Abstract
Lmx1b is a homeodomain transcription factor that regulates dorsal identity during limb development. Lmx1b knockout (KO) mice develop distal ventral-ventral limbs. Although induction of Lmx1b is linked to Wnt7a expression in the dorsal limb ectoderm, the downstream targets of Lmx1b that accomplish limb dorsalization are unknown. To identify genes targeted by Lmx1b, we compared gene arrays from Lmx1b KO and wildtype mouse limbs during limb dorsalization, i.e., 11.5, 12.5, and 13.5 days post coitum.
We identified 54 target genes differentially expressed in all three stages. Several skeletal targets, including Emx2, Matrilin1 and Matrilin4, demonstrated a loss of scapular expression in the Lmx1b KO mice, supporting a role for Lmx1b in scapula development. Furthermore, the relative abundance of extracellular matrix-related soft tissue targets regulated by Lmx1b, such as collagens and proteoglycans, suggests a mechanism which includes changes in the extracellular matrix composition to accomplish limb dorsalization.
Our study provides the most comprehensive characterization of genes regulated by Lmx1b during limb development to-date and provides targets for further investigation.
Keywords: Limb development, mouse, patterning, Lmx1b, microarray
INTRODUCTION
The limb is a complex organ that requires unique multi-axis asymmetry for function. The limb emerges from the lateral plate mesoderm as a bulge of undifferentiated mesoderm covered by ectoderm. In the mouse model at 11.5 days post coitum (dpc) the limb bud mesoderm is diffuse and relatively uniform. Centrally, the mesoderm condenses to form cartilage anlagen and peripheral mesoderm condenses to form tendon precursors. Joint segmentation begins as a zone of high cell density (an interzone) within the mesodermal condensation (Craig et al., 1987, Dalgleish, 1964). The joint capsule and associated ligaments and tendon attachments develop from condensations surrounding the interzone regions (Mitrovic, 1978). This process proceeds in a proximal-to-distal fashion. By 13.5 dpc, the skeletal elements, joints, ligaments and tendon precursors of the stylopod (humerus/femur), zeugopod (radius-ulna/tibia fibula) and autopod (hand/foot) are largely defined.
Development of the limb bud can be described along three axes. The proximal-distal axis depicts the progressive outgrowth of the three limb segments: stylopod, zeugopod and autopod. The anterior-posterior axis defines the patterning of the digits (e.g., from thumb to little finger) and zeugopod (e.g., radius and ulna). The dorsal-ventral axis delineates the extensor and flexor compartments of the limb. The limb has marked asymmetry in structure and function along the dorsal-ventral axis despite being composed of similar elements. This limb asymmetry is under the control of Lmx1b, a dorsalizing homeodomain-containing transcription factor. Lmx1b knockout (KO) mice (Chen et al., 1998) display distal ventral-ventral morphology in the bone, muscle and soft tissues. In humans, single allele mutation of LMX1B causes Nail Patella Syndrome (NPS) (Dreyer et al., 1998) with incomplete dorsalization of extremities, i.e., loss of the patella, deficient nails, and joint malformations. However, limb-specific downstream target genes of Lmx1b have yet to be clearly defined.
The molecular control of dorsalization is initiated by Wnt7a expression in the limb bud ectoderm (Riddle et al., 1995). Progressive expression of the En-1 transcription factor in the ventral ectoderm restricts Wnt7a to the dorsal ectoderm (Cygan et al., 1997, Loomis et al., 1998). Secretion of dorsally-restricted Wnt7a into the underlying mesoderm induces Lmx1b expression limited to the dorsal mesoderm (Vogel et al., 1995). During progressive joint and tendon formation (11.5dpc – 13.5dpc), the expression of Lmx1b proximally decreases and becomes restricted to differentiated tissues while retaining intense expression in the distal less-differentiated dorsal mesoderm (Dreyer et al., 2004).
The restriction of Lmx1b to dorsal tendons and joints during differentiation suggests a role in tendon/joint formation, but no direct link between Lmx1b and any tendon- or joint-related genes have been described. In this report, we used microarray analysis to identify differentially expressed genes during joint, ligament and tendon formation in the presence (wildtype mice) or absence (Lmx1b KO mice) of Lmx1b. Progressive stages were analyzed and compared in an effort to minimize stage-specific differences and accentuate Lmx1b-specific targets. Our analysis identified genes regulated by Lmx1b that are involved in asymmetric nerve, bone, joint, ligament, and tendon formation during dorsalization.
MATERIALS and METHODS
Lmx1b KO mouse
Lmx1b knockout (KO) mice were a kind gift of Randy L. Johnson (Chen et al., 1998). Lmx1b homozygous mouse embryos were obtained by mating heterozygous male mice with heterozygous female mice. We time-dated matings using noon on the day that the vaginal plug was found as 0.5 days post coitum (dpc). At 11.5 and 12.5 dpc, embryos were harvested and the limb buds with the limb girdles were isolated. Embryos at 13.5dpc were also harvested and their distal limb buds (zeugopods and autopods) were isolated. Embryos were genotyped to confirm Lmx1b homozygosity (−/− or +/+).
Gene expression array
RNA from embryonic forelimbs and hindlimbs of wild type (WT) and Lmx1b KO mice was harvested using the Rneasy Kit (Qiagen). RNA was pooled to decrease genetic variability, i.e., six limbs at 11.5 dpc, three limbs at 12.5 dpc and six limbs at 13.5 dpc. Duplicate samples were generated using different embryos for each stage and then hybridized to the Affymetrix GeneChip® Mouse Genome 430 2.0 Array (UCI, Irvine, CA). Microarray data was submitted to Gene Expression Omnibus and can be located under series accession number GSE34732. The data was normalized using RMA and analyzed using the Comprehensive R and Bioconductor based web service for microarray data analysis (Rainer et al., 2006). Genes differentially expressed between WT and Lmx1b KO mice with pvalue >0.05 were not considered significant and were not further analyzed.
In order to enhance gene discovery, all genes with significant differential expression were examined to compare expression differences at all three stages. Genes which demonstrated a 2-fold change at any stage were considered for further analysis.
Validation by Real-time PCR (Quantitative PCR (Q-PCR))
Complementary DNA (cDNA) was transcribed from limb mRNA at each stage using both Lmx1b KO and WT mice. PCR products were sequenced to determine specificity (Supplemental Data Table 2). Differential expression was determined by Real-time PCR (Q-PCR) measuring SYBR Green I fluorescence with the Roche Lightcycler 2.0 and Roche-recommended reagents. Triplicate Lightcycler runs with duplicate samples were compared to simultaneous PGK runs to normalize fold changes between Lmx1b KO and WT expression. Gene targets were considered validated if Q-PCR data confirmed microarray data with at least a 2-fold change at any stage.
