Abstract
Regulation of T4-specific mRNA synthesis was studied during leucine starvation of a leucine-requiring stringent Escherichia coli B strain. This was done by imposing starvation prior to T4 infection and then letting RNA synthesis proceed for different time periods. Rifampin or streptolydigin was added to stop further RNA synthesis, and protein synthesis was restored by addition of leucine. Samples were withdrawn at different times, and the enzyme-forming capacities found that, during conditions which elicit the stringent response in uninfected bacteria, immediate early mRNA is not stringently regulated. This conclusion contradicts the earlier conclusion of others, obtained by measuring incorporation of radioactive uracil; this is explained by the observation of Edlin and Neuhard (1967), confirmed and extended by us to the T4-infected cell, that the incorporation of uracil into RNA of a stringent strain is virtually blocked by amino acid starvation, whereas that of adenine continues at 30 to 50% of the rate seen in the presence of the required amino acid.
Full text
PDF

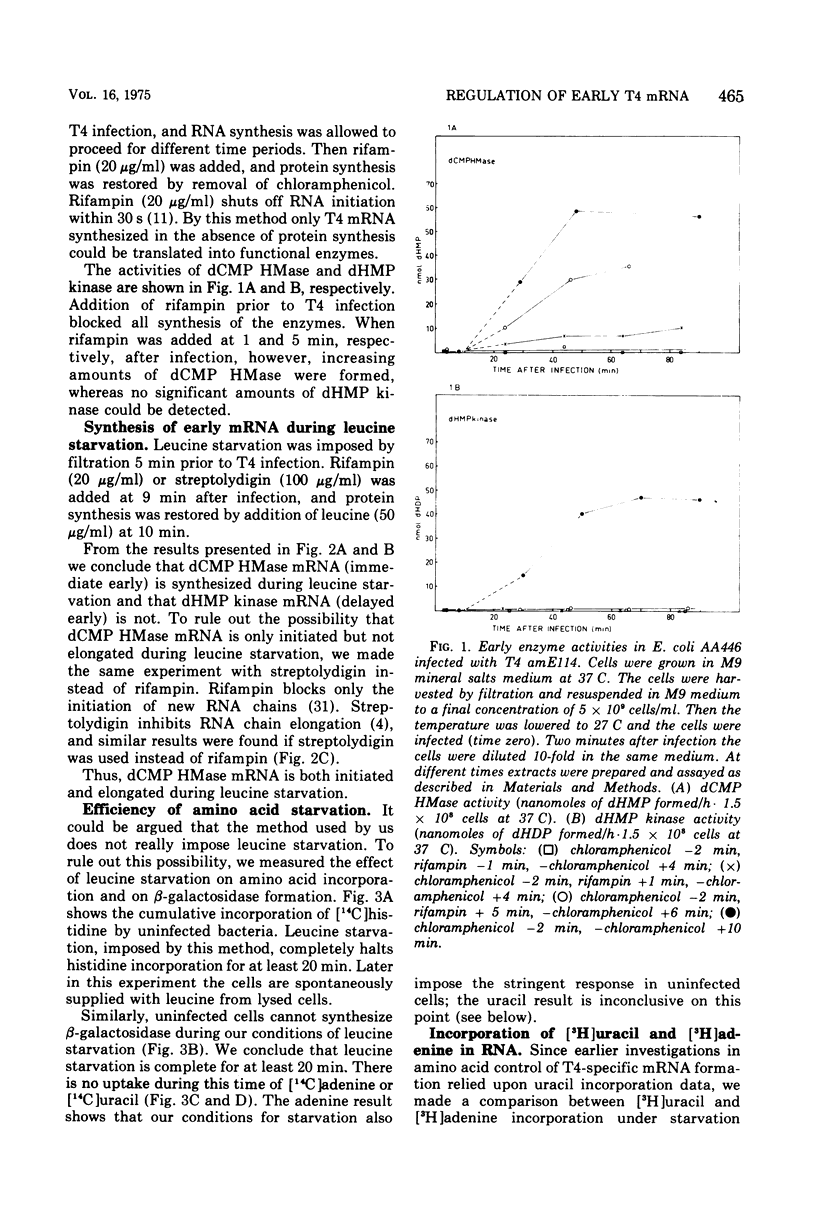

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
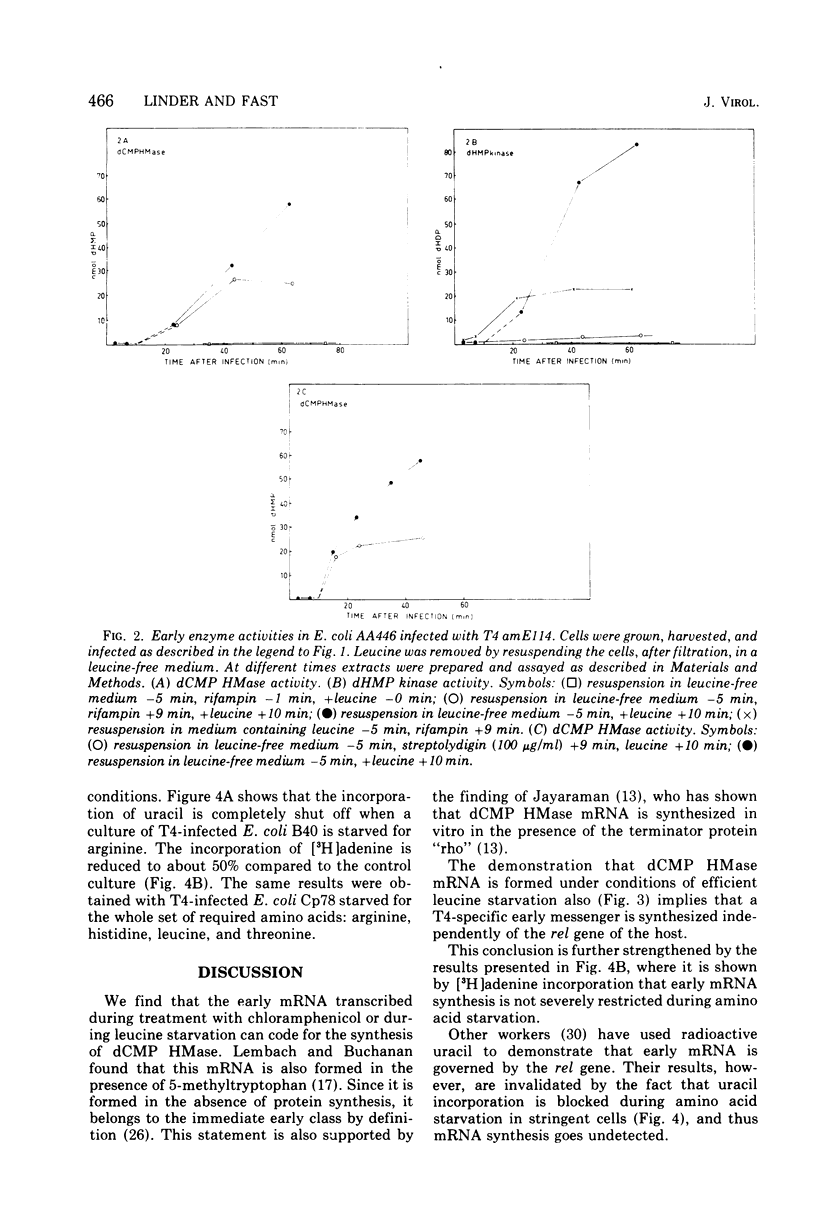
- Black L. W., Gold L. M. Pre-replicative development of the bacteriophage T4: RNA and protein synthesis in vivo and in vitro. J Mol Biol. 1971 Sep 14;60(2):365–388. doi: 10.1016/0022-2836(71)90300-7. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Cassani G., Burgess R. R., Goodman H. M., Gold L. Inhibition of RNA polymerase by streptolydigin. Nat New Biol. 1971 Apr 14;230(15):197–200. doi: 10.1038/newbio230197a0. [DOI] [PubMed] [Google Scholar]
- Donini P., Edlin G. rna synthesis in t4 infected Escherichia coli during amino acid starvation. Virology. 1972 Oct;50(1):273–276. doi: 10.1016/0042-6822(72)90370-4. [DOI] [PubMed] [Google Scholar]
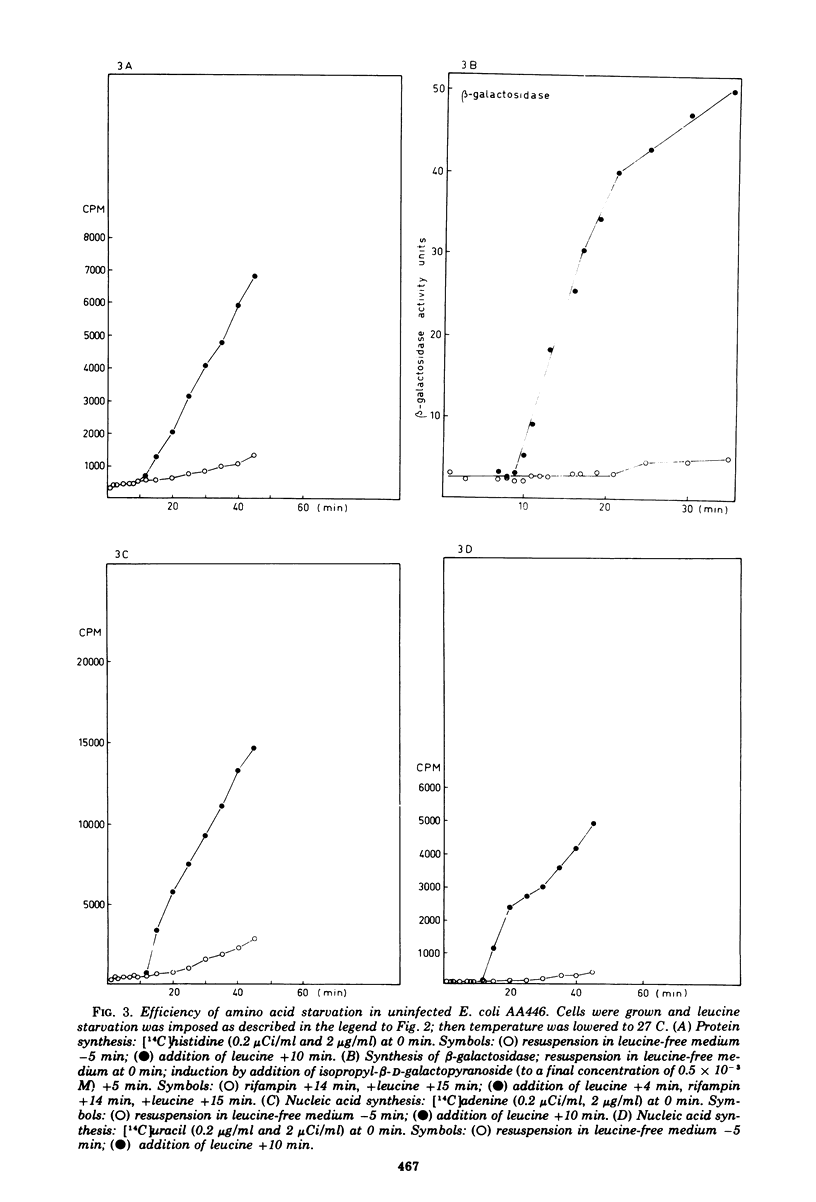
- EDLIN G. GENE REGULATION DURING BACTERIOPHAGE T4 DEVLOPMENT. I. PHENOTYPIC REVERSION OF T4 AMBER MUTANTS BY 5-FLUOROURACIL. J Mol Biol. 1965 Jun;12:363–374. doi: 10.1016/s0022-2836(65)80260-1. [DOI] [PubMed] [Google Scholar]
- Edlin G., Donini P. Synthesis of guanosine 5'-diphosphate, 2'-(or 3'-) diphosphate and related nucleotides in a variety of physiological conditions. J Biol Chem. 1971 Jul 10;246(13):4371–4373. [PubMed] [Google Scholar]
- Edlin G., Neuhard J. Regulation of nucleoside triphosphate pools in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):225–230. doi: 10.1016/0022-2836(67)90328-2. [DOI] [PubMed] [Google Scholar]
