Abstract
Aims
Chronic chorioamnionitis is a histological manifestation of maternal anti-fetal cellular rejection. Failure of graft survival being the most catastrophic event in organ transplantation, we hypothesized that fetal death could be a consequence of rejection of the mother against the fetus. This study was conducted to assess evidence of cellular and antibody-mediated rejection in fetal death cases.
Methods and results
Placental histology was reviewed for the presence of chronic chorioamnionitis in unexplained preterm fetal death (n=30) and preterm live birth (n=103) cases. Amniotic fluid CXCL10 concentrations were measured by specific immunoassay. Chronic chorioamnionitis was more frequent in fetal death cases than in live birth cases (60.0% versus 37.9%; P<0.05) and fetal death cases had a higher median amniotic fluid CXCL10 concentration than live birth cases (2.0 ng/ml versus 1.8 ng/ml, P<0.05), after adjusting for gestational age at amniocentesis. Maternal anti-human leucocye antigen (HLA) class II seropositivity determined by flow cytometry was higher in fetal death cases compared to live birth cases (35.7% versus 10.9%; P<0.05).
Conclusions
Chronic chorioamnionitis is a common pathology of unexplained preterm fetal death. The novel findings herein suggest strongly that cellular and antibody-mediated anti-fetal rejection of the mother is associated with fetal death (graft failure) in human pregnancy.
Keywords: anti-HLA antibody, chronic chorioamnionitis, fetal death, pregnancy, rejection
Introduction
As more than 3.2 million stillbirths are being reported worldwide every year,1 fetal death is one of the most challenging and devastating obstetric complications. Meticulous placental and autopsy examinations are important in the identification of the causes of unexpected fetal death.2 However, variable fractions of fetal death (ranging from 25% to 60%) cannot be explained, regardless of clinical and pathological examination, including autopsies.3 Of note, small-for-gestational-age (SGA) pregnancy is associated frequently with fetal death,4 and evidence suggests strongly that fetal death is associated with a maternal systemic anti-angiogenic state, which is characterized by decreased plasma concentration of pro-angiogenic molecules and increased plasma concentration of anti-angiogenic molecules.5,6 We have reported decreased concentration of placental growth factor (PlGF) and elevated concentrations of anti-angiogenic molecules, such as soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) and soluble endoglin (sEng), in the maternal circulation at the time of diagnosis of unexplained fetal death cases.5 A subsequent longitudinal analysis showed that the pattern of dysregulation of angiogenic and anti-angiogenic factors in fetal death is distinct from that of pre-eclampsia. Patients destined to develop fetal death have higher PlGF and lower sVEGFR-1 and sEng plasma concentrations in the first trimester than do normal pregnancies; this is reversed in the mid- and third trimesters.6 Therefore, it is highly likely that ongoing, chronic biological perturbations in the feto–maternal compartments precede this catastrophic event.
Chronic chorioamnionitis is defined as amniotrophic infiltration of maternal T cells into the chorioamniotic membranes.7,8 It is associated commonly with preterm labour and preterm prelabour rupture of membranes with increased amniotic fluid (AF) CXCL10 concentration and CXCL9, CXCL10 and CXCL11 mRNA overexpression in the chorioamniotic membranes, all of which are T cell chemokines.9 CXCL9, CXCL10 and CXCL11 exert their effects by binding to CXCR3, which is present in T cells and natural killer cells.10,11 CXCR3 is a G protein-coupled surface receptor, having seven transmembrane α-helical structures. Its N-terminal extracellular domain is critical for ligand binding while the intracellular C-terminal domain is involved in signal transduction on receptor activation by ligand (CXCL9, CXCL10, CXCL11) binding.12 Sharing a common pathogenesis with villitis of unknown aetiology (VUE), chronic chorioamnionitis is considered a histological manifestation of maternal anti-fetal cellular rejection occurring in the chorioamniotic membranes.
The fetus is a semi-allogeneic graft to the mother, and its survival is a key parameter of successful reproduction. We hypothesized that a subset of fetal death might be analogous to the failure of graft survival in organ transplantation. Evidence supports that CXCL9, CXCL10 and CXCL11 are functionally involved in graft rejection. Pre- and post-transplant CXCL9 and CXCL10 concentrations in patients' sera have predictive value for acute rejection in cardiac and renal allograft loss.13–16 CXCL11 is a dominant chemokine in recruiting CXCR3+ T cells into allogeneic skin graft in mice.17 The purpose of this study was to determine whether the frequency of chronic chorioamnionitis is higher in fetal death than in a gestational age-matched group of live births.
Materials and methods
Patient Population and Study Materials
All participating patients delivered at Hutzel Women's Hospital, Detroit, Michigan, USA, and provided written informed consent. The Institutional Review Boards of the participating institutions approved the collection of clinical information and use of biological materials for research purposes. We selected cases in which amniotic fluid samples were available from the Bank of Biological Materials of the Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, and abnormalities or major structural anomalies. Autopsies were performed in all unexplained fetal death cases (n=30), and major pathology which could explain fetal death was not found. Preterm labour was defined by the presence of regular uterine contractions with cervical dilation that led to delivery before 37 weeks of gestation. The SGA cases were defined as less than the 10th percentile in birth weight for gestational age. Amniotic fluid samples were obtained by transabdominal amniocentesis from women who underwent amniocentesis for clinical indications. Samples were kept at −80°C until analysed by immunoassay.
Histopathological Examination
Histopathological assessment of the placental lesions was performed based upon the diagnostic criteria proposed by the Perinatal Section of the Society for Pediatric Pathology.18–21 Chronic chorioamnionitis was diagnosed when lymphocytic infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue was present, as described previously.9 The extent of inflammation in chronic chorioamnionitis was defined as grade 1 when there were three or more foci of, or patchy, inflammation, and grade 2 when diffuse inflammation was bserved. 9 The stage of inflammation was evaluated as stage I if amniotropic lymphocytic infiltration was confined to the chorionic trophoblast layer without the involvement of the chorioamniotic connective tissue, and stage II if lymphocytic infiltration into the chorioamniotic connective tissue was noted.9 The haematoxylin and eosin (H&E) sections of the chorioamniotic membranes roll (n=2), umbilical cord (n=2) and placental disc (n =3) were examined. Placental findings were reviewed by one pathologist (C.J.K.), who was masked to all clinical information except for the gestational age at delivery.
Immunohistochemistry
To confirm that lymphocytic infiltrates into chorioamniotic membranes are T cells, immunohistochemistry was conducted in representative examples of both live birth cases (n=5) and fetal death cases (n=5) using 5-μm-thick paraffin sections. Primary antibodies used were murine monoclonal anti-CD3 (1:50 diluted; Dako, Carpinteria, CA, USA) and anti-CD8 (1:100 diluted; Dako) and immunostaining was performed using the Ventana Discovery automatic staining system (Ventana Medical Systems, Tucson, AZ, USA). The Discovery DAB Map Kit (Ventana Medical Systems) was used to detect the chromogen reaction.
Enzyme-Linked Immunosorbent Assay (Elisa)
Amniotic fluid samples obtained by transabdominal amniocentesis were centrifuged at 1300 g for 10 min and stored at −80°C until use. The AF concentrations of interleukin (IL-6) and CXCL10 were measured by specific ELISA (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions.
Flow Cytometry
Flow cytometric evaluation of panel-reactive anti-HLA class I and class II antibodies in maternal serum samples was performed using the FlowPRA®-I and FlowPRA®-II screening tests (One Lambda Inc., Canoga Park, CA, USA), respectively, according to the manufacturer's instructions. Briefly, 20 μl of serum samples were incubated with microbeads for 30 min at room temperature with gentle rotation. The beads were washed three times with 1 ml of FlowPRA wash buffer, and centrifuged at 9000 g for 2 min. The beads were incubated subsequently with 100 μl of fluorescein isothiocyanate (FITC)-conjugated F(ab)2 fragment of Fcγ fragment-specific goat anti-human immunoglobulin (Ig)G for 30 min. The beads were washed and 0.5 ml of fixing solution [0.5% formaldehyde in phosphate-buffered saline (PBS)] was added. The FL1 fluorescence of 5000 events was analysed using BD LSRII flow cytometry (BD Biosciences, San Jose, CA, USA). Samples with panel reactivity of 10% or more were considered positive for the screening test of panel-reactive anti-HLA antibodies.22,23
Statistical Analysis
Univariate analysis was performed with an unpaired t-test or a Mann–Whitney U test for continuous variables and with χ2 tests or Fisher's exact tests for categorical variables, as appropriate. Analysis of covariance was conducted for comparisons of AF IL-6 and CXCL10 concentrations among groups to adjust for gestational age at amniocentesis. Logistic regression analysis was conducted to examine the effect of the placental histopathological lesions and other clinical variables (e.g. maternal age, drug abuse, smoking, race, gravida, maternal medical diseases such as hypertensive disorders and diabetes mellitus, gestational age at delivery, SGA and fetal gender) on the occurrence of fetal death. Statistical analysis was performed using spss version 15.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered significant.
Results
Frequency Of Chronic Chorioamnionitis
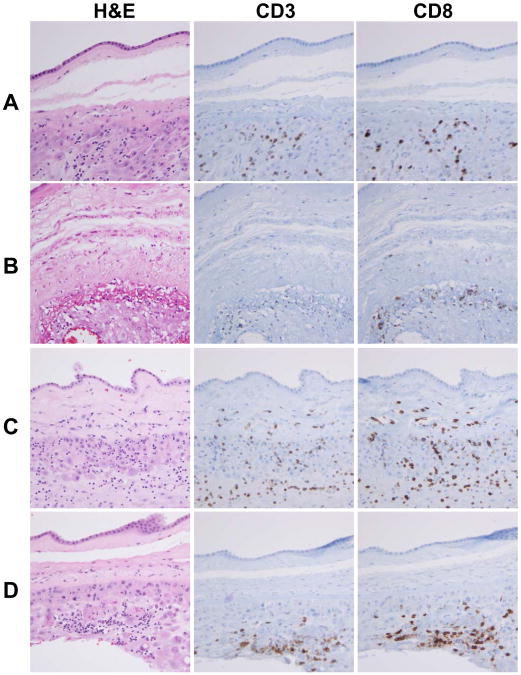
The demographics and clinical characteristics of patients are summarized in Table 1. Gestational age at amniocentesis, amniocentesis to delivery interval, gravida, parity and the use of antenatal steroids were among the clinical parameters which showed significant differences between fetal death and live birth cases. Of note, the percentage of SGA cases was significantly higher in fetal death (36.7%) than in the live birth group (15.5%) (P<0.05; Table 1). Representative histological features of chronic chorioamnionitis cases showing amniotropic T cell infiltration in the chorioamniotic membranes in fetal death cases are shown in Figure 1. Chronic chorioamnionitis typically showed margination of lymphocytes in the choriodecidual interface with foci of infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue. Immunohistochemistry for CD3 and CD8 in representative live birth (n=5) and fetal death (n=5) cases confirmed that CD8+ cytotoxic T cells are a major subset of lymphocytic infiltrates. Among the chronic chorioamnionitis cases (n=57), 63.2% (n=36) of the cases showed focal or patchy lymphocytic infiltrations compatible with grade 1 inflammation and 36.8% (n=21) of cases had grade 2 inflammation in the chorioamniotic membranes. In addition, 22.8% (n=13) of cases had stage I inflammation limited to the chorionic trophoblast layer and 77.2% (n=44) of the cases had stage II inflammation involving the chorioamniotic connective tissue layer.
Table 1. Demographic and clinical characteristics of study population.
| Preterm Live birth n=103 | preterm Fetal death n=30 | P- value | |
|---|---|---|---|
| Maternal age (years)* | 23 (16–44) | 25.5 (16–35) | NS |
| Primigravida (%) | 23.3 (24/103) | 43.3 (13/30) | 0.031 |
| Nullipara (%) | 36.9 (38/103) | 56.7 (7/30) | 0.053 |
| Gestational age at delivery (weeks)* | 31.9 (20.4–36.4) | 31.0 (21.6–36.9) | NS |
| Birth weight (g)* | 1588 (280–2920) | 1431.5 (304–3400) | NS |
| Small for gestational age (%) | 15.5 (16/103) | 36.7 (11/30) | 0.011 |
| Baby gender (male) (%) | 53.4 (55/103) | 56.7 (17/30) | NS |
| Antenatal steroid use (%) | 83.5 (86/103) | 0.0 (0/30) | <0.001 |
| Caesarean delivery (%) | 35.9 (39/103) | 0.0 (0/30) | <0.001 |
| Hypertensive disorders in pregnancy (%) | 6.8 (7/103) | 3.3 (1/30) | NS |
| Diabetes mellitus (%) | 1.9 (2/103) | 3.3 (1/30) | NS |
| Smoking (%) | 25.2 (26/103) | 23.3 (7/30) | NS |
| Drug abuse (%) | 19.4 (20/103) | 20.0 (6/30) | NS |
| Gestational age at amniocentesis (weeks)* | 28.6 (15.4–35.9) | 30.9 (21.6–36.9) | 0.046 |
| Amniocentesis to delivery interval (days)* | 6.0 (0–89) | 0.7 (0–3) | <0.001 |
Median (range).
Figure 1.
Immunohistological features of chronic chorioamnionitis in preterm live birth and fetal death cases. T cell infiltrates are largely composed of CD8+ T cells. A, A preterm live birth case showing infiltration of CD8+ T cells into the chorionic trophoblast layer. B, A preterm live birth case with destruction and thinning of chorionic trophoblast layer by infiltrating CD8+ T cells. C, Infiltration of CD8+ T cells into the chorionic connective tissue layer in a case of fetal death. D, A fetal death case shows margination of CD8+ T cells at the choriodecidual interface with foci of scattered lymphocytic infiltration into the chorionic trophoblast layer.
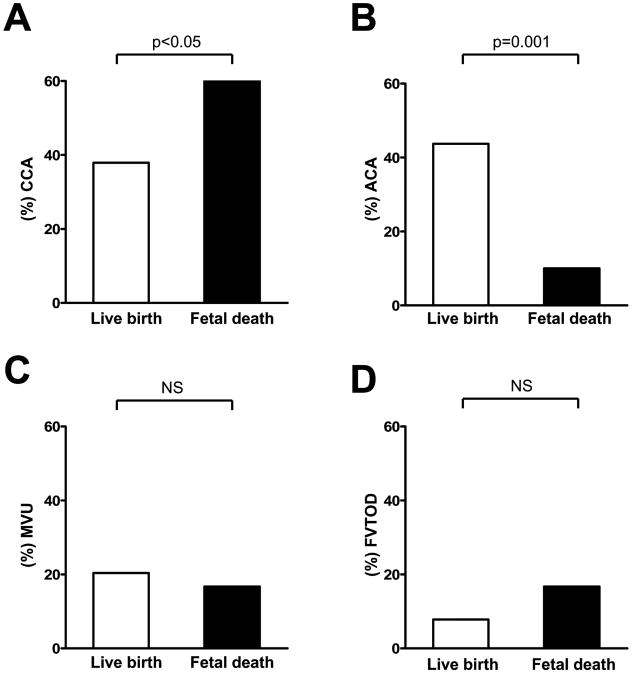
Chronic chorioamnionitis was more frequent in fetal death cases than in live birth cases (60% versus 37.9%, respectively; P<0.05) (Figure 2A). Among cases with chronic chorioamnionitis, the proportions of grade 2 inflammation and stage II inflammation in fetal death cases were not different from those in live birth cases (for grade 2 inflammation: 38.9% versus 35.9%; for stage II inflammation: 77.8% versus 76.9%), whereas acute chorioamnionitis was more frequent in live birth cases than in fetal death cases (43.7% versus 10.0%, respectively; P=0.001) (Figure 2B). After adjusting for clinical parameters, including maternal age, drug abuse, smoking, race, gravida, maternal medical diseases such as hypertensive disorders and diabetes mellitus, gestational age at delivery, SGA and fetal gender, which could have influenced the occurrence of fetal death in utero, the presence of chronic chorioamnionitis was an independent risk factor for fetal death [odds ratio (OR)=2.59, 95% confidence interval (CI) 1.01–6.66, P<0.05]; and the presence of acute chorioamnionitis had a protective effect against fetal death in utero (OR=0.09, 95% CI 0.02–0.40, P=0.001). The frequencies of the placental findings, consistent with maternal vascular underperfusion (20.4% versus 16.7%) and fetal vascular thrombo-occlusive disease (7.8% versus 16.7%), were not different between live birth and fetal death cases (Figure 2C,D). The frequency of VUE in live birth and fetal death cases was similar (8.7% and 10.0%, respectively).
Figure 2.
Comparisons of frequencies of placental lesions between preterm live birth cases and preterm fetal death cases. Chronic chorioamnionitis is more frequent in fetal death cases (A, P<0.05), while acute chorioamnionitis is more frequent in live birth cases (B, P =0.001). Conversely, no difference was noted in the findings consistent with maternal vascular underperfusion (C) and fetal vascular thrombo-occlusive disease (D). CCA: chronic chorioamnionitis; ACA: acute chorioamnionitis; MVU: maternal vascular underperfusion; FVTOD: fetal vascular thrombo-occlusive disease; NS: not significant.
Amniotic Fluid IL-6 and Cxcl10 Concentrations
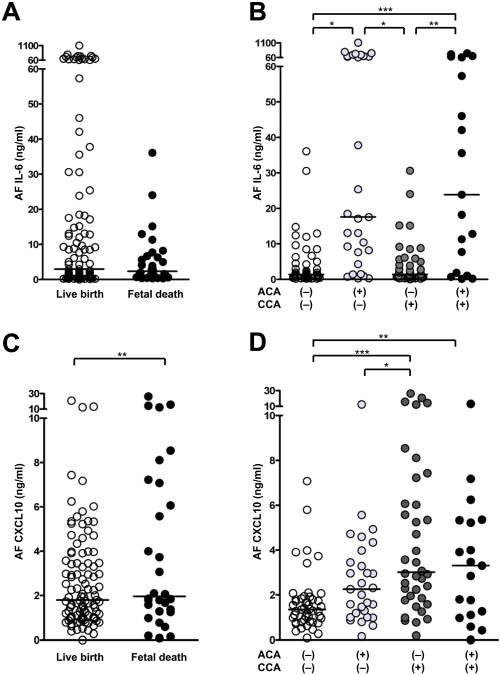
Amniotic fluid samples in all cases of live birth (n=103) and fetal death (n=30) were assayed for IL-6 and CXCL10. AF IL-6 concentration was not different between live birth (median: 3.0 ng/ml, range: 0.1–1,101.0 ng/ml) and fetal death (median: 2.4 ng/ml, range: 0.3–36.1 ng/ml), after adjusting for gestational age at amniocentesis (Figure 3A). When the cases were compared according to the presence or absence of acute and chronic chorioamnionitis, the presence of acute chorioamnionitis was associated with an elevated AF IL-6 concentration after adjusting for gestational age at amniocentesis (Figure 3B). The median AF IL-6 concentration was significantly higher in cases with isolated acute chorioamnionitis (n=29) (median: 17.6 ng/ml, range: 0.3–1–101.0 ng/ml) than in those without chorioamnionitis (n=47) (median: 1.4 ng/ml, range: 0.2–36.1 ng/ml) and in those with isolated chronic chorioamnionitis (n=38) (median: 1.4 ng/ml, range: 0.1–30.6 ng/ml) (P<0.05, for each). The median AF IL-6 concentration was also higher in cases with concomitant acute and chronic chorioamnionitis (n=19) (median: 23.9 ng/ml, range: 0.1–360.1 ng/ml) than in those without chorioamnionitis and in those with isolated chronic chorioamnionitis (P<0.01, for each), whereas a higher AF CXCL10 concentration was observed in fetal death (median: 2.0 ng/ml, range: 0.1–26.3 ng/ml) than in live birth (median: 1.8 ng/ml, range: 0.0–20.8 ng/ml), after adjusting for gestational age at amniocentesis (P<0.01; Figure 3C). As is the case with acute chorioamnionitis, chronic chorioamnionitis was associated with the elevation of AF CXCL10 concentration, after adjusting for gestational age at amniocentesis (Figure 3D). Cases with isolated chronic chorioamnionitis had a higher median AF CXCL10 concentration (median: 3.0 ng/ml, range: 0.2–23.3 ng/ml) than those without chorioamnionitis (median: 1.3 ng/ml, range: 0.1–7.1 ng/ml) and those with isolated acute chorioamnionitis (median: 2.3 ng/ml, range: 0.2–12.4 ng/ml) (P<0.05, for each). AF CXCL10 concentrations in chronic chorioamnionitis cases with grade 1 inflammation (median: 2.9 ng/ml, range: 0.0–15.7 ng/ml) and grade 2 inflammation (median: 3.3 ng/ml, range: 0.8–26.3 ng/ml) were higher than in cases without chronic chorioamnionitis (median: 1.5 ng/ml, range: 0.1–12.4 ng/ml) (P<0.01, for each). Similarly, chronic chorioamnionitis cases with stage I inflammation (median: 5.3 ng/ml, range: 1.0–15.7 ng/ml) and stage II inflammation (median, 2.8 ng/ml: range, 0.0–26.3 ng/ml) had higher median AF CXCL10 concentrations than cases without chronic chorioamnionitis (P<0.01, for each). Cases with concomitant acute and chronic chorioamnionitis (median: 3.3 ng/ml, range: 0.0–13.2 ng/ml) also had a significantly higher median of AF CXCL10 concentration than those without chorioamnionitis (P<0.01).
Figure 3.
Amniotic fluid (AF) concentrations of interleukin (IL)-6 and CXCL10 in live birth and fetal death cases and in acute and chronic chorioamnionitis cases. A, AF IL-6 concentration is not different between cases with live birth and fetal death. B, The presence of acute chorioamnionitis, regardless of chronic chorioamnionitis, is associated with a robust increase in IL-6. C, Fetal death cases have a higher median AF CXCL10 concentration than live birth cases (P<0.05). D, Median AF CXCL10 concentrations are elevated in cases with isolated chronic chorioamnionitis and cases with concomitant acute and chronic chorioamnionitis, compared to cases without chorioamnionitis (P<0.05, for each). All comparisons of AF IL-6 and CXCL10 concentrations are performed after adjusting for gestational age at amniocentesis. *P<0.05; **P<0.01; ***P<0.001. AF: amniotic fluid; CCA: chronic chorioamnionitis; ACA: acute chorioamnionitis.
Panel-Reactive Anti-Hla Antibodies In Maternal Sera
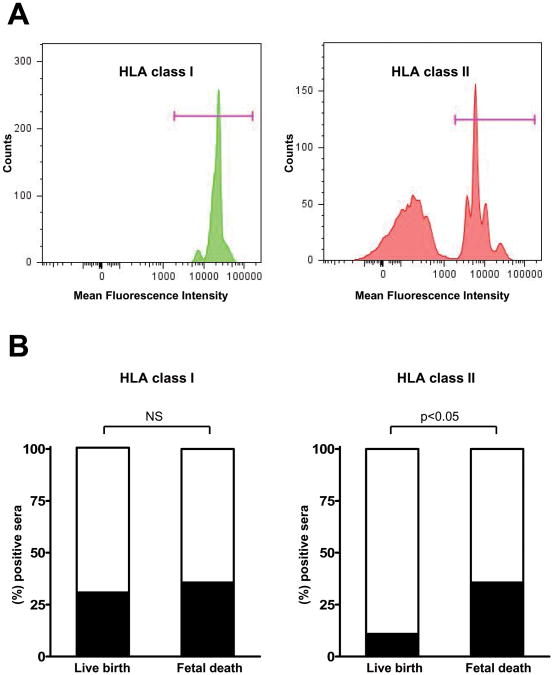
Given that chronic chorioamnionitis displays pathological features of cellular rejection, and that both cellular and humoral antibody-mediated rejection mechanisms are important in the development of allograft rejection, we screened for the presence of panel-reactive anti-HLA class I and class II antibodies in maternal circulation. Flow cytometric analysis was performed in maternal serum samples obtained at the time of delivery in preterm live birth cases (n=57) and preterm fetal death cases (n=18). Flow cytometry demonstrated clearly that some patients have panel-reactive anti-HLA antibodies in their circulation. Maternal seropositivity for anti-HLA class I antibodies was not different between preterm live birth and fetal death cases (30.9% versus 35.7%, respectively; Figure 4A). However, the seropositivity for anti-HLA class II antibodies was significantly different between preterm live birth and fetal death cases (10.9% versus 35.7%; P<0.05; Figure 4B).
Figure 4.
Panel-reactive anti-human leucocyte antigen (HLA) antibodies in maternal sera. A, Flow cytometric analysis clearly shows anti-HLA class I (left) and anti-HLA-class II (right) antibodies in maternal sera of a fetal death case. B, Seropositivity for anti-HLA class II but not anti-HLA class I antibodies is significantly higher in mothers with fetal death compared to those who gave preterm live births (P<0.05). NS: not significant.
Discussion
Fetal death could be associated with several maternal and feto–placental conditions such as infection, alloimmunization and congenital anomalies.24,25 Korteweg et al., using the Dutch Tulip cause of death classification for perinatal mortality, proposed that the main causes of fetal death are placental disorders (64.9%), congenital anomalies (5.3%), infection (1.9%), other (4.8%) and unknown (23.1%).26,27 The primary finding of our study is that chronic chorioamnionitis is a major placental lesion of preterm fetal death, which might explain a major subset of unexplained fetal death cases. We also demonstrate, for the first time, that panel-reactive anti-HLA class II antibodies, which are evidence of anti-fetal antibody-mediated rejection, are found more frequently in maternal sera of fetal death than in live birth cases.
Immunohistological findings observed in the present study confirmed that chronic chorioamnionitis is CD8+ cytotoxic T cell-rich inflammation. While the severity (grade and stage of inflammation) of chronic chorioamnionitis was not different between cases of live birth and fetal death, we have also found that amniotic fluid CXCL10 concentration is higher even in grade 1 or stage I inflammation. This is consistent with the findings in our previous analysis of chronic chorioamnionitis in various obstetric settings, in that even histologically mild cases have a robust elevation of amniotic fluid CXCL10 concentration.9 Therefore, meticulous histological evaluation to detect low-grade, low-stage chronic chorioamnionitis would be important.
In addition to T cell chemokine activity, interferon (IFN)-γ-inducible CXCL9, CXCL10 and CXCL11 share anti-angiogenic properties on CXCR3+ vascular endothelial cells.28,29 CXCL10 antagonizes the actions of VEGF on tube formation in Matrigel™, and also cell motility of CXCR3+ endothelial cells.30 It also induces regression of newly formed vessels via activation of CXCR3 on endothelial cells.31 An intriguing observation in this study is that the proportion of SGA cases is significantly greater in fetal death, while the frequency of placental lesions consistent with maternal vascular underperfusion or fetal vascular thrombo-occlusive disease was not different between fetal death and live birth cases. This implies that the mechanism of SGA in fetal death cases cannot be attributed solely to placental underperfusion. Villitis of unknown aetiology is the villous counterpart of chronic chorioamnionitis characterized by maternal T cell infiltration into fetal chorionic villi, and we have demonstrated systemic elevation of CXCL9, CXCL10 and CXCL11 in both maternal and umbilical cord plasma in cases with VUE.32,33 Villitis of unknown aetiology can accompany obliterative fetal vasculopathy of the placental villous tree and SGA showing potential impact of increased anti-angiogenic chemokines.34,35 Previous studies also have shown lower birth weight percentile and fetal growth restriction as features of cases with chronic chorioamnionitis.7,8 In this context, the systemic anti-angiogenic milieu in the fetus could explain the development of SGA in some fetal deaths, although its causal relationship and pathobiology require further studies.
Panel-reactive anti-HLA antibodies (anti-paternal/fetal antibodies) have been studied mainly in the settings of recurrent spontaneous abortions and miscarriages.36–38 In this study, we found for the first time that anti-HLA antibodies were detected more frequently in maternal circulation of fetal death than in live birth cases. In addition to chronic chorioamnionitis, this is strong evidence for the presence of maternal anti-fetal rejection, which could be a fundamental derangement associated with or causally related to fetal death. In this context, the clinicopathological findings described meticulously in the original study of chronic chorioamnionitis is significant and has profound implications. Gersell et al. reported that seven of 17 patients with chronic chorioamnionitis had a history of spontaneous abortion, fetal death or preterm birth.7 These three conditions could actually be different manifestations of a common pathophysiology: maternal anti-fetal rejection. The fetus is protected from maternal immune responses by trophoblasts which mainly express fewer polymorphic HLA-G molecules.39,40 Maternal sensitization to HLA antigens can result from previous pregnancy, transfusion, feto–maternal haemorrhage and potential fetal cell trafficking into the mother.41–43 The significant difference in seropositivity of anti-HLA class II but not anti-HLA class I antibodies between fetal death and live birth groups is intriguing, because HLA class II antigens are not expressed in the cells at the very site of the feto–maternal interface, the trophoblasts. However, anti-HLA antibodies which cross the placenta are found in fetal circulation,44 and neonatal thrombocytopenia by maternal anti-HLA antibodies has been reported as a side effect following allogenic leucocyte immunization in women with recurrent abortions.45 Therefore, further investigation is required to determine whether maternal anti-HLA antibodies induce distinct types of fetal damage in addition to known alloimmune reactions such as red-cell alloimmunization and alloimmune thrombocytopenia.46,47
Several previous studies in humans and mice have indicated that the activation of the complement system leads to preterm birth, pre-eclampsia and fetal death.48 Concentrations of C5a and fragment Bb, arkers of the activation of the alternative pathway of the complement system, are elevated in women with preterm labour.48,49 More importantly, in a murine model of peri-implantation pregnancy loss and intrauterine growth restriction, Girardi et al. have shown that C5a generated by the activation of the alternative pathway of the complement system is an essential trigger of fetal injury, and that complement activation products induce increased release of sVEGFR-1 from mouse splenic macrophages.50 These findings are consistent with the changes in maternal plasma pro- and anti-angiogenic factors, including elevation of sVEGFR-1. In conjunction with this observation in mice, it has been demonstrated that the median plasma concentration of C5a is higher in patients with fetal death than in normal pregnant women.51 Therefore, it is also possible that activation of the classical pathway of the complement system by complement-fixing anti-HLA antibodies functions as a link between an anti-angiogenic state and fetal death.