Whole Mount In Situ hybridization (WMISH)
Sense and antisense digoxigen-labeled RNA probes ranging from 600bp – 1000bp were generated for each of the genes validated by Real-time PCR. Probes were sequenced to ensure specificity (Supplemental Data Table 2). Aggrecan probe sequence was generously provided by the Nancy Schwartz lab (Cortes et al., 2009). Lmx1b KO and WT mouse embryos were harvested at 12.5 dpc and fixed overnight in MEMFA (0.1M MOPS, 2mM EGTA, 1mM MgSO4, 3.7% formaldehyde). WMISH was performed as described (Yamada et al., 1999) with each probe hybridized at 60°C, and embryos were washed post hybridization at 65°C.
RESULTS
Determination of Candidate genes by Comparative Microarray
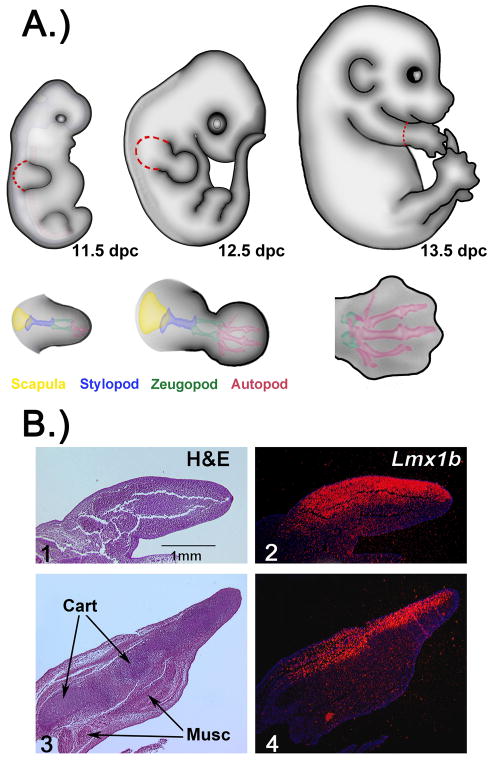
In an effort to identify targets of Lmx1b involved in limb dorsalization, we isolated limb bud tissue with robust Lmx1b expression between 11.5 days post coitum (dpc) and 13.5dpc. At 11.5 dpc, expression of Lmx1b is throughout the dorsal limb bud mesoderm (Fig 1B). Thus, we harvested the entire limb bud as depicted in Fig 1A. The tissue harvested includes presumptive limb girdle elements (e.g., scapula) and tissue that will become all three segments of the limb (stylopod, zeugopod and autopod). Expression of Lmx1b at 12.5 dpc begins to localize to the condensing tendons proximally, while in distal less-differentiated dorsal mesenchyme, expression remains diffuse (Dreyer et al., 2004). At this stage, we also harvested the entire limb including limb girdle elements (Fig. 1A). Lmx1b expression at 13.5 dpc wanes proximally but remains strong in the autopod (Fig. 1B). Therefore, the autopod was harvested at 13.5 dpc to coincide with strong Lmx1b expression (Fig. 1A). In order to diminish biologic variability and enhance Lmx1b-associated changes, RNA was pooled from several different embryos/litters. Genes with significant (p<0.05) differential expression were included in our data for this study.
Figure 1. Depiction of Methods.
A.) Depiction of the three developmental stages analyzed by microarray. Presumptive structures are color-coded at each embryonic stage depicting the progressive differentiation of the limb. Dashed red lines are drawn to illustrate the limb tissue harvested for gene array experiments. (dpc- days post coitum) B) Section in situ hybridization for Lmx1b at 11.5 dpc (2) with expression pseudocolored red demonstrating restricted dorsal expression. By 13.5 dpc (4), proximal expression of Lmx1b is less intense and localized to condensing musculo-tendonous tissue; distal Lmx1b expression remains diffuse in the dorsal mesoderm. H&E of corresponding limbs show the amount of relative tissue differentiation at each stage(1,2). (musc- muscle) (cart-cartilage)
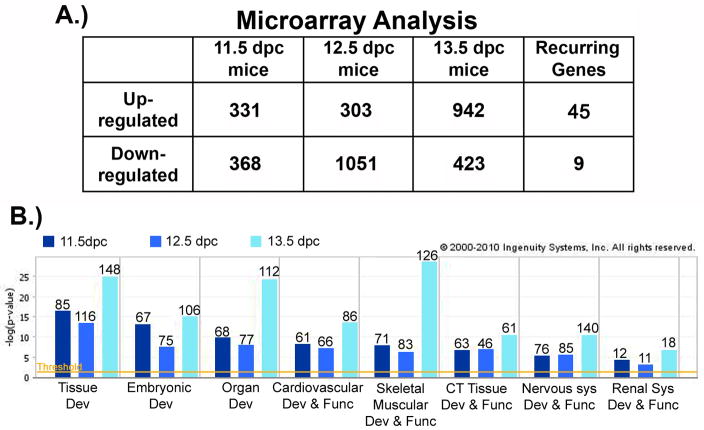
At 11.5 dpc, we identified 331 genes that were up-regulated in the presence of Lmx1b, while 368 genes were down-regulated. Lmx1b up-regulated the expression of 303 genes and down-regulated 1051 genes at 12.5 dpc. By 13.5 dpc, Lmx1b up-regulated 942 genes, and down-regulated 423 genes (Fig. 2A).
Figure 2. Analysis of Microarray Data.
A.) The number of genes with significant (p < 0.05) differential expression between WT and Lmx1b KO microarray analyses for each stage. The last column identifies the subset of genes differentially expressed in all three stages. B.) Graphical representation of ingenuity pathway assistant (IPA) analysis that identifies developmental processes and system networks containing the genes that are differentially expressed at each stage. The relative significance determined by IPA defined parameters is listed on the y-axis. The number of genes in each network is listed at the top of each bar. The P value < 0.05 is denoted by the orange threshold line.
To identify functionally relevant developmental pathways or networks used by Lmx1b, we analyzed our dataset with the Ingenuity Pathway Assistant (IPA) software (Fig. 2B). When a comparison analysis is performed across stages, the data cluster into several networks affected by Lmx1b. These include skeletal, connective tissue and nervous system developmental networks which correlate with morphology disrupted in the Lmx1b knockout (KO) mouse.
Many of the targets discovered at individual stages may represent stage specific changes. In order to enrich genes downstream of Lmx1b during limb dorsalization, we identified genes with similar changes in expression for all three stages. We discovered 45 up-regulated genes and 9 down-regulated genes across all three stages. These genes were considered Lmx1b targets. Similar studies by Kania et al., used a 1.4-fold change; we chose a more stringent criterion since we have a larger data set incorporating several embryonic stages. For further analysis, we required candidate genes to have at least a 2-fold change at one or more stages. In addition, we selected genes that we suspected to be involved with limb development pathways. Table 1 lists the 23 genes which met this criterion, along with the fold change demonstrated at each stage.
Table 1.
A partial list of up and down-regulated genes identified by gene array across all three stages. Gene symbols and descriptions are listed along with the fold change at each stage. All data points listed in the table have a p value of < 0.05. Genes included in this partial list had a two fold change at one or more stages by gene array analysis. The final column indicates Q-PCR validation of this inclusion criteria.
Partial list of genes from microarray analysis regulated by Lmx1b during all three experimental stages
| UP-REGULATED | |||||
|---|---|---|---|---|---|
| Description | Symbol | 11.5 FC | 12.5 FC | 13.5 FC | Validated |
| Empty spiracles homolog 2 (Drosophila) | Emx2 | 2.57 | 5.30 | 2.21 | Yes |
| Keratocan | Kera | 3.56 | 13.40 | 7.82 | Yes |
| Lumican | Lum | 2.56 | 3.67 | 2.33 | Yes |
| Decorin | Dcn | 1.43 | 2.10 | 2.57 | Yes |
| Aggrecan 1 | Agc1 | 1.53 | 1.88 | 2.02 | Yes |
| Matrilin 1, cartilage matrix protein 1 | Matn1 | 2.09 | 3.07 | 2.36 | Yes |
| Matrilin 4 | Matn4 | 1.41 | 1.60 | 2.03 | Yes |
| Growth differentiation factor 5 | Gdf5 | 1.61 | 2.10 | 2.10 | Yes |
| Procollagen, type IX, alpha 3 | Col9a3 | 1.51 | 1.53 | 2.07 | No |
| Procollagen, type XI, alpha 2 | Col11a2 | 1.37 | 1.57 | 2.02 | Yes |
| Cerebellin 2 precursor protein | Cbln2 | 1.54 | 2.45 | 2.21 | Yes |
| Lysyl oxidase | Lox | 1.59 | 2.45 | 1.45 | Yes |
| Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 | Efemp1 | 1.46 | 1.68 | 2.37 | No |
| Noggin | Nog | 1.46 | 1.87 | 2.30 | Yes |
| Leukocyte cell derived chemotaxin 1 | Lect1 | 2.69 | 2.44 | 1.81 | Yes |
| Scinderin | Scin | 1.41 | 2.22 | 2.48 | Yes |
| Apolipoprotein D | Apod | 1.70 | 2.97 | 2.28 | Yes |
| Fam | MGC99845 | 1.45 | 1.60 | 2.70 | Yes |
| Retinoic acid receptor, beta | Rarb | 1.51 | 2.04 | 1.45 | No |
| DOWN-REGULATED | |||||
| Regulatory factor X, 4 | Rfx4 | 1.99 | 2.73 | 1.60 | Yes |
| RIKEN cDNA 1700109F18 gene | Rik | 1.31 | 2.49 | 2.09 | No |
| Myeloid ecotropic viral integration site 1 | Meis1 | 1.31 | 1.41 | 2.24 | No |
| AF4/FMR2 family, member 3 | Aff3 | 1.40 | 2.02 | 1.43 | Yes |
Validation of Candidate Lmx1b Target Genes by Real-time PCR
Candidate genes derived by microarray analysis were confirmed by Real-time PCR (Q-PCR) using SYBR green fluorescence. Q-PCR data met the previously described 2-fold change criteria for 18 candidate genes (Fig. 3). Four genes (denoted in red) fell below our threshold and were not examined further. The fold-change at 12.5 dpc was greater than at other stages for most of the Lmx1b related targets. This finding suggests that 12.5 dpc is a crucial time during dorsalization, with Lmx1b exerting its greatest influence on gene targets. Thus, we examined the expression patterns of candidate genes in the WT and Lmx1b mutant mice at 12.5 dpc.
Figure 3. Graphical representation of Real-time PCR (Q-PCR).
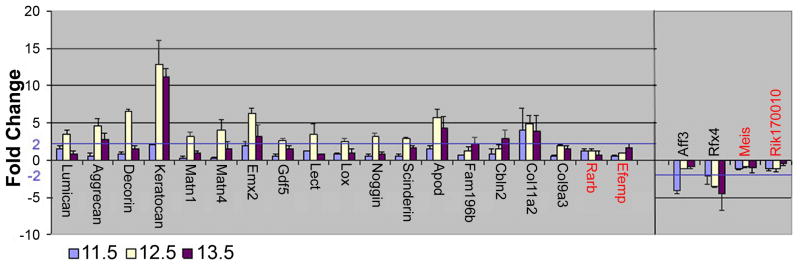
The fold change of each gene was measured at each stage; a 2-fold threshold is denoted by a blue line. Q-PCR validated microarray data for genes shown in black type. Genes denoted in red failed to meet validation criteria. The greatest fold changes are consistently seen at stage 12.5, therefore this stage was used for in situ hybridization analysis. * Col9a3 produced a 1.9-fold change at 12.5 dpc. Since several other collagen related targets were also validated, Col9a3 was analyzed further.
Confirmation of Candidate Lmx1b Target Genes by Whole Mount in situ Hybridization (WMISH)
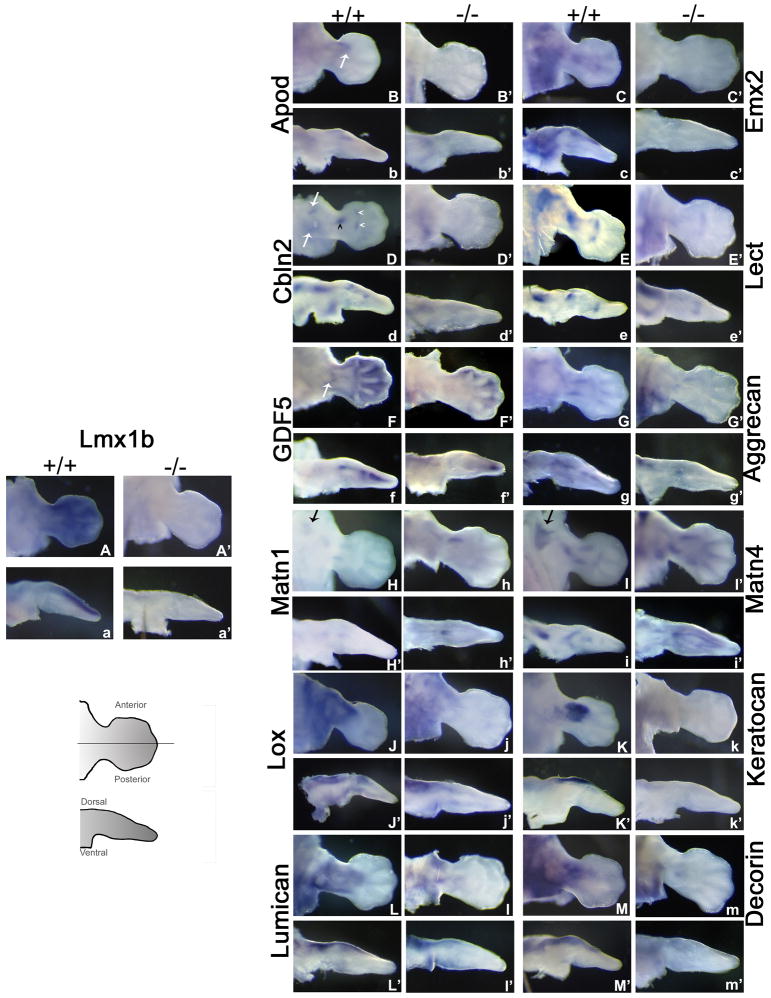
WMISH was utilized to determine differential expression for 19 genes. Their expression patterns are briefly described below and illustrated in figures 4, 5, and 6, along with the expression pattern of Lmx1b (Fig 4A,a, & A′,a′).