- Fast R., Sköld O. Pyrimidine-ribonucleotide pools and their turnover in phage T4-infected Escherichia coli cells. Eur J Biochem. 1973 Sep 21;38(1):40–45. doi: 10.1111/j.1432-1033.1973.tb03030.x. [DOI] [PubMed] [Google Scholar]
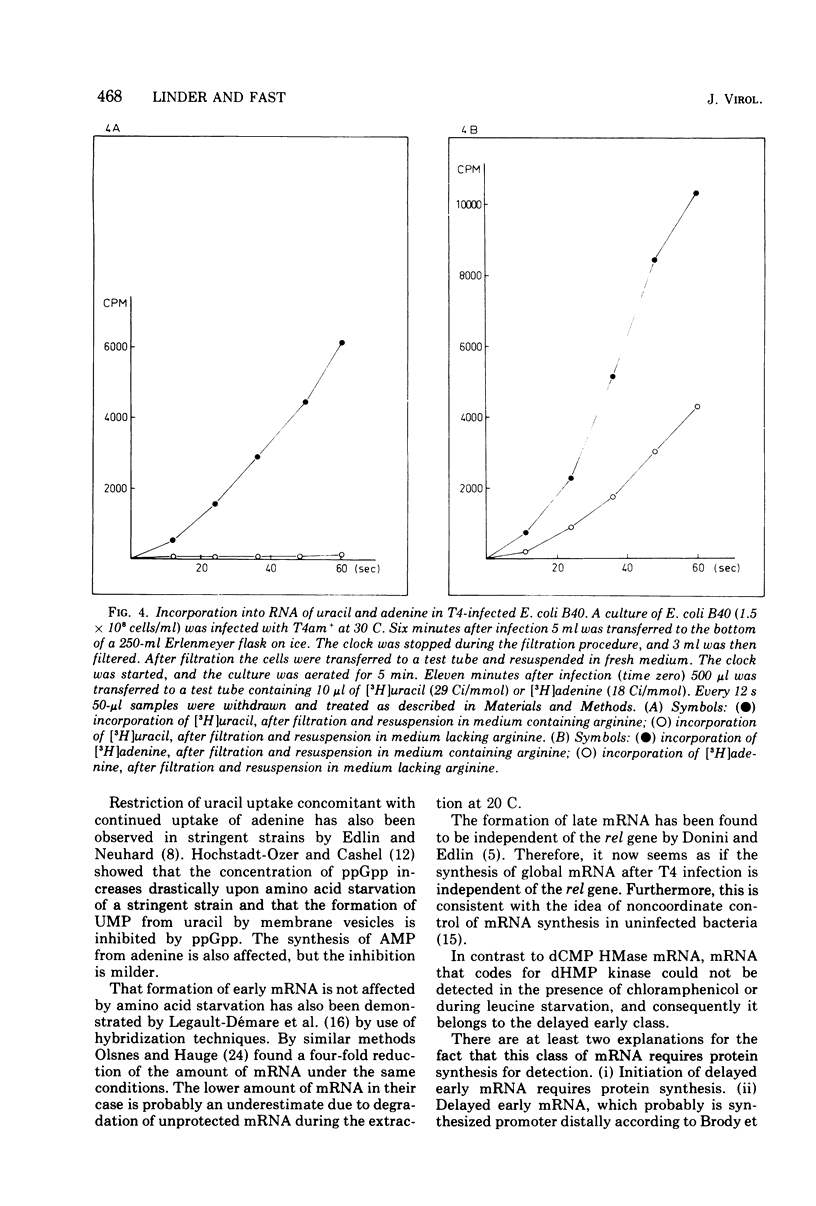
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Schleif R. In vivo experiments on the mechanism of action of L-arabinose C gene activator and lactose repressor. J Mol Biol. 1973 Nov 5;80(3):433–444. doi: 10.1016/0022-2836(73)90414-2. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Cashel M. The regulation of purine utilization in bacteria. V. Inhibition of purine phosphoribosyltransferase activities and purine uptake in isolated membrane vesicles by guanosine tetraphosphate. J Biol Chem. 1972 Nov 10;247(21):7067–7072. [PubMed] [Google Scholar]
- Jayaraman R. Transcription of bacteriophage T4 DNA by Escherichia coli RNA polymerase in vitro: identification of some immediate-early and delayed-early genes. J Mol Biol. 1972 Sep 28;70(2):253–263. doi: 10.1016/0022-2836(72)90537-2. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D., Morse D. E. Loss of dispensable endonuclease activity in relief of polarity by suA. Nat New Biol. 1971 Jun 16;231(24):214–217. doi: 10.1038/newbio231214a0. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Legault-Démare L., Malhié A., Gros F. Synthèse des messagers précoces phagiques chez Escherichia coli infecté par T4 durante une carence spécifique en aminoacide. Eur J Biochem. 1969 Apr;8(4):482–488. doi: 10.1111/j.1432-1033.1969.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Guertin M. Regulation of mRNA utilization and degradation by amino-acid starvation. Nat New Biol. 1971 Aug 11;232(2):165–169. doi: 10.1038/newbio232165a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E. Polarity induced by chloramphenicol and relief by suA. J Mol Biol. 1971 Jan 14;55(1):113–118. doi: 10.1016/0022-2836(71)90285-3. [DOI] [PubMed] [Google Scholar]
- NOMURA M., OKAMOTO K., ASANO K. RNA metabolism in Escherichia coli infected with bacteriophage T4. Inhibition of host ribosomal and soluble RNA synthesis by phage and effect of chloromycetin. J Mol Biol. 1962 May;4:376–387. doi: 10.1016/s0022-2836(62)80018-7. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Hauge J. G. Amino acid control of RNA synthesis in T4-infected Escherichia coli. Eur J Biochem. 1968 Dec;7(1):128–136. doi: 10.1111/j.1432-1033.1968.tb19583.x. [DOI] [PubMed] [Google Scholar]
- Pato M. L., Bennett P. M., von Meyenburg K. Messenger ribonucleic acid synthesis and degradation in Escherichia coli during inhibition of translation. J Bacteriol. 1973 Nov;116(2):710–718. doi: 10.1128/jb.116.2.710-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. F., Cohen P. S., Ennis H. L. Properties of phage T4 messenger RNA synthesized in the absence of protein synthesis. Virology. 1972 Apr;48(1):201–206. doi: 10.1016/0042-6822(72)90127-4. [DOI] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Schachner M., Seifert W., Zillig W. A correlation of changes in host and T 4 bacteriophage specific RNA synthesis with changes of DNA-dependent RNA polymerase in Escherichia coli infected with bacteriophage T 4 . Eur J Biochem. 1971 Oct 26;22(4):520–528. doi: 10.1111/j.1432-1033.1971.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld O. Effect of the "ribonucleic acid control" locus in Escherichia coli on T4 bacteriophage-specific ribonucleic acid synthesis. J Virol. 1970 Jun;5(6):718–725. doi: 10.1128/jvi.5.6.718-725.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. C., Greenberg G. R. Tetrahydrofolate-dependent labilization of the hydrogen atom on carbon 5 of 5'-deoxycytidylate, a step in the deoxycytidylate hydroxymethylase reaction. J Biol Chem. 1967 Mar 25;242(6):1307–1313. [PubMed] [Google Scholar]