A limitation of this study is the definition of unexplained fetal death. Fetal death is considered unexplained when a cause cannot be identified by fetal, maternal, placental and obstetric factors.3 Bonetti et al. have reported that scrupulous clinicopathological analyses, including fetal autopsies and placental examinations, could explain more than 80% of fetal death cases.2 However, establishment of causality with placental lesions in fetal death is difficult unless there are unequivocal findings such as rupture of vasa previa and abruption. Similarly, in their recent proposal the Stillbirth Collaborative Research Network also excluded isolated acute histological chorioamnionitis and SGA from direct causes of death and listed only extensive and severe placental changes among possible and probable causes of fetal death.52 The placental lesions such as acute chorioamnionitis and maternal vascular underperfusion are actually far more common in live births.6 In the present study, we defined clinically unexplained fetal death as fetal death without definite causes such as placental abruption, torsion and/or stricture of umbilical cord sufficient to cause fetal death, and fetal chromosomal abnormalities or major structural anomalies. Another issue in the interpretation of the results of this study is the significant difference in the amniocentesis-to-delivery interval between fetal death cases (study group) and live birth cases (control group). This seems to be an unavoidable problem due to a difference in clinical presentation. All maternal serum samples were also obtained only at the time of delivery; thus, the role of changing dynamics of maternal anti-HLA antibodies across gestation could not be determined. Therefore, further observations on a longitudinal basis would be necessary in the future.
Collectively, the findings of the present study suggest strongly that a substantial proportion of preterm fetal deaths has a biological signature of maternal anti-fetal rejection. Chronic chorioamnionitis is a major placental lesion in clinically unexplained preterm fetal death. Identification of the deleterious effects of anti-angiogenic T cell chemokines (CXCL9, CXCL10, CXCL11) in maternal, fetal and placental compartments would be a key to understanding the pathogenesis of unexplained preterm fetal death. Assessment of potential immunopathological consequences associated with alloimmune anti-HLA antibodies will be another important area for future research, and a recent observation by Nielsen et al., that the presence of these antibodies in early pregnancy is associated with a reduced likelihood of a live birth, could be an important piece of supportive evidence for the proposal described herein.38
Acknowledgments
This work was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Abbreviations
- AF
amniotic fluid
- HLA
human leucocyte antigen
- PlGF
growth factor
- SGA
small-for-gestational-age
- sEng
soluble endoglin
- sVEGFR-1
soluble vascular endothelial growth factor receptor-1
- VUE
villitis of unknown aetiology
References
- 1.Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet. 2006;367:1487–1494. doi: 10.1016/S0140-6736(06)68586-3. [DOI] [PubMed] [Google Scholar]
- 2.Bonetti LR, Ferrari P, Trani N. The role of fetal autopsy and placental examination in the causes of fetal death: a retrospective study of 132 cases of stillbirths. Arch Gynecol Obstet. 2010 Jan 6; doi: 10.1007/s00404-009-1317-4. [DOI] [PubMed] [Google Scholar]
- 3.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 4.Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ. 2005;331:1113–1117. doi: 10.1136/bmj.38629.587639.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinoza J, Chaiworapongsa T, Romero R, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med. 2007;20:495–507. doi: 10.1080/14767050701413022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Chaiworapongsa T, Erez O. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010 May 12; doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991;10:217–229. [PubMed] [Google Scholar]
- 8.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:1457–1461. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Lopez MA, Sanchez-Madrid F, Rodriguez-Frade JM, et al. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Invest. 2001;81:409–418. doi: 10.1038/labinvest.3780248. [DOI] [PubMed] [Google Scholar]
- 12.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann NY Acad Sci. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 13.Crescioli C, Buonamano A, Scolletta S, et al. Predictive role of pretransplant serum CXCL10 for cardiac acute rejection. Transplantation. 2009;87:249–255. doi: 10.1097/TP.0b013e3181919f5d. [DOI] [PubMed] [Google Scholar]
- 14.Matz M, Beyer J, Wunsch D, et al. Early post-transplant urinary IP-10 expression after kidney transplantation is predictive of short- and long-term graft function. Kidney Int. 2006;69:1683–1690. doi: 10.1038/sj.ki.5000343. [DOI] [PubMed] [Google Scholar]
- 15.Rotondi M, Rosati A, Buonamano A, et al. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. Am J Transplant. 2004;4:1466–1474. doi: 10.1111/j.1600-6143.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 16.Lazzeri E, Rotondi M, Mazzinghi B, et al. High CXCL10 expression in rejected kidneys and predictive role of pretransplant serum CXCL10 for acute rejection and chronic allograft nephropathy. Transplantation. 2005;79:1215–1220. doi: 10.1097/01.tp.0000160759.85080.2e. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Xu W, Xu L, et al. I-TAC is a dominant chemokine in controlling skin intragraft inflammation via recruiting CXCR3+ cells into the graft. Cell Immunol. 2010;260:83–91. doi: 10.1016/j.cellimm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 19.Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:443–452. doi: 10.1007/s10024-004-2020-x. [DOI] [PubMed] [Google Scholar]
