fried I, Koch C. Local field potentials and spikes in the human medial temporal lobe are selective to image category. J Cogn Neurosci. 2007;19:479–492. doi: 10.1162/jocn.2007.19.3.479. [DOI] [PubMed] [Google Scholar]
- Kreiman G, Hung CP, Kraskov A, Quiroga RQ, Poggio T, DiCarlo JJ. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron. 2006;49:433–445. doi: 10.1016/j.neuron.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003a;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003b;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO2 coupling measured with calibrated BOLD fMRI: sources of bias. Neuroimage. 2007;36:1110–1122. doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lokkegaard A, Nyengaard JR, West MJ. Stereological estimates of number and length of capillaries in subdivisions of the human hippocampal region. Hippocampus. 2001;11:726–740. doi: 10.1002/hipo.1088. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. The Royal Society of London Biological Sciences. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Vazquez A, Wang P, Kim SG. Trial-by-trial relationship between neural activity, oxygen consumption, and blood flow responses. Neuroimage. 2008;40:442–450. doi: 10.1016/j.neuroimage.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Lauritzen M. Temporal coupling between neuronal activity and blood flow in rat cerebellar cortex as indicated by field potential analysis. J Physiol. 2000;523(Pt 1):235–246. doi: 10.1111/j.1469-7793.2000.t01-1-00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgoren N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512(Pt 2):555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Traynelis SF, Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990;249:674–677. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, Spencer DD, Constable RT. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex. 2008;18:1843–1855. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Nase G, Singer W, Monyer H, Engel AK. Features of neuronal synchrony in mouse visual cortex. J Neurophysiol. 2003;90:1115–1123. doi: 10.1152/jn.00480.2002. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275–1285. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB. On the relationship of synaptic activity to macroscopic measurements: does co-registration of EEG with fMRI make sense? Brain Topogr. 2000;13:79–96. doi: 10.1023/a:1026683200895. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Corina DP, Corrigan N, Schoenfield-McNeill J, Poliakov A, Zamora L, Zanos S. Neuronal correlates of functional magnetic resonance imaging in human temporal cortex. Brain. 2009 doi: 10.1093/brain/awp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain Work and Brain Imaging. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc Natl Acad Sci U S A. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Battaglia FP, Chawla MK, Ekstrom AD, Gerrard JL, Lipa P, Rosenzweig ES, Worley PF, Guzowski JF, McNaughton BL, Barnes CA. Independence of firing correlates of anatomically proximate hippocampal pyramidal cells. J Neurosci. 2001;21:RC134. doi: 10.1523/JNEUROSCI.21-05-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Rekkas PV, Westerveld M, Skudlarski P, Zumer J, Pugh K, Spencer DD, Constable RT. Neural correlates of temporal-order judgments versus those of spatial-location: deactivation of hippocampus may facilitate spatial performance. Brain Cogn. 2005;59:103–113. doi: 10.1016/j.bandc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Restom K, Perthen JE, Liu TT. Calibrated fMRI in the medial temporal lobe during a memory-encoding task. Neuroimage. 2008;40:1495–1502. doi: 10.1016/j.neuroimage.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Arroyos R, Gaztelu JM, Zaplana J, Dajas F, Garcia-Austt E. Hippocampal and entorhinal glucose metabolism in relation to cholinergic theta rhythm. Brain Res Bull. 1993;32:171–178. doi: 10.1016/0361-9230(93)90071-i. [DOI] [PubMed] [Google Scholar]
- Scharrer E. Vascularization and vulnerability of the cornu ammonis in the opposum. Archives of Neurological Psychiatry. 1940;19:308–318. [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MC. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage. 2009;44:1224–1238. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Schlemmer HP, Pichler BJ, Schmand M, Burbar Z, Michel C, Ladebeck R, Jattke K, Townsend D, Nahmias C, Jacob PK, Heiss WD, Claussen CD. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008;248:1028–1035. doi: 10.1148/radiol.2483071927. [DOI] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex. 2008;18:1814–1827. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman SL, Astur RS. Factors affecting the hippocampal BOLD response during spatial memory. Behav Brain Res. 2008;187:433–441. doi: 10.1016/j.bbr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci U S A. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457:475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J Neurosci. 2008;28:10961–10971. doi: 10.1523/JNEUROSCI.1956-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick DS, Nunnally RL, Smith JC, Clark W, Mills DJ, Cohen MS, Judy JW. Towards a microcoil for intracranial and intraductal MR microscopy. Conf Proc IEEE Eng Med Biol Soc. 20082008:2047–2050. doi: 10.1109/IEMBS.2008.4649594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen K, Offenhauser N, Lauritzen M. Principal neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J Physiol. 2004;560:181–189. doi: 10.1113/jphysiol.2004.068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uecker A, Barnes CA, McNaughton BL, Reiman EM. Hippocampal glycogen metabolism, EEG, and behavior. Behav Neurosci. 1997;111:283–291. doi: 10.1037//0735-7044.111.2.283. [DOI] [PubMed] [Google Scholar]
- Ureshi M, Matsuura T, Kanno I. Stimulus frequency dependence of the linear relationship between local cerebral blood flow and field potential evoked by activation of rat somatosensory cortex. Neurosci Res. 2004;48:147–153. doi: 10.1016/j.neures.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. The Human Parahippocampal Region in Alzheimer's Disease, Dimentia, and Ageing. In: Witter MP, Wouterlood F, editors. The Parahippocampal Region: Organization and Role in Cognitive Function. Oxford: Oxford University Press; 2002. pp. 271–295. [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007;10:1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Waydo S, Kraskov A, Quian Quiroga R, Fried I, Koch C. Sparse representation in the human medial temporal lobe. J Neurosci. 2006;26:10232–10234. doi: 10.1523/JNEUROSCI.2101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]