Abstract
Estrogen regulates hippocampal dendritic spine density and synapse number in an N-methyl-d-aspartate (NMDA) receptor-dependent manner, and these effects may be of particular importance in the context of age-related changes in endocrine status. We investigated estrogen's effects on axospinous synapse density and the synaptic distribution of the NMDA receptor subunit, NR1, within the context of aging. Although estrogen induced an increase in axospinous synapse density in young animals, it did not alter the synaptic representation of NR1, in that the amount of NR1 per synapse was equivalent across groups. Estrogen replacement in aged female rats failed to increase axospinous synapse density; however, estrogen up-regulated synaptic NR1 compared with aged animals with no estrogen. Therefore, the young and aged hippocampi react differently to estrogen replacement, with the aged animals unable to mount a plasticity response generating additional synapses, yet responsive to estrogen with respect to additional NMDA receptor content per synapse. These findings have important implications for estrogen replacement therapy in the context of aging.
At the turn of the century, the life expectancy of American women was roughly equivalent to the average age of the onset of menopause (1). Presently, there is a 30-year discrepancy between these two demographic indices, with a life expectancy of ≈80 years and the average onset of menopause remaining in the early fifties (1). As such, it is critical that we understand the interaction of reproductive senescence with the aging of other systems, particularly the nervous system. The regulation of the reproductive axis by estrogens has been characterized in great detail (2). However, estrogens also impact synaptic communication in brain regions involved in cognitive processing, such as the hippocampus (3), and these effects may be of particular importance in the context of aging when both circulating estrogen levels change and hippocampal-dependent functions decline. With respect to its nonreproductive functions, estrogen improves verbal memory and the capacity for learning new associations in both naturally and surgically menopausal women (4). In rats, although findings are somewhat controversial (5–7), learning was enhanced during the proestrus phase (i.e., high estrogen) of the estrous cycle compared with the estrus phrase (i.e., low estrogen) (8). Another study found improved learning and memory on the Morris water maze task in ovariectomized animals with intrahippocampal estrogen injection compared with saline injection (9).
Our current understanding of estrogen effects on synaptic plasticity in the hippocampus is based almost exclusively on data from young animals. For example, dendritic spine density in CA1 pyramidal cells is sensitive to naturally occurring estrogen fluctuations in young animals (10), as well as experimentally induced estrogen depletion and replacement (11–14). Recent evidence suggests that estrogens mediate these morphological changes through N-methyl-d-aspartate (NMDA) receptors. Estradiol increases NMDA agonist binding, as well as the NR1 levels in CA1 dendrites (15, 16). Moreover, estrogen-induced increases in dendritic spine density are blocked by NMDA receptor antagonists (17, 18), and electrophysiological properties of NMDA receptor-mediated transmission are altered by estrogens (19–21).
For the hypothalamus, it is well documented that aged animals respond differently than young animals to estrogen deprivation (22–25). In addition, dendritic spines on the granule cells of the dentate gyrus respond differently to estrogen replacement in middle-aged and young animals (26). However, effects of estrogen on the CA1 region of aged animals remain unexplored. More importantly, the direct effects of estrogen on the synaptic representation of the NMDA receptor have not been investigated and, as such, the degree to which the observed synaptic effects of estrogen on CA1 pyramidal cells in young animals applies to aged subjects is unknown. Therefore, the present study was designed to examine the effects of estrogen on the synaptic distribution of the NMDA receptor subunit, NR1, and the degree to which estrogen-induced synaptic changes in CA1 hippocampal pyramidal cells are equivalent in young and aged females. The analyses revealed fundamental age-related differences in estrogen-induced synaptic plasticity that have important implications for estrogen replacement in the context of menopause.
Materials and Methods
Animals.
Nine young (3–4 month; ≈225 gm) and nine aged (23–24 month; ≈350 gm) female Sprague–Dawley rats were purchased from Harlan Sprague–Dawley (Indianapolis, IN). Young rats were virgins, and aged rats were either virgins or retired breeders. Animals were housed in a temperature-controlled room (12-h light/dark cycle; lights on at 0700). Food and water were available ad libitum. All experiments were conducted in accordance with Guidelines for the Care and Use of Experimental Animals, by using protocols approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine.
Surgical Procedures.
The estrous cycle status of the young and aged rats was not monitored before ovariectomy. Presumably all young rats were cycling and all aged rats were acyclic (i.e., constant estrous or diestrus), as was demonstrated previously in our laboratory in a different cohort of animals (27). Although our aged rats were most likely exposed to elevated and unopposed estradiol before ovariectomy, we determined that the vehicle and estrogen regimen after ovariectomy were effective by examining the uterus from each animal. In both young and aged rats treated with a vehicle, uteri were very small and atrophied, and those that received estrogen displayed uterine hypertrophy. Bilateral ovariectomy was performed under isofluorane anesthesia. After 7 days, a silastic capsule (capsule dimensions: inner diameter 1.96 mm; outer diameter 3.18 mm) filled with either 17-β estradiol (10% in cholesterol) or cholesterol was implanted s.c. under anesthesia. Young animals received an implant that was 1 cm in length and aged animals received an implant that was 2 cm in length. Different implant lengths were used for animals of different ages to account for differences in body weights (28, 29), presumably resulting in comparable estrogen levels in young and aged rats. Moreover, we used a similar estrogen replacement in paradigm in a longer-term ovariectomy in aged animals and observed a similar uterine response and the circulating estrogen levels were within a physiological range (30).
Tissue Processing and Perfusion.
Animals were anesthetized with 30% chloral hydrate and perfused transcardially with 2% dextran in 0.1 M phosphate buffer (PB) (pH 7.4, 50 ml/min) for 1 min, followed by 4% paraformaldehyde and 0.125% glutaraldehyde in PB for 10–15 min. The carcasses were examined to confirm complete removal of both ovaries and the presence of the s.c. implant. The uterus of each animal was also examined to determine whether the estrogen replacement was effective, with uterine hypertrophy seen only for estrogen-treated animals. No animals in the present study had obvious pituitary tumors, incomplete removal of ovaries, or lack of implant. The brains were removed and postfixed overnight. Two blocks from the dorsal hippocampus (≈1 μm thick) were randomly selected from each animal and processed for postembedding immunogold.
Postembedding Immunogold.
Freeze substitution and low-temperature embedding of the specimens were performed as described previously (31–33). Slices were cryoprotected by immersion in increasing concentrations of glycerol in phosphate buffer (10, 20, and 30%) and were plunged rapidly in liquid propane cooled by liquid nitrogen (−190°C) in a Universal Cryofixation System KF80 (Reichert–Jung, Vienna). The samples were immersed in 1.5% uranyl acetate (for en bloc fixation) in anhydrous methanol (−90°C, 24 h) in a cryosubstitution Automatic Freeze-Substitution System unit (Leica, Vienna). The temperature increased in steps of 4°C/h from −90 to −45°C. The samples were washed with anhydrous methanol and infiltrated with Lowicryl HM20 resin (Electron Microscopy Sciences, Fort Washington, PA) at −45°C with a progressive increase in the ratio of resin to methanol for 1 h each, followed by pure Lowicryl (overnight). Polymerization was performed with UV light (360 nm) at −45°C for 48 h, followed by 24 h at room temperature. An area in the stratum radiatum of CA1 (≈150–200 μm from the cell bodies) was sectioned. Pairs of ultrathin sections (≈70–80 nm in thickness, as determined by interference colors) were cut by diamond knife on a Reichert–Jung ultramicrotome and mounted on slot grids for spine density analysis. Another series of two adjacent sections were collected and mounted on a nickel mesh grid for immunogold analysis. Sections for the spine density analysis were counterstained with 1.0% uranyl acetate and Reynolds lead citrate and then viewed on a JEOL 1200EX electron microscope. The mesh grids with ultrathin sections for the immunolabeling studies were treated with a saturated solution of NaOH in absolute ethanol, rinsed, and incubated in the following solutions at room temperature: 0.1% sodium borohydride and 50 mM glycine, and then in Tris-buffered saline (TBS) containing 2% human serum albumin (HSA). Single immunolabeling was performed, and sections were incubated with primary antibody [54.1 (34); 10 μg/ml] in the above diluent overnight, washed, and incubated in secondary gold-tagged (10 nm) antibody in TBS (2% HSA and polyethyleneglycol 20,000; 5 mg/1 ml). Sections were washed and dried, counterstained with 1% uranyl acetate and Reynolds lead citrate, and viewed on the electron microscope. Images were captured by using the Advantage charge-coupled device camera (Advanced Microscopy Techniques, Danvers, MA). Controls omitting the primary antibody were performed, and no immunogold labeling was observed. The specificity of this primary antibody has been demonstrated in numerous experimental paradigms (15, 35, 36).
Disector Analysis of Axospinous Synapse Density.
Axospinous synapse counts were performed by using IGL trace (Harris Laboratory; Boston University, Boston). A disector analysis of synapse density, as previously described (12, 37–39), was performed within the stratum radiatum of CA1 (Fig. 1). A total area of ≈1,950 μm2 was analyzed with an average section thickness of 75 nm, generating a volume thickness of 146 μm (3). Approximately 150–300 synapses per animal were recorded.
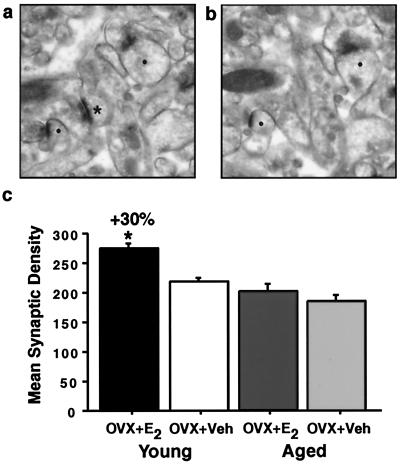
Figure 1.
Synapse density in stratum radiatum of CA1 in young and aged estrogen (OVX + E2)- and vehicle (OVX + Veh)-treated groups. Ultrastructural images from a representative young animal illustrating the disector method. For each animal, 15 pairs of adjacent digital images were taken. One section was considered the reference (a) and the other the look-up (b). Only synaptic profiles (*) contained in the reference but not in the corresponding look-up planes were counted. [Note: (●), Solid circles denote profiles contained in both planes.] This procedure was repeated by switching the reference and look-up sections. (c) Analysis revealed a significant increase in young OVX + E2 compared with OVX + Veh (P < 0.005) but no difference between aged OVX + E2 and OVX + Veh (P > 0.38). The young OVX + E2 were significantly different from both aged groups (both P values < 0.0001).
Synaptic Bin Analysis for NR1.
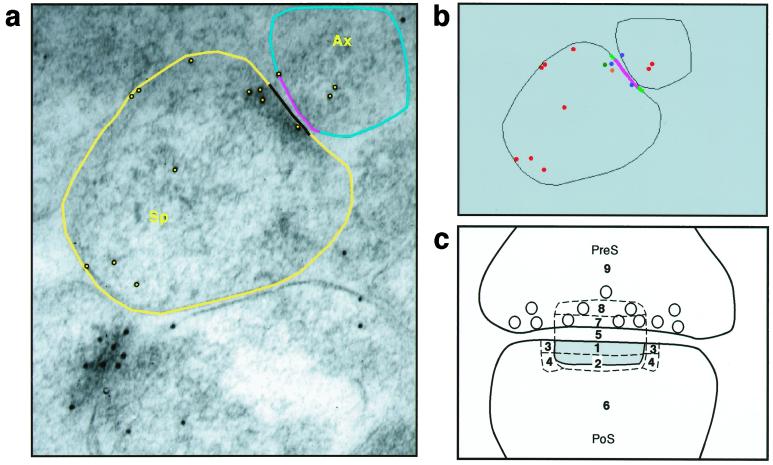
The immunogold particle density and distribution were analyzed by using software developed in our laboratory with Sun Microsystem's (Mountain View, CA) java development kit, Ver. 1.2.2, and java advanced imaging toolkit, Ver. 1.0.2 (i.e., SynBin; Fig. 2), on the basis of principles regarding proximity to membranes articulated by Ottersen and colleagues (40, 41). Accordingly, the position of each gold particle is determined as it relates to the post- and presynaptic membrane structures. The program analyzes the resulting data map and objectively assigns each gold particle to a given bin, with bin sizes and targeted synaptic domains established prospectively. Through this process, a precise gold particle/bin density emerges that is an accurate reflection of gold particle distribution and density in different compartments of the synaptic complex.
Figure 2.
Bin analysis of synaptic NR1. Images illustrate software used for this analysis. (a) Outlines of the relevant synaptic components, i.e., presynaptic and postsynaptic membranes, length of the postsynaptic density (PSD), and length of the presynaptic membrane that corresponds to the length of the PSD. (b) The program provides a data map of the distribution of gold particles relative to these membranes. (c) A schematic diagram of different postsynaptic (PoS) compartments. Two major postsynaptic bins were designated: Bin 1 is 0–30 nm from the inner leaflet of the postsynaptic membrane, and Bin 2 is 30–60 nm from the postsynaptic membrane. Bins 3 and 4 are side bins, 15 nm lateral to Bins 1 and 2. Bin 5 is the synaptic cleft; Bin 6 is a cytoplasmic bin that includes gold particles >60 nm from the postsynaptic membrane. Two major presynaptic (PreS) zones were designated: Bin 7 is 0–30 nm from the inner border of the presynaptic membrane; Bin 8 is 30–60 nm from the presynaptic membrane. Bin 9 is a presynaptic bin that includes gold >60 nm from the presynaptic membrane. Sp, spine; Ax, Axon.
Gold particle analysis was done on 50–75 randomly chosen spines per animal. Synapses cut obliquely that lacked clear visualization and delineation of classic synaptic structures, such as pre- and postsynaptic membranes, a synaptic cleft, and postsynaptic density, were excluded from the quantitative analysis. For the present analysis, 30 nm was chosen for the bin width because it assures high resolution yet comfortably accommodates the theoretical limit of resolution, i.e., 25 nm. The following zones were defined for each synapse: (i) two postsynaptic bins: the first one was 0–30 nm from the inner leaflet of the postsynaptic membrane and the second 30–60 nm from the postsynaptic membrane; (ii) side bins that were 15 nm lateral to both of the postsynaptic bins; (iii) the synaptic cleft; (iv) a cytoplasmic bin that included gold particles >60 nm from the postsynaptic membrane; (v) two presynaptic zones, one extending 0–30 nm from the inner border of the presynaptic membrane and an additional bin extending 30–60 nm from the inner border of the presynaptic membrane; and (vi) a presynaptic bin that included gold >60 nm from the presynaptic membrane. With such a design, gold particles in the 0- to 30-nm postsynaptic density bin are unquestionably synaptic in location, whereas all other postsynaptic bins may include particles representing nonsynaptic pools of NR1. In addition, the lateral bins (i.e., Bins 3 and 4 in Fig. 2c) were used to establish a “buffer zone” at the lateral edge of the synapse to account for gold particles at the edge (i.e., within 15 nm) that might be labeling proteins associated with the postsynaptic density.
Quantitative and Statistical Analysis.
Statistical analyses were performed by using statview 5.0 (Abacus Concepts, Berkeley, CA). Potential group differences in spine number and number of gold particles per synaptic compartment between young ovariectomized vehicle- and estrogen-treated animals, as well as aged ovariectomized vehicle- and estrogen-treated rats, were evaluated by unpaired t tests. Significance was set at P < 0.05.
Results
To determine whether the young and aged hippocampi respond similarly to estrogen manipulation, we analyzed axospinous synapse density in CA1. Qualitative observation of the sections from the young and aged animals revealed no gross anatomical differences between the groups. We observed a significant increase (30%; P < 0.005; Fig. 1c) in the density of axospinous synapses in the estrogen-treated compared with vehicle-treated young animals. This result is consistent with previous studies demonstrating increases in the density of spines and synapses with estrogen in young animals (11–14). Unlike the young animals, the synapse density between the aged estrogen- and vehicle-treated groups was similar (P > 0.40; Fig. 1c). These data demonstrate that the estrogen-induced increase in dendritic spine density is attenuated in aged animals. We also observed a significant difference in the spine density between the young estrogen-treated animals and aged animals (both P values < 0.0001), indicating that there is an age-related decrease in spine density and showing that estrogen treatment does not reverse this decline under conditions in which it did elevate spine density in young animals and caused uterine hypertrophy in both young and aged animals.
Estrogen replacement in young animals has been shown to increase the intradendritic levels of NR1, the obligatory subunit for a functional NMDA receptor (15). Therefore, we determined the degree to which such an estrogen-induced increase in dendritic NR1 reflected increased NR1 per synapse of CA1 pyramidal neurons or increased NR1 to serve more synapses without a shift in NR1 per synapse. Qualitative observation of CA1 in young and aged animals revealed that all hormonally manipulated groups had synapses with gold particles located in the postsynaptic density, synaptic cleft, and spine head (Figs. 2 and 3 a–d), in general agreement with previous observations (42, 43). Occasionally, gold particles were also found to be located presynaptically (Figs. 2 and 3 a–d). Only synapses that contained two or more gold particles within the postsynaptic density and/or cleft were considered labeled in the NR1 quantitative analysis. We observed that over 90% of the asymmetrical synapses in all of the young and aged groups contained NR1 labeling (data not shown). Statistical analysis revealed no significant differences between any groups in this respect (both P values > 0.20), and this percentage of NR1-labeled synapses is in good agreement with other studies (42, 43).
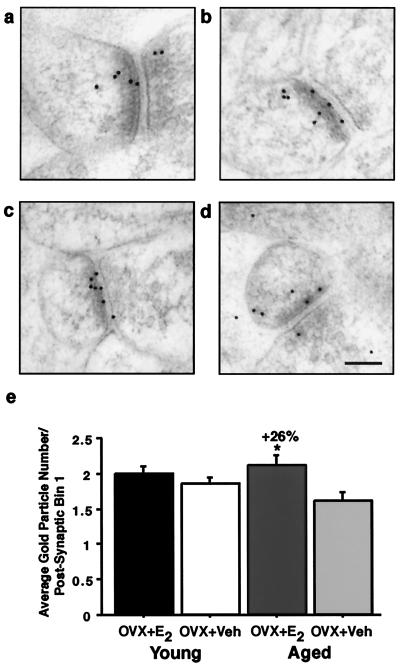
Figure 3.
Bin analysis of NR1 in young and aged OVX + E2 and OVX + Veh. Images from young (a and b) and aged (c and d) animals illustrating the distribution of the NR1 postembedding immunogold. (e) Analysis revealed no significant differences in the synaptic distribution of NR1 between the young (a) OVX + E2 and (b) OVX + Veh (P > 0.15). However, the aged (c) OVX + E2 had significantly more NR1 per postsynaptic Bin 1 compared with (d) OVX + Veh (P < 0.05). (Bar = 100 nm.)
To quantify the synaptic distribution of NR1, we used software that we developed (i.e., SynBin; Fig. 2). Our analysis revealed that no shifts in NR1 levels occurred in any compartment of the dendritic spine of young animals after estrogen replacement (all P values > 0.15; Fig. 3e). However, we observed that aged rats with estrogen replacement have significantly more NR1 (26%; P < 0.05; Fig. 3e) in the postsynaptic compartment within 30 nm of the postsynaptic membrane compared with vehicle-treated animals. This bin represents the pool of receptor that is consistently located within the boundaries of the postsynaptic density and thus is unequivocally synaptic. No other pool of receptor displayed a significant difference between the aged estrogen- and vehicle-treated groups (all P values > 0.22). In fact, the aged vehicle-treated animals had the lowest NR1 representation in Bin 1 (i.e., within 30 nm of the postsynaptic membrane) of all four groups, with the difference between the aged vehicle-treated and young estrogen-treated rats representing a strong trend yet failing to reach statistical significance (P = 0.053).
We also determined whether there was an effect of estrogen on the average length of the postsynaptic density (i.e., the active synaptic zone) that might contribute to the estrogen-related shift observed in aged animals. We observed no difference in the length of the postsynaptic density between the young estrogen- (243 nm) and vehicle-treated (232 nm) animals (P > 0.61; data not shown). Analysis of the aged animals revealed no significant effect of estrogen treatment on the length of the postsynaptic density between the estrogen- (241 nm) or vehicle-treated (243 nm) groups (P > 0.84). In addition, we observed no significant correlation between the length of the postsynaptic density and NR1 distribution within the young and aged groups (r = 0.07; P > 0.79; data not shown). This is consistent with a previous study reporting no correlation with the length of the postsynaptic density and NMDA receptor levels (43) and suggests that the age-related shift in NR1 after short-term estrogen treatment is not because of a shift in the length of the postsynaptic density.
Discussion
In this study, we used quantitative ultrastructural approaches to reveal age-related differences in estrogen-induced synaptic plasticity. We found a significant decrease in axospinous synapse density within CA1 of young compared with aged rats, similar to the age-related decrease reported in the dentate gyrus of male rats (44). Furthermore, estrogen treatment did not reverse this decline under conditions in which it did elevate spine density in younger animals and cause hypertrophy of an atrophied uterus in both young and aged animals. Moreover, estrogen treatment was able to increase NMDA receptors in both young and aged CA1, with the important difference that it increased NMDA receptors in proportion to new spines in younger rats but increased NMDA receptors per axospinous synapse in aged animals without increasing spine density (Fig. 4). These fundamentally different responses to estrogen may enhance NMDA-mediated transmission in both situations but may carry a different degree of risk for excitotoxicity mediated by NMDA receptors.
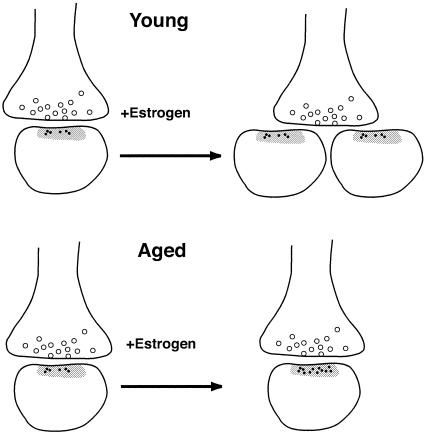
Figure 4.
A schematic of estrogen-induced plasticity in young and aged animals. Estrogen treatment increases NR1 expression per synapse in aged hippocampus, whereas it increases spine number but not synaptic NR1 in young female rat hippocampus. Small black dots represent immunogold particles labeling NR1 associated with postsynaptic Bin 1; open circles are synaptic vesicles; the gray zone indicates the postsynaptic density.
Our data on estrogen's effects on plasticity in the aged hippocampus suggest that the estrogen-induced morphological plasticity is attenuated in aged subjects compared with young animals. It has been well documented for the reproductive axis that the hypothalamus of aged animals has a blunted response to estrogen deprivation or replacement compared with young animals (45–52). This may be caused, at least in part, by the condition of constant estrous or diestrous, because both conditions involve constant levels of unopposed estrogen rather than the normal fluctuations of estradiol and progesterone in the ovarian cycle (53–55), and this condition in the aged animal may underlie the blunted spine response we observed. Other reflections of hippocampal plasticity may be attenuated with age as well. Previous studies have described a decreased sprouting response and synaptogenesis in the hippocampus, as well as a reduced up-regulation of growth-associated proteins in the hilus of aged animals after a perforant path lesion (56–62), and we observed an attenuated up-regulation of NR1 in the dentate gyrus of aged animals after a perforant path lesion (M.M.A, A. H. Gazzaley, and J.H.M., unpublished observations). Although these data suggest that some elements of synaptic plasticity are blunted in aged animals, they do not address plasticity at the level of NMDA receptor subunit representation at the synapse. The present results demonstrate a critically important capacity for synaptic plasticity in aged animals: the aged female rats treated with estrogen had significantly more NR1 per synapse than those receiving a vehicle and, in fact, had a level of NR1 per synapse very similar to the young groups. This increase was selective to the postsynaptic bin that was within 30 nm of the postsynaptic membrane, i.e., the bin that was consistently associated with the postsynaptic density. In addition, that there were no effects of estrogen on the length of the postsynaptic density between groups suggests that the estrogen-related NR1 increase in aged subjects is not because of an overall increase in the postsynaptic density length but rather because of an increase in NR1 representation per synapse.