- 20.Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 21.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta. 2005;26:S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Bartel G, Wahrmann M, Exner M, et al. Determinants of the complement-fixing ability of recipient presensitization against HLA antigens. Transplantation. 2007;83:727–733. doi: 10.1097/01.tp.0000256337.18347.aa. [DOI] [PubMed] [Google Scholar]
- 23.Betkowski AS, Graff R, Chen JJ, Hauptman PJ. Panel reactive antibody screening practices prior to heart transplantation. J Heart Lung Transplant. 2001;20:205. doi: 10.1016/s1053-2498(00)00440-x. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010;375:1482–1490. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy UM, Goldenberg R, Silver R, et al. Stillbirth classification – developing an international consensus for research: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;114:901–914. doi: 10.1097/AOG.0b013e3181b8f6e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korteweg FJ, Erwich JJ, Holm JP, et al. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol. 2009;114:809–817. doi: 10.1097/AOG.0b013e3181b72ebe. [DOI] [PubMed] [Google Scholar]
- 27.Korteweg FJ, Gordijn SJ, Timmer A, Holm JP, Ravise JM, Erwich JJ. A placental cause of intra-uterine fetal death depends on the perinatal mortality classification system used. Placenta. 2008;29:71–80. doi: 10.1016/j.placenta.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani P, Annunziato F, Lasagni L, et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasagni L, Francalanci M, Annunziato F, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98:617–625. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodnar RJ, Yates CC, Rodgers ME, Du X, Wells A. IP-10 induces dissociation of newly formed blood vessels. J Cell Sci. 2009;122:2064–2077. doi: 10.1242/jcs.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JS, Romero R, Kim MR, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto–maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–1446. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol. 2005;192:264–271. doi: 10.1016/j.ajog.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Giacomucci E, Bulletti C, Polli V, Prefetto RA, Flamigni C. Immunologically mediated abortion (IMA) J Steroid Biochem Mol Biol. 1994;49:107–121. doi: 10.1016/0960-0760(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 37.Rogenhofer N, Toth B, Kiessig S, et al. Enzyme linked immunosorbent assay (ELISA) as screening method for anti-paternal allo-antibodies in patients with recurrent pregnancy loss (RPL) Eur J Obstet Gynecol Reprod Biol. 2008;136:155–159. doi: 10.1016/j.ejogrb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen HS, Witvliet MD, Steffensen R, et al. The presence of HLA-antibodies in recurrent miscarriage patients is associated with a reduced chance of a live birth. J Reprod Immunol. 2010 Jul 3; doi: 10.1016/j.jri.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Hunt JS, Langat DL. HLA-G: a human pregnancy-related immunomodulator. Curr Opin Pharmacol. 2009;9:462–469. doi: 10.1016/j.coph.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–1937. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen F, Zuelzer WW, Gustafson DC, Evans MM. Mechanisms of isoimmunization. I The transplacental passage of fetal erythrocytes in homospecific pregnancies. Blood. 1964;23:621–646. [PubMed] [Google Scholar]
- 42.Sebring ES, Polesky HF. Fetomaternal hemorrhage: incidence, risk factors, time of occurrence, and clinical effects. Transfusion. 1990;30:344–357. doi: 10.1046/j.1537-2995.1990.30490273444.x. [DOI] [PubMed] [Google Scholar]
- 43.van Kampen CA, Versteeg-van der Voort Maarschalk MF, Langerak-Langerak J, van BE, Roelen DL, Claas FH. Pregnancy can induce long-persisting primed CTLs specific for inherited paternal HLA antigens. Hum Immunol. 2001;62:201–207. doi: 10.1016/s0198-8859(01)00209-9. [DOI] [PubMed] [Google Scholar]
- 44.King KE, Kao KJ, Bray PF, et al. The role of HLA antibodies in neonatal thrombocytopenia: a prospective study. Tissue Antigens. 1996;47:206–211. doi: 10.1111/j.1399-0039.1996.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka T, Umesaki N, Nishio J, et al. Neonatal thrombocytopenia induced by maternal anti-HLA antibodies: a potential side effect of allogenic leukocyte immunization for unexplained recurrent aborters. J Reprod Immunol. 2000;46:51–57. doi: 10.1016/s0165-0378(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan C. Alloimmune thrombocytopenia of the fetus and the newborn. Blood Rev. 2002;16:69–72. doi: 10.1054/blre.2001.0187. [DOI] [PubMed] [Google Scholar]
- 47.Moise KJ. Fetal anemia due to non-Rhesus-D red-cell alloimmunization. Semin Fetal Neonatal Med. 2008;13:207–214. doi: 10.1016/j.siny.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Soto E, Romero R, Richani K, et al. Anaphylatoxins in preterm and term labor. J Perinat Med. 2005;33:306–313. doi: 10.1515/JPM.2005.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaisbuch E, Romero R, Erez O, et al. Activation of the alternative pathway of complement is a feature of pre-term parturition but not of spontaneous labor at term. Am J Reprod Immunol. 2010;63:318–330. doi: 10.1111/j.1600-0897.2009.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richani K, Romero R, Soto E, et al. Unexplained intrauterine fetal death is accompanied by activation of complement. J Perinat Med. 2005;33:296–305. doi: 10.1515/JPM.2005.052. [DOI] [PubMed] [Google Scholar]
- 52.Dudley DJ, Goldenberg R, Conway D, et al. A New system for determining the causes of stillbirth. Obstet Gynecol. 2010;116:254–260. doi: 10.1097/AOG.0b013e3181e7d975. [DOI] [PMC free article] [PubMed] [Google Scholar]