Figure 4. Whole Mount in situ Hybridization of Targets Up-regulated by Lmx1b.
WMISH for Q-PCR validated up-regulated genes at 12.5 dpc. Cartoon provides orientation for pictures, line along AP axis depicts cut performed for dorsoventral pictures. Dorsal views along with cross sections are shown for each gene. Expression patterns for each gene are as follows: Capital letter (e.g., A) denotes expression in WT limbs along the dorsal surface, capital letter with apostrophe (e.g., A′) denotes expression in Lmx1b KO limbs along the dorsal surface. Small letter (e.g., a) marks WT limb sections while small letter with apostrophe (e.g., a′) indicates KO limb sections. B, white arrow indicates anterior Apod expression. D Cbln expression in the autopod (white arrowhead), zeugopod (black arrowhead), and proximal/stylopod (white arrows). F, Gdf5 in the olecranon (white arrow). H and I, black arrows both indicate scapular stain for Matn1 and Matn4, respectively.
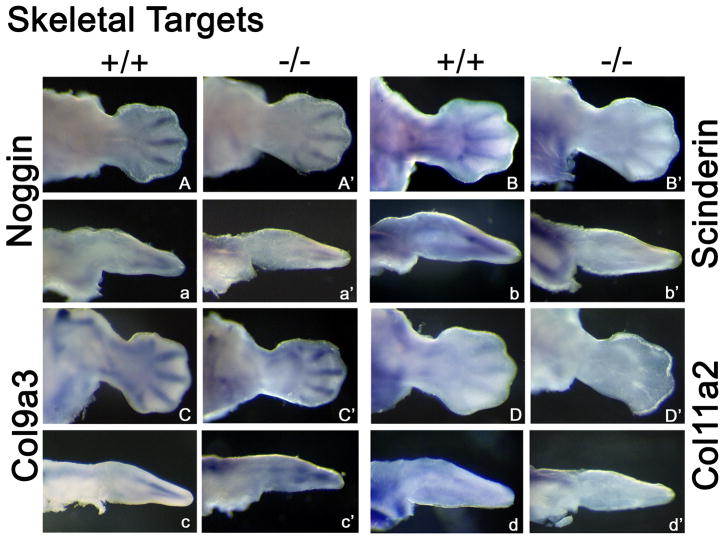
Figure 5. Whole Mount in situ Hybridization of Skeletal Targets Up-regulated by Lmx1b.
Expression patterns for each skeletal related gene at 12.5 dpc are as follows. Capital letter (e.g., A) denotes WT expression along the dorsal surface. Capital letter with apostrophe (e.g., A′) denotes WT ventral expression while (A”) denotes cut sections. Small letter (e.g., a) marks WT limb sections while small letter with apostrophe (e.g., a′) indicates Lmx1b KO limb sections. All skeletal genes show a decrease in expression in the Lmx1b KO limb.
Figure 6. Whole Mount in situ Hybridization of Targets Down-regulated by Lmx1b.
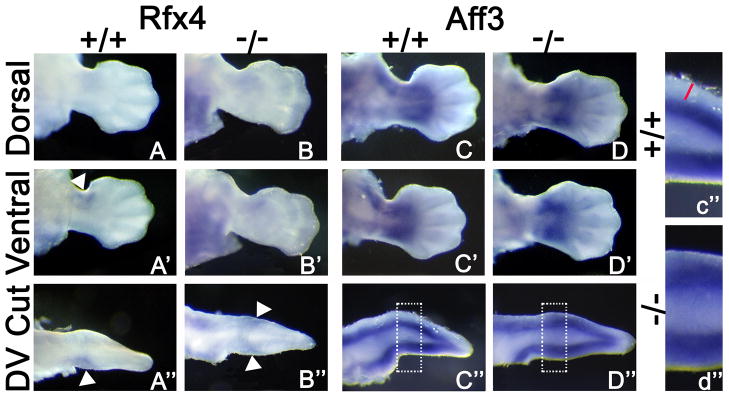
WMISH for Q-PCR validated down-regulated genes at 12.5 dpc, cut sections performed as illustrated by cartoon in Fig. 4. Rfx4 is expressed dorsally (A) and ventrally (A′, white arrowhead; A”, cut section, white arrowhead) WT limb. In the Lmx1b KO limb, Rfx4 is expressed dorsally (B) and ventrally (B′). A cut section shows duplicated expression (B”, white arrowhead). C and C′ show dorsal and ventral Aff3 expression in WT limb, respectively. C” displays enhanced ventral Aff3 expression, magnification area denoted by the white box demonstrates absence of Aff3 in the dorsal subectodermal mesoderm (red line). D and D′ show dorsal and ventral expression of Aff3 in the Lmx1b KO limb, respectively. D” magnified in d″, Aff3 is enhanced both dorsally and ventrally limb.
Apolipoprotein D (ApoD) is a carrier glycoprotein that is part of the lipocalin superfamily. In humans, ApoD is a minor component of plasma high density lipoproteins. Apod has been reported as a potential downstream target of Lmx1b with dorsal ectodermal expression of ApoD demonstrated at 13.5 dpc (Gu & Kania, 2010). We found expression of ApoD proximally in the dorsal limb mesoderm (Figure 4B,b, & B′,b′) and accentuated at the anterior presumptive wrist boundary in the presence of Lmx1b at 12.5 dpc (Fig. 4B, white arrow). We also confirmed the previously reported expression within the dorsal ectoderm at 13.5 dpc (data not shown), indicating a stage-specific shift in ApoD expression.
We found Empty spiracles 2 (Emx2), a transcription factor encoding gene linked to the formation of the scapula (a dorsal limb girdle structure), to be expressed diffusely within the dorsal limb girdle at 12.5 dpc (Fig. 4C,c). Emx2 expression also extends from the limb girdle out into the dorsal autopod mesoderm. In Lmx1b KO mice, expression of Emx2 is absent in both the scapular and dorsal limb mesoderm (Fig. 4C′,c′).
Cerebellin 2 (Cbln2) is a cerebellum-specific, membrane-associated hexadecapeptide derived from Cerebellin 1. Leukocyte cell-derived chemotaxin 1 (Lect1) is a cartilage-specific glycoprotein also known as Chondromodulin I. Interestingly in our WMISH Cbln2 and Lect1 both exhibit focal points of intense dorsal expression (Fig. 4D,d, D′,d′, E,e, & E′,e′). Cbln2 displays focal anterior and posterior limb girdle expression in the dorsal mesoderm (Fig. 4D white arrows). There is also concentrated expression overlying the proximal zeugopod (black arrowhead) and two discrete zones of dorsal expression at the zeugopod-autopod boundary (white arrowheads). Lect1 has strong focal dorsal expression over the stylopod and a pattern similar to Cbln2 expression along the zeugopod-autopod boundary (Fig. 4E). The limbs of Lmx1b KO mice show near absence of Cbln2 and Lect1 expression at all of these sites (Fig. 4D′d′ and E′,e′ respectively).
Several genes up-regulated by Lmx1b localize to the developing skeleton. Growth differentiation factor 5 (Gdf5) is a member of the TGF-beta superfamily of secreted growth factors. Gdf5 expression in WT limbs localizes to developing joints, interdigital regions and dorsal mesoderm along the zeugopod (Fig. 4F,f), and is prominent in the developing elbow joint (Fig. 4F, white arrow). In the Lmx1b KO mouse limb, Gdf5 expression is absent from the area associated with the olecranon and reduced within interdigital regions (Fig. 4F′). Matrilin 1 (Matn1) and Matrilin 4 (Matn4) are cartilage matrix proteins. Aggrecan (Agc) is a chondroitin sulfate proteoglycan, Agc, Matn1, and Matn4 expression surrounds condensing cartilage in the limb and the scapula at 12.5 dpc (Fig. 4G,g,H,h,I,i respectively). Interestingly, the scapula lacks Agc, Matn1, and Matn4 in Lmx1b KO mice (Fig. 4G′g′,H′,h′,I′,I′ respectively). Agc and Matn1 is also absent in the ulnar anlagen, while Matn4 in the ulnar anlagen is absent distally. Additional skeleton-related genes identified by our gene array show symmetrical expression along the dorsal-ventral axis and include Noggin, Scinderin, Collagen9a3 and Collagen11a2. (Fig. 5). These genes display decreased expression in Lmx1b KO mice.
Lysyl oxidase 1 (Lox) encodes a connective tissue-related enzyme that is expressed strongly in the dorsal mesoderm from the limb girdle to the distal zeugopod (Fig. 4J,j). Lox in the Lmx1b KO mouse is greatly reduced (Fig. 4J′,j′). A cluster of connective tissue-related proteoglycans was also identified in our gene array as potential candidates: Keratocan (Kera), Lumican (Lum), and Decorin (Dec). Kera is expressed intensely in the dorsal zeugopod mesoderm (Fig. 4K,k) and is undetectable in Lmx1b KO limbs (Fig. 4K′,k′). Lum and Dec demonstrate strong dorsal mesodermal expression with very light staining of the proximal ventral mesoderm in WT limbs (Fig. 4L,l,M,m). Lmx1b KO limbs display greatly reduced dorsal expression of Lum and Dec; the residual staining is symmetrical and consistent with the light ventral expression seen in WT limbs (Fig. 4L′,l′,M′,m′).
The up-regulated target Family with sequence similarity 196, member B (Fam196b) is a predicted gene which was not detectable by WMISH.
The two additional transcription factors that were identified by our gene array were down regulated. Regulatory factor X,4 (Rfx4) is a winged helix transcription factor, which is expressed in the ventral limb mesoderm proximally and anteriorly (Fig. 6A,A′,A″ white arrowheads), and is duplicated in the dorsal mesoderm of the Lmx1b mutant (Fig. 6B,B′B″ white arrowheads). The nuclear transcription factor AF4/FMR2 family, member 3 (Aff3) shows broad ventral expression within the presumptive soft tissue of the zeugopod and autopod, surrounding the condensing cartilage and extending to the ectoderm, while on the dorsal aspect, the subectodermal expression is lacking (Fig. 6C,C′,C″,c″ red line). Lmx1b KO limbs reveal broad symmetrical Aff3 expression patterns along the dorsal and ventral mesoderm (Fig. 6D,D′,D″,d″).
DISCUSSION
Lmx1b is a homeodomain transcription factor known to be necessary and sufficient for dorsalizing distal limb structures including skeletal elements, nerves, tendons and ligaments. Loss of Lmx1b expression in Lmx1b KO mice causes scapular hypoplasia, distal ulnar hypoplasia, and loss of soft tissue dorsalization, i.e., symmetrical ventral-ventral or flexor-flexor ligaments, tendons and muscles (Chen & Johnson, 2002). Retroviral mediated overexpression of LMX1B in limb mesoderm generates a dorsal-dorsal distal phenotype (Vogel et al., 1995). Therefore, it is expected that Lmx1b regulates many diverse targets during limb dorsalization. Supporting this concept, our microarray analysis revealed clusters of target genes associated with bone, connective tissue, and nerve development.
Lmx1b genes associated with skeletal development
Our microarray data included several skeletal targets: Noggin, Growth differentiation factor 5 (Gdf5), Aggrecan (Agc), Scinderin, Collagen9a3, Collagen11a2, Matrilin1 (Matn1) and Matrilin4 (Matn4). Of these targets, Gdf5, Agc, Matn1 and Matn4 demonstrate asymmetric expression in normal mice that is disrupted in Lmx1b KO mice (Fig. 4F′,G′,H′,I′).
Gdf5 is a regulator of joint and cartilage formation (Buxton et al., 2001). In the 12.5 dpc limb, Gdf5 is expressed within the developing joints and interdigital spaces (Fig. 4F). Notably, the developing elbow joint is an asymmetric structure with a prominent dorsal joint interface. Correspondingly, Gdf5 expression includes a strip of dorsal mesoderm (Fig. 4F,white arrow). In Lmx1b KO embryos, this expression is absent, consistent with our microarray and Real-time PCR analysis. In a previous report, no difference in Gdf5 expression was seen in autopod joints between normal and Lmx1b KO mice, however the elbow was not examined (Dreyer et al., 2004). Although Lmx1b may regulate Gdf5 expression, Gdf5 KO mice do not present with obvious dorsoventral abnormalities; instead, loss of Gdf5 in mice and humans causes shortened limbs with abnormal joint formation and ossification of digits (Storm et al., 1994). Lmx1b plays a role in elbow development, since over 90% of patients with haploinsufficiency present with elbow dysplasia (Bongers et al., 2002). Thus, the loss of Gdf5 expression in Lmx1b KO mice may signify a contribution to or reflection of deficient elbow dorsalization.
Agc is a highly expressed structural proteoglycan associated with cartilage development and maintenance. In the cartilage matrix deficiency mouse (CMD), wherein Agc is spontaneously/naturally deleted, there is marked limb hypoplasia with no apparent dorsal-ventral limb axis abnormalities (Watanabe & Yamada, 2002). However, in our review of the published data, Agc deficient mice do appear to have more pronounced ulnar hypoplasia with ulnar deviation at the wrist. Lmx1b KO mice show a loss of Agc expression within the presumptive ulna (Fig. 4G′). This deficiency in Agc expression in the posterior cartilage anlagen may contribute to the ulnar hypoplasia seen in the Lmx1b KO mouse.
Matrilin (Matn) genes exhibit overlapping expression patterns; in particular Matn1 and Matn4 are expressed in cartilaginous tissues where they bind collagen and proteoglycans to aid in skeletogenesis. Similar to Agc in the Lmx1b KO limb, Matn1 expression is lacking in the presumptive ulna (Fig. 4H′). In contrast, Matn4 expression is present within the proximal ulna anlagen, but is absent distally (Fig. 4I′).
Ulna formation is under the control of Sonic Hedgehog (Shh) (Ros et al., 2003). The differential expression patterns of Agc, Matn1 and Matn4 in response to Lmx1b suggest that posterior cartilage proteoglycan regulation may be a collective target of Lmx1b and Shh. Furthermore, these findings support the concept that Lmx1b may work in concert with other patterning factors along different axes, such as Shh, to generate asymmetrical structures.
Lmx1b regulated genes associated with connective tissue development
A large second group of target genes uncovered by our analysis express within the dorsal limb mesoderm and, therefore, may be regulated by Lmx1b to pattern dorsal soft tissues. These include: Empty spiracles homolog 2 (Emx2), Apolipoprotein D (ApoD), Lysyl oxidase (Lox), Keratocan (Kera), Lumican (Lum), and Decorin (Dec).
Emx2 expression extends along the dorsal mesoderm of the zeugopod, continuing distally into the autopod and digits (Fig. 4C). This expression is absent in Lmx1b KO limbs (Fig. 4C′). Overexpression of Emx2 in the chick model induced a single posterior or mirrored digit on the anterior border (Pröls et al., 2004). A role for Lmx1b in the integration of Shh signals has been suggested by Tzchori and co-workers (Tzchori et al., 2009), although no disruption of Shh signaling is evident in Lmx1b KO limbs (Chen et al., 1998). As suggested for posterior proteoglycans, it is possible that Lmx1b and Shh work in combination to also regulate digit-specific Emx2 activity. Alternatively, Emx2 and Shh could work in concert to establish dorsal digit identity. Irrespectively, overexpression of Emx2 in the limb appears to mimic or induce ectopic Shh expression.
ApoD is a multi-ligand, multi-functional transporter (Rassart et al., 2000). The expression of ApoD at 13.5 dpc has been reported in the dorsal limb ectoderm (Gu & Kania, 2010). We confirmed dorsal ectoderm expression at 13.5 dpc; however, at 12.5 dpc, we found ApoD expression in the anterior dorsal mesoderm (Fig. 4B). With its stage-specific shift from dorsal limb mesoderm to ectoderm and loss of expression in Lmx1b KO limbs, it is unclear what role ApoD might play in limb development since ApoD KO mice have no reported limb abnormalities (Ganfornina et al., 2008).
Lox is a soft tissue associated target involved in collagen formation/maturation. Lox expression is broad in the proximal dorsal mesoderm extending out into the zeugopod (Fig. 4J). Lox expression at 12.5dpc overlaps Lmx1b expression in the zeugopod; however this expression is absent in the Lmx1b KO limb (Fig. 4J′). The role of this differential expression in response to Lmx1b is unclear. Since Lox is a copper binding enzyme involved in crosslinking collagen and elastin (Kagan & Trackman, 1991), it is possible that dorsal tendons/ligaments in mice have a composition of elastin and collagen that differs from ventral tendons and/or ligaments.
Kera, Dec, and Lum are proteoglycans associated with connective tissue development. Dec and Lum are associated with collagen fibrillogenesis and degeneration of the eye, skin and tendons. The ability to slow, but not to stop, the process of collagen formation allows for the homogeneous spacing between collagen fibrils during development. Not surprisingly, Dec KO (Danielson et al., 1997) and Lum KO mice (Chakravarti et al., 1998) present with irregularly spaced collagen, which causes weak or abnormal connective tissue i.e. weak tendons with irregular attachment sites, and skin fragility. Lmx1b KO mice display a marked reduction of Dec and Lum expression in the distal dorsal mesoderm with limited expression remaining in the proximal ventral and dorsal mesoderm (Fig. 4L,M). Kera has been studied in the embryonic eye and adult tendon where it is utilized for collagen fibril development and maintenance (Liu et al., 2003, Conrad & Conrad, 2003). Kera expression in the Lmx1b KO was undetectable (Fig. 4K′). In addition, expression of Collagen9a3 and Collagen11a2 (Fig. 5C,D) is reduced in Lmx1b KO limbs(Fig. 5C′,D′). The collective differential expressions suggest that Lmx1b plays an active role in collagen formation and maintenance, which may, in part, confer the dorsal character of these structures.
Lmx1b regulated genes associated with neuronal development and angiogenesis
Cerebellin 2 (Cbln2) and Leukocyte cell-derived chemotaxin 1 (Lect) demonstrate a strikingly similar focal pattern of expression of the dorsal zeugopod and autopod (Fig. 4D,E), which is greatly reduced in the Lmx1b KO (Fig. 4D′,E′). Lmx1b guides the initial branching and subsequent trajectory of dorsal motor axons (Kania et al., 2000). Interestingly, Cbln2 is abundant in the embryonic brain (Miura et al., 2006) and has been linked to connectivity in sensory neurons (Reiner et al.). Therefore, Lmx1b may work in conjunction with localized dorsal Cbln2 expression to guide motor axon trajectory and branching. Lect functions as an inhibitor of angiogenesis in the growth plate (Shukunami et al., 1999). Lect in the dorsal limb may assist Lmx1b in pattering blood vessels by restricting vasculogenesis of targeted locations associated with innervation and joint formation.
