Abstract
Fishes can play important functional roles in the nutrient dynamics of freshwater systems. Aggregating fishes have the potential to generate areas of increased biogeochemical activity, or hotspots, in streams and rivers. Many of the studies documenting the functional role of fishes in nutrient dynamics have focused on native fish species; however, introduced fishes may restructure nutrient storage and cycling freshwater systems as they can attain high population densities in novel environments. The purpose of this study was to examine the impact of a non-native catfish (Loricariidae: Pterygoplichthys) on nitrogen and phosphorus remineralization and estimate whether large aggregations of these fish generate measurable biogeochemical hotspots within nutrient-limited ecosystems. Loricariids formed large aggregations during daylight hours and dispersed throughout the stream during evening hours to graze benthic habitats. Excretion rates of phosphorus were twice as great during nighttime hours when fishes were actively feeding; however, there was no diel pattern in nitrogen excretion rates. Our results indicate that spatially heterogeneