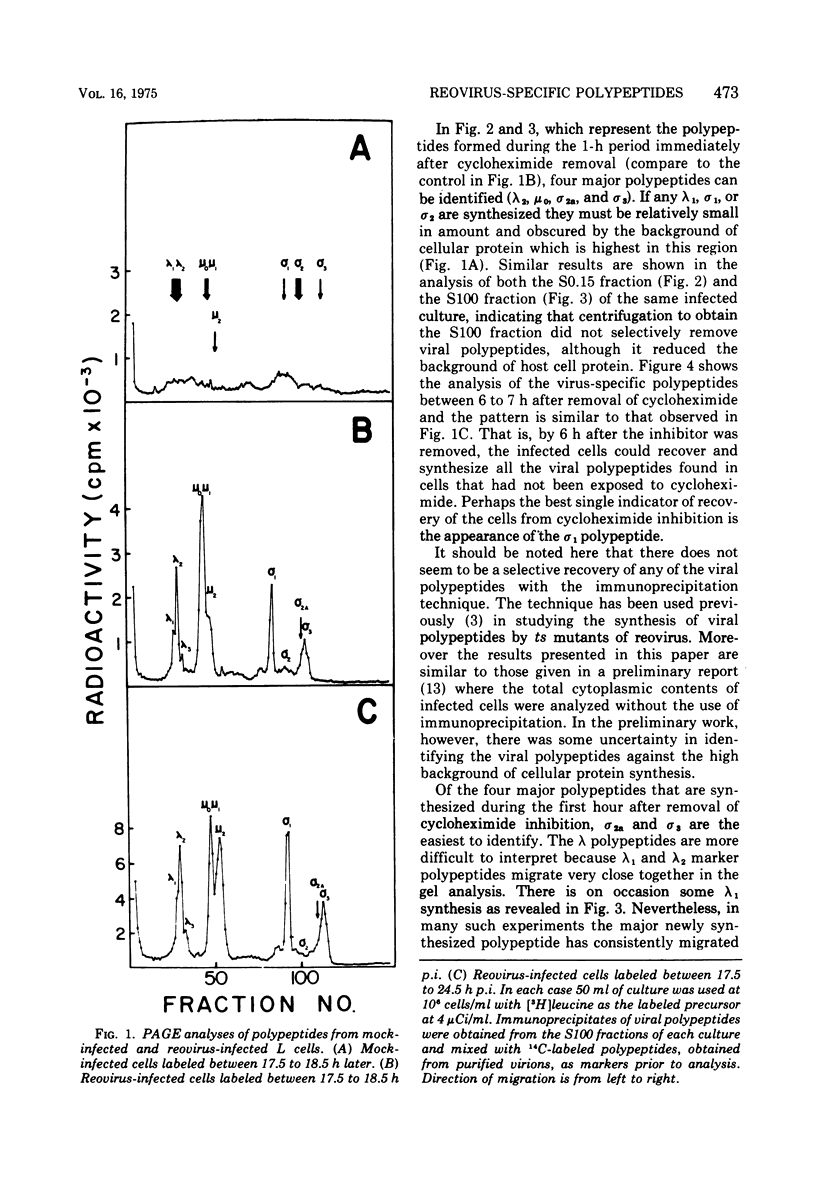
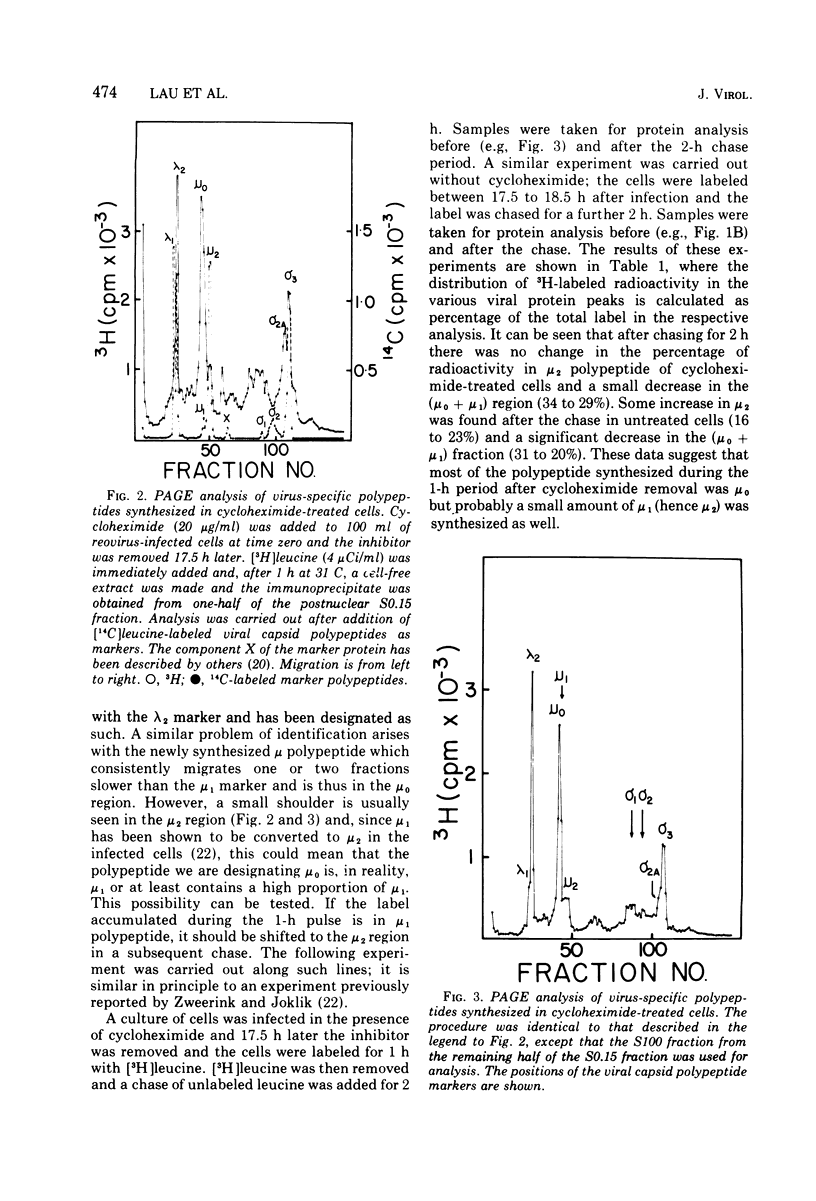
Abstract
When L cells are infected with reovirus in the presence of cycloheximide neither virus-specific polypeptides nor viral double-stranded RNA are synthesized. There is some synthesis of viral single-stranded RNA, transcribed mainly from segments L1, M3, S3, and S4 of the 10 viral genomic segments, and in previous work this has been termed the early mRNA pattern. In an attempt to determine whether these early transcripts are functional mRNA's, the transcripts were allowed to accumulate for a period of 17.5 h at 31 C in cycloheximide-treated cells. The cycloheximide was removed and the cells were exposed for various periods to radioactive amino acids to label any virus-specific polypeptides that might be synthesized. An immunoprecipitation technique was used to separate the viral polypeptides from cellular extracts and this precipitate was then analyzed on sodium dodecyl sulfate-polyacrylamide gels. Within 30 min of cycloheximide removal, four major polypeptides (lambda2, mu0, sigma2a, and sigma3) and two minor polypeptides (lambda1 and mu2) were found. In infected cells without cycloheximide eight viral polypeptides (lambda1, lambda2, mu0, mu2, sigma1, sigma2, sigma2a, sigma3) were found at 17.5 h after infection and the same pattern was found between 3 to 4 h after removal of cycloheximide which had been present for 17.5 h after infection. The latter result shows that the cycloheximide inhibition is reversible and that the cells readily recovered and synthesized the normal complement of viral polypeptides. In one set of experiments cordycepin was added to infected cells immediately after the removal of cycloheximide at 17.5 h to inhibit the synthesis of new viral transcripts. During the succeeding 4 h in the presence of cordycepin, the pattern of protein synthesis was the same as that obtained during the 30 min after cycloheximide removal. It is concluded that the polypeptides formed right after removal of cycloheximide are the translation products of transcripts accumulated during cycloheximide treatment and, therefore, that these transcripts are functional viral mRNA's.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral RNA. Virology. 1972 Dec;50(3):799–809. doi: 10.1016/0042-6822(72)90434-5. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Laskov R., Scharff M. D. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral peptides. Virology. 1972 Oct;50(1):209–215. doi: 10.1016/0042-6822(72)90361-3. [DOI] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. 3. Evidence that mutants of group D ("RNA-negative") are structural polypeptide mutants. Virology. 1972 Oct;50(1):282–286. doi: 10.1016/0042-6822(72)90373-x. [DOI] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. I. Patterns of gene expression by mutants of groups C, D, and E. Virology. 1972 Oct;50(1):189–201. doi: 10.1016/0042-6822(72)90359-5. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Kennedy T. D., Lane B. G. Wheat-embryo ribonucleates. III. Modified nucleotide constituents in each of the 5.8S, 18S and 26S ribonucleates. Can J Biochem. 1974 Dec;52(12):1110–1123. doi: 10.1139/o74-155. [DOI] [PubMed] [Google Scholar]
- Matsuhisa T., Joklik W. K. Temperature-sensitive mutants of reovirus. V. Studies on the nature of the temperature-sensitive lesion of the group C mutant ts447. Virology. 1974 Aug;60(2):380–389. doi: 10.1016/0042-6822(74)90333-x. [DOI] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Millward S., Graham A. F. Control of transcription of the reovirus genome. Nucleic Acids Res. 1974 Mar;1(3):373–385. doi: 10.1093/nar/1.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevec L., Watanabe Y., Gauntt C. J., Graham A. F. Transcription of the genomes of type 1 and type 3 reoviruses. J Virol. 1968 Apr;2(4):289–297. doi: 10.1128/jvi.2.4.289-297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Schonberg M., Levin D. H., Acs G. The reovirus replicative cycle: conservation of parental RNA and protein. Proc Natl Acad Sci U S A. 1970 Sep;67(1):275–281. doi: 10.1073/pnas.67.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Graham A. F. Structural units of reovirus ribonucleic acid and their possible functional significance. J Virol. 1967 Aug;1(4):665–677. doi: 10.1128/jvi.1.4.665-677.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kudo H., Graham A. F. Selective inhibition of reovirus ribonucleic acid synthesis by cycloheximide. J Virol. 1967 Feb;1(1):36–44. doi: 10.1128/jvi.1.1.36-44.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., McDowell M. J., Joklik W. K. Essential and nonessential noncapsid reovirus proteins. Virology. 1971 Sep;45(3):716–723. doi: 10.1016/0042-6822(71)90185-1. [DOI] [PubMed] [Google Scholar]