Lmx1b regulated genes associated with the developing scapula
The scapula, a component of the limb girdle, also develops as a dorsal structure. Scapular development is a complex process. Signals from the lateral plate mesoderm pattern the head of the scapula while Hox genes from somites create a segmental pattern along the blade (Huang et al., 2000, Huang et al., 2006). Several knockout (KO) mouse models display varying degrees of scapular aplasia. Emx2 (Pellegrini et al., 2001) and Wnt-βcatenin (Hill et al., 2006) KO mice display a complete loss of the scapula, while Pax1 KO mice develop hypoplastic scapulae lacking the acromion (Wallin et al., 1994). Tbx15 KO mice develop a foramen in the blade (Singh et al., 2005), Alx3 KO and Cart1 KOs (Brouwer et al., 2003) reduce the rostral blade. Despite these mechanistic insights, a comprehensive list of genes and how they interact during scapula formation remains unclear. Expression of several genes discovered in our study localize to the developing scapula in normal mice. Other than Emx2, the targets identified in our analyses have not previously been associated with scapular formation. Agc, Lum, Dec, and Lox show a reduction of scapular expression in Lmx1b KO mice (Fig. 4C′,G′,L′,M′). Emx2, Matn1, and Matn4 exhibit a striking loss of scapular expression (Fig. 4H′,I′). Since Lmx1b KO mice have hypoplastic scapulae, we suggest Lmx1b works in concert with these collective targets to augment scapular development.
Genes down-regulated by Lmx1b during limb development
We discovered two genes down-regulated by Lmx1b in the dorsal limb bud. Regulatory factor X, 4 (Rfx4) encodes for a transcription factor and has proximal anterior expression in the ventral mesoderm (Fig. 6A′). This localized expression is duplicated in the Lmx1b KO limb (Fig. 6B″). Rfx transcription factors have been associated with CNS development and may direct proximal nerve migration in the limb. A recent report found that an intraflagellar transport protein, ift172, is a target of Rfx4 regulation, and in a spontaneous mouse mutation, disrupted Shh and Gli signaling in the brain and spinal cord (Ashique et al., 2009). Rfx4 expression was also observed in the limb, but no skeletal abnormalities were reported (Ashique et al., 2009). Soft tissue or nerve patterning, however, was not examined. Thus, it is unclear what role this gene and the regulation of cilia may have on dorsal-ventral patterning.
AF4/FMR2 family, member 3 (Aff3) is expressed in both the dorsal and ventral mesoderm but has broader ventral expression during normal mouse limb development that includes the subectodermal mesoderm (Fig. 6C″,c″). In the Lmx1b KO mouse, Aff3 expression is equally accentuated in both the dorsal and ventral mesoderm (Fig. 6D″,d″). Aff3 was initially discovered in association with lymphoid tissue (Ma & Staudt, 1996), a more recent limb study detected expression at 11.5dpc and 13.5 dpc (Gyurján et al., 2011). The presence of Aff3 within the developing limb and Q-PCR detected down-regulation by Lmx1b, suggests a role for Aff3 in dorsoventral patterning.
Summary
Previous studies have performed microarray analysis to discover downstream Lmx1b targets. Krawchuk and Kania (2008) used proximal WT and Lmx1b KO 11.5 dpc hindlimb tissue. Keratocan was the only target discovered coincident with our study; however, Keratocan was considered “not significant or inconclusive”. Our data and data from others (Randy Johnson, personal communication) find Keratocan significantly elevated in comparative WT and Lmx1b KO mouse limb gene arrays. We propose Keratocan is a significant Lmx1b target important to dorsal tendon formation. A second microarray analysis was also published using 13.5 dpc limbs (Krawchuk & Kania, 2008, Gu & Kania, 2010). This report revealed ApoD as a downstream target and Cbln2 as a “target of interest”. These two genes were also detected by our microarray study, supporting their role as Lmx1b targets.
The persistent asymmetrical elevation of a cluster of proteoglycans during limb dorsalization is a noteworthy discovery from our data and may provide clues to the mechanical mechanism of dorsal morphogenesis. Kurpios and co-workers recently demonstrated differential proteoglycan content within left and right dorsal mesentery that caused asymmetric cell density, and the initiation of directional gut rotation (Kurpios et al., 2008). Thus, one mechanism Lmx1b may use to orchestrate dorsalization is the regulation of proteoglycans that modulate cell shape, density and migration.
Our multi-stage microarray data expands previous investigations to include skeletal, connective tissue, neuronal, and angiogenic targets regulated by Lmx1b during limb dorsalization. Several of these genes exhibit the Lmx1b binding site as described by Morello and coworkers (Morello et al., 2001), suggesting direct Lmx1b regulation. Although Lmx1b is necessary and sufficient to accomplish distal limb dorsalization (Chen et al., 1998), many of the genes in our data sets demonstrate a basal ventral expression. This finding supports the concept of Lmx1b functioning as a selector transcription factor in a multi-factor regulatory network as recently suggested by Qui and coworkers (Qiu et al., 2009).
Supplementary Material
Acknowledgments
We are grateful to Dr. Randy Johnson for sharing gene array data from e11.5 WT and Lmx1b KO mouse limbs. We would also like to thank Drs. Soriano and Payne for critical review of the manuscript. This work was supported in part by a grant from the National Institutes of Child Health and Human Development (NICHD) HD 39421(KCO).
References
- Ashique A, Choe Y, Karlen M, et al. The Rfx4 transcription factor modulates Shh signaling by regional control of ciliogenesis. Science signaling. 2009;2:ra70. doi: 10.1126/scisignal.2000602. [DOI] [PubMed] [Google Scholar]
- Bongers EM, Gubler MC, Knoers NV. Nail-patella syndrome. Overview on clinical and molecular findings. Pediatric Nephrology. 2002;17:703–712. doi: 10.1007/s00467-002-0911-5. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Ten Berge D, Wiegerinck R, Meijlink F. The OAR/aristaless domain of the homeodomain protein Cart1 has an attenuating role in vivo. Mechanisms of development. 2003;120:241–252. doi: 10.1016/s0925-4773(02)00416-1. [DOI] [PubMed] [Google Scholar]
- Buxton P, Edwards C, Archer CW, Francis-West P. Growth/differentiation factor-5 (GDF-5) and skeletal development. The Journal of Bone and Joint Surgery. 2001;83:S23. [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, Lamantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. The Journal of cell biology. 1998;141:1277. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Johnson RL. Interactions between dorsal-ventral patterning genes lmx1b, engrailed-1 and wnt-7a in the vertebrate limb. International Journal of Developmental Biology. 2002;46:937–942. [PubMed] [Google Scholar]
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nature genetics. 1998;19:51–55. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- Conrad AH, Conrad GW. The keratocan gene is expressed in both ocular and non-ocular tissues during early chick development. Matrix Biology. 2003;22:323–337. doi: 10.1016/s0945-053x(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Cortes M, Baria AT, Schwartz NB. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development. 2009;136:1697. doi: 10.1242/dev.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig FM, Bentley G, Archer CW. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987;99:383. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Cygan JA, Johnson RL, Mcmahon AP. Novel regulatory interactions revealed by studies of murine limb pattern in Wnt-7a and En-1 mutants. Development. 1997;124:5021. doi: 10.1242/dev.124.24.5021. [DOI] [PubMed] [Google Scholar]