That the synaptic distribution of NR1 is equivalent across young estrogen- and vehicle-treated groups suggests that the increase in the intradendritic levels of this subunit (15) serves to provide adequate NR1 to the additional spines induced by estrogen replacement. Thus, the estrogen-induced enhancements of NMDA-mediated transmission, i.e., larger NMDA-mediated long-term potentiation (LTP) (19, 20), cannot be explained by increased synaptic NMDA receptors; however, it is possible that more axospinous synapses with equivalent NMDA receptor per synapse might underlie some of these physiological changes. It has been documented that structural synaptic modifications (i.e., increases in the number of perforated axospinous synapses with multiple completely partitioned transmission zones) occur after LTP induction (63). Although the exact function of structural synaptic modifications is unknown, they may represent a more efficacious synapse. It is also possible that synaptic changes in the other NMDA receptor subunit levels, such as NR2A and NR2B, might also be contributing to the enhancements in NMDA-mediated receptor LTP (19, 20), as well as increased Ca2+ influx through NMDA receptors (21). Studies have shown that recombinantly expressed heteromeric NR1-NR2 subunit receptors have distinct subunit-dependent biophysical and physiological properties that vary, depending on their stoichiometry, in the strength of the Mg2+ block, sensitivity to modulation by glycine-reducing agents, and phosphorylation, desensitization, and offset decay kinetics, as well as affinities for antagonists and agonists (64). Future studies need to be directed towards examining the synaptic distribution of other NMDA receptor subunits after estrogen manipulation in young and aged animals.
Although the nature of estrogen-dependent synaptic plasticity differs in young and aged CA1, both scenarios might affect NMDA receptor-mediated transmission. Although the functional implications of these age-related differences in estrogen-mediated effects on the synapse have yet to be clarified, estrogen has been demonstrated to affect cognitive performance in both animal models and women (4–9). The different mechanisms of plasticity displayed in young and aged females in response to estrogen may have implications for vulnerability to excitotoxicity as well. The spine has well-characterized calcium buffering capabilities (65, 66) that likely play a protective role in glutamatergic transmission, yet such capacity may be taxed by an increase in NMDA receptor representation per spine and synapse. This suggests that the form of estrogen-induced plasticity evident in aged subjects may render them more vulnerable to excitotoxicity mediated by NMDA receptors. This prediction is consistent with a study demonstrating that aged female rats are more susceptible to NMDA-mediated excitotoxicity than young animals (67).
Conclusions
These data demonstrate that the effects of estrogen on CA1 synapses must be viewed in the context of brain aging. The synaptic scenario most conducive to NMDA receptor-mediated effects in CA1 is that of the young female with estrogen treatment, in that this condition results in the highest synaptic density, and the synapses have a representation of NR1 equivalent to or higher than all of the other groups. Young females without estrogen retain the same NR1 per synapse but have fewer axospinous synapses. With age, there is a loss of axospinous synapses in CA1, and this loss is not reversible or apparently even impacted by the presence of estrogen in this paradigm. However, aged animals without estrogen display an additional compromise in NMDA receptor-mediated CA1 synapses: their synapses exhibit a low synaptic representation of NMDA receptors. Thus, although estrogen impacts the aged CA1 synapse in a manner that might help preserve hippocampal function, it does so in the context of a synaptic density compromised by age.
Acknowledgments
We thank T. Oung, N. Riley, and A. Leonard for technical assistance, Dr. W. Lou for statistical help, Drs. P. Hof and A. Gore for assistance with experimental design, and Drs. P. Rapp and C. Mobbs for helpful discussion and comments regarding the manuscript. This research was made possible by funds granted to J.H.M. from the National Institute for Aging (PO1 AG16765) and the National Institute of Mental Health (MH57571).
Abbreviation
- NMDA
N-methyl-d-aspartate
References
- 1.Lamberts S W J, van den Beld A W, van der Lely A-J. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 2.Fink G. Sci Prog. 1986;70:403–423. [PubMed] [Google Scholar]
- 3.Woolley C S. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 4.Sherwin B B. Neurology. 1997;48:S21–S26. doi: 10.1212/wnl.48.5_suppl_7.21s. [DOI] [PubMed] [Google Scholar]
- 5.Berry B, McMahan R, Gallagher M. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- 6.Stackman R W, Blasberg M E, Langan C J, Clark A S. Neurobiol Learn Mem. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- 7.Wilson I A, Puoliväli J, Heikkinen T, Riekkinen P., Jr Eur J Pharmacol. 1999;381:93–99. doi: 10.1016/s0014-2999(99)00583-x. [DOI] [PubMed] [Google Scholar]
- 8.Warren S G, Juraska J M. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- 9.Packard M G, Teather L A. NeuroReport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- 10.Woolley C W, Gould E, Frankfurt M, McEwen B S. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould E, Woolley C S, Frankfurt M, McEwen B S. J Neurosci. 1990;4:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolley C S, McEwen B S. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolley C S, McEwen B S. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 14.Woolley C S, Wenzel H J, Schwartzkroin P A. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Gazzaley A H, Weiland N G, McEwen B S, Morrison J H. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiland N G. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- 17.Murphy D D, Segal M. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolley C S, McEwen B S. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foy M R, Xu J, Xie X, Brinton R D, Thompson R F, Berger T W. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 20.Córdoba Montoya D A, Carrer H F. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- 21.Pozzo-Miller L D, Inque T, Murphy D D. J Neurophysiol. 1999;81:1404–1411. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- 22.Finch C E, Felicio L S, Mobbs C V, Nelson J F. Endocrine Rev. 1984;5:467–497. doi: 10.1210/edrv-5-4-467. [DOI] [PubMed] [Google Scholar]
- 23.Lu J K, Anzalone C K, Lapolt P S. Neurobiol Aging. 1994;15:541–544. doi: 10.1016/0197-4580(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 24.Rubin B S. Biol Reprod. 2000;63:968–976. doi: 10.1095/biolreprod63.4.968. [DOI] [PubMed] [Google Scholar]
- 25.Wise P M, Kashon M L, Krajnak K M, Rosewell K L, Cai A, Scarbrough K, Harney J P, McShane T, Lloyd J, Weiland N G. Rec Prog Hormone Res. 1997;52:279–305. [PubMed] [Google Scholar]
- 26.Miranda P, Williams C L, Einstein G. J Neurosci. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams, M. M., Morrison, J. H. & Gore, A. C. (2001) Exp. Neurol., in press.
- 28.Lauber A H, Romano G J, Mobbs C V, Howells R D, Pfaff D W. Brain Res Mol Brain Res. 1990;8:47–54. doi: 10.1016/0169-328x(90)90008-2. [DOI] [PubMed] [Google Scholar]
- 29.Funabashi T, Kleopoulos S P, Kimura F, Mobbs C V. Gen Comp Endocrinol. 1998;112:364–371. doi: 10.1006/gcen.1998.7139. [DOI] [PubMed] [Google Scholar]
- 30.Adams, M. M., Oung, T., Morrison, J. H. & Gore, A. C. (2001) Exp. Neurol., in press. [DOI] [PubMed]
- 31.Chaudhry F A, Lehre K P, van Lookeren Campagne M, Ottersen O P, Danbolt N C, Storm-Mathisen J. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 32.Hjelle O P, Chaudhry F A, Ottersen O P. Eur J Neurosci. 1994;6:794–804. doi: 10.1111/j.1460-9568.1994.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 33.Van Lookeren Campagne M, Oestreicher A B, van der Krift T P, Gispen W H, Verkleij A J. J Histochem Cytochem. 1991;39:1267–1279. doi: 10.1177/39.9.1833448. [DOI] [PubMed] [Google Scholar]
- 34.Siegel S J, Janssen W G, Gasic G P, Jahn R, Heinemann S F, Morrison J H. Proc Natl Acad Sci USA. 1994;91:564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazzaley A H, Siegel S J, Kordower J H, Mufson E J, Morrison J H. Proc Natl Acad Sci USA. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazzaley A H, Benson D L, Huntley G W, Morrison J H. J Neurosci. 1997;17:2006–2017. doi: 10.1523/JNEUROSCI.17-06-02006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeGroot D M G, Bierman E P B. J Neurosci Methods. 1986;18:79–101. doi: 10.1016/0165-0270(86)90113-5. [DOI] [PubMed] [Google Scholar]
- 38.Rampon C, Tang Y-P, Goodhouse J, Shimizu E, Kyin M, Tsien J Z. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 39.Sterio D C. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 40.Blackstad T W, Karagülle T, Ottersen O P. Comput Biol Med. 1990;20:15–34. doi: 10.1016/0010-4825(90)90041-m. [DOI] [PubMed] [Google Scholar]
- 41.Ruud H K, Blackstad T W. Comput Biomed Res. 1999;32:93–122. doi: 10.1006/cbmr.1999.1508. [DOI] [PubMed] [Google Scholar]
- 42.Racca C, Stephenson F A, Streit P, Roberts J D B, Somogyi P. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen O P. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 44.Geinisman Y, de Toledo-Morrell L, Morrell F, Persina I S, Rossi M. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 45.Wise P M, Ratner A. Neuroendocrinology. 1980;30:15–19. doi: 10.1159/000122968. [DOI] [PubMed] [Google Scholar]
- 46.Rubin B S, Elkind-Hirsch K, Bridges R S. Neurobiol Aging. 1985;6:309–315. doi: 10.1016/0197-4580(85)90009-0. [DOI] [PubMed] [Google Scholar]
- 47.Hwang C, Pu H-F, Hwang C-Y, Liu J-Y, Yao H-C, Tung Y-F, Wang P S. Neuroendocrinology. 1990;52:127–132. doi: 10.1159/000125562. [DOI] [PubMed] [Google Scholar]
- 48.Steger R W, Sonntag W E, Peluso J J, Meites J. Neurobiol Aging. 1983;4:53–57. doi: 10.1016/0197-4580(83)90054-4. [DOI] [PubMed] [Google Scholar]
- 49.Belisle S, Bellabarba D, Lehoux J-G. Mech Ageing Dev. 1990;52:207–217. doi: 10.1016/0047-6374(90)90125-y. [DOI] [PubMed] [Google Scholar]
- 50.Joshi D, Billiar R B, Miller M M. Proc Soc Exp Biol Med. 1995;209:237–244. doi: 10.3181/00379727-209-43898. [DOI] [PubMed] [Google Scholar]
- 51.Gee D M, Flurkey K, Mobbs C V, Sinha Y N, Finch C E. Endocrinology. 1984;114:685–693. doi: 10.1210/endo-114-3-685. [DOI] [PubMed] [Google Scholar]
- 52.Mobbs C V, Gee D M, Finch C E. Endocrinology. 1984;115:1653–1662. doi: 10.1210/endo-115-5-1653. [DOI] [PubMed] [Google Scholar]
- 53.Steger R W, Peluso J J. Exp Aging Res. 1982;8:203–208. doi: 10.1080/03610738208260367. [DOI] [PubMed] [Google Scholar]
- 54.Rubin B S, Lee C E, King J C. Biol Reprod. 1994;51:1264–1272. doi: 10.1095/biolreprod51.6.1264. [DOI] [PubMed] [Google Scholar]
- 55.Lu K H, Hopper B R, Vargo T M, Yen S S C. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 56.West J R. Brain Res Bull. 1984;12:323–330. doi: 10.1016/0361-9230(84)90060-1. [DOI] [PubMed] [Google Scholar]
- 57.Schauwecker P E, Cheng H-W, Serquinia R M P, Mori N, McNeill T H. J Neurosci. 1995;15:2462–2470. doi: 10.1523/JNEUROSCI.15-03-02462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheff S W, Bernardo L S, Cotman C W. Brain Res. 1980;199:21–38. doi: 10.1016/0006-8993(80)90227-9. [DOI] [PubMed] [Google Scholar]
- 59.Hoff S F, Sheff S W, Bernardo L S, Cotman C W. J Comp Neurol. 1982a;205:246–252. doi: 10.1002/cne.902050304. [DOI] [PubMed] [Google Scholar]
- 60.Hoff S F, Sheff S W, Cotman C W. J Comp Neurol. 1982b;205:253–259. doi: 10.1002/cne.902050305. [DOI] [PubMed] [Google Scholar]
- 61.Cotman C W, Scheff S W. Mech Ageing Dev. 1979;9:103–117. doi: 10.1016/0047-6374(79)90124-6. [DOI] [PubMed] [Google Scholar]
- 62.Stone D J, Rozovsky I, Morgan T E, Anderson C P, Lopez L M, Shick J, Finch C E. Exp Neurol. 2000;165:46–57. doi: 10.1006/exnr.2000.7455. [DOI] [PubMed] [Google Scholar]
- 63.Geinisman Y. Cereb Cortex. 2000;10:952–962. doi: 10.1093/cercor/10.10.952. [DOI] [PubMed] [Google Scholar]
- 64.Sucher N J, Awobuluyi M, Choi Y, Lipton S A. Trends Pharmacol Sci. 1996;17:348–355. [PubMed] [Google Scholar]
- 65.Calverley R K S, Jones D G. Brain Res Rev. 1990;15:215–249. doi: 10.1016/0165-0173(90)90002-6. [DOI] [PubMed] [Google Scholar]
- 66.Harris K M. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- 67.Auer R N. Stroke. 1996;27:743–746. doi: 10.1161/01.str.27.4.743. [DOI] [PubMed] [Google Scholar]