- Dalgleish AE. Development of the limbs of the mouse. Dept. of Anatomy; 1964. [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. The Journal of cell biology. 1997;136:729. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer SD, Naruse T, Morello R, et al. Lmx1b expression during joint and tendon formation: localization and evaluation of potential downstream targets. Gene expression patterns. 2004;4:397–405. doi: 10.1016/j.modgep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Dreyer SD, Zhou G, Baldini A, et al. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nature genetics. 1998;19:47–50. doi: 10.1038/ng0598-47. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Do Carmo S, Lora JM, et al. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell. 2008;7:506–515. doi: 10.1111/j.1474-9726.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WXW, Kania A. Identification of genes controlled by LMX1B in E13. 5 mouse limbs. Developmental Dynamics. 2010;239:2246–2255. doi: 10.1002/dvdy.22357. [DOI] [PubMed] [Google Scholar]
- Gyurján I, Sonderegger B, Naef F, Duboule D. Analysis of the dynamics of limb transcriptomes during mouse development. BMC developmental biology. 2011;11:47. doi: 10.1186/1471-213X-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal -catenin during murine limb patterning. Development. 2006;133:1219. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- Huang R, Christ B, Patel K. Regulation of scapula development. Anatomy and embryology. 2006;211:65–71. doi: 10.1007/s00429-006-0126-9. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Patel K, Wilting J, Christ B. Dual origin and segmental organisation of the avian scapula. Development. 2000;127:3789. doi: 10.1242/dev.127.17.3789. [DOI] [PubMed] [Google Scholar]
- Kagan H, Trackman P. Properties and function of lysyl oxidase. American journal of respiratory cell and molecular biology. 1991;5:206. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102:161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- Krawchuk D, Kania A. Identification of genes controlled by LMX1B in the developing mouse limb bud. Developmental Dynamics. 2008;237:1183–1192. doi: 10.1002/dvdy.21514. [DOI] [PubMed] [Google Scholar]
- Kurpios NA, Ibaòes M, Davis NM, et al. The direction of gut looping is established by changes in the extracellular matrix and in cell: cell adhesion. Proceedings of the National Academy of Sciences. 2008;105:8499. doi: 10.1073/pnas.0803578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Birk DE, Hassell JR, Kane B, Kao WWY. Keratocan-deficient mice display alterations in corneal structure. Journal of Biological Chemistry. 2003;278:21672. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- Loomis CA, Kimmel RA, Tong CX, Michaud J, Joyner AL. Analysis of the genetic pathway leading to formation of ectopic apical ectodermal ridges in mouse Engrailed-1 mutant limbs. Development. 1998;125:1137. doi: 10.1242/dev.125.6.1137. [DOI] [PubMed] [Google Scholar]
- Ma C, Staudt L. LAF-4 encodes a lymphoid nuclear protein with transactivation potential that is homologous to AF-4, the gene fused to MLL in t (4; 11) leukemias. Blood. 1996;87:734. [PubMed] [Google Scholar]
- Mitrovic D. Development of the diarthrodial joints in the rat embryo. American Journal of Anatomy. 1978;151:475–485. doi: 10.1002/aja.1001510403. [DOI] [PubMed] [Google Scholar]
- Miura E, Iijima T, Yuzaki M, Watanabe M. Distinct expression of Cbln family mRNAs in developing and adult mouse brains. European Journal of Neuroscience. 2006;24:750–760. doi: 10.1111/j.1460-9568.2006.04950.x. [DOI] [PubMed] [Google Scholar]
- Morello R, Zhou G, Dreyer SD, et al. Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nature genetics. 2001;27:205–208. doi: 10.1038/84853. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Pantano S, Fumi MP, Lucchini F, Forabosco A. Agenesis of the scapula in Emx2 homozygous mutants. Developmental biology. 2001;232:149–156. doi: 10.1006/dbio.2001.0159. [DOI] [PubMed] [Google Scholar]
- Pröls F, Ehehalt F, Rodriguez-Niedenführ M, He L, Huang R, Christ B. The role of Emx2 during scapula formation. Developmental biology. 2004;275:315–324. doi: 10.1016/j.ydbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Qiu Q, Chen H, Johnson RL. Lmx1b expressing cells in the mouse limb bud define a dorsal mesenchymal lineage compartment. Genesis. 2009;47:224–233. doi: 10.1002/dvg.20430. [DOI] [PubMed] [Google Scholar]
- Rainer J, Sanchez-Cabo F, Stocker G, Sturn A, Trajanoski Z. CARMAweb: comprehensive R-and bioconductor-based web service for microarray data analysis. Nucleic Acids Research. 2006;34:W498. doi: 10.1093/nar/gkl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E, Bedirian A, Do Carmo S, et al. Apolipoprotein d. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 2000;1482:185–198. doi: 10.1016/s0167-4838(00)00162-x. [DOI] [PubMed] [Google Scholar]
- Reiner A, Yang M, Cagle MC, Honig MG. Localization of cerebellin 2 in late embryonic chicken brain: Implications for a role in synapse formation and for brain evolution. The Journal of Comparative Neurology. doi: 10.1002/cne.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT6a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995;83:631–640. doi: 10.1016/0092-8674(95)90103-5. [DOI] [PubMed] [Google Scholar]
- Ros MA, Dahn RD, Fernandez-Teran M, et al. The chick oligozeugodactyly (ozd) mutant lacks sonic hedgehog function in the limb. Development. 2003;130:527. doi: 10.1242/dev.00245. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Iyama K, Inoue H, Hiraki Y. Spatiotemporal pattern of the mouse chondromodulin-I gene expression and its regulatory role in vascular invasion into cartilage during endochondral bone formation. International Journal of Developmental Biology. 1999;43:39–50. [PubMed] [Google Scholar]
- Singh MK, Petry M, Haenig B, Lescher B, Leitges M, Kispert A. The T-box transcription factor Tbx15 is required for skeletal development. Mechanisms of development. 2005;122:131–144. doi: 10.1016/j.mod.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh T, Copeland N, Jenkins NA, Kingsley DM, Lee S. Limb alterations in brachypodism mice due to mutations in a new member of the TGFb-superfamily. Nature. 1994;368:639–642. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Tzchori I, Day TF, Carolan PJ, et al. LIM homeobox transcription factors integrate signaling events that control three-dimensional limb patterning and growth. Development. 2009;136:1375. doi: 10.1242/dev.026476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, Rodriguez C, Warnken W. Izpisua-Belmonte JC (1995) Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature. 1995;378:716–720. doi: 10.1038/378716a0. [DOI] [PubMed] [Google Scholar]
- Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Yamada Y. Chondrodysplasia of gene knockout mice for aggrecan and link protein. Glycoconjugate journal. 2002;19:269–273. doi: 10.1023/A:1025344332099. [DOI] [PubMed] [Google Scholar]
- Yamada M, Szendro PI, Prokscha A, Schwartz RJ, Eichele G. Evidence for a role of Smad6 in chick cardiac development. Developmental biology. 1999;215:48–61. doi: 10.1006/dbio.1999.9419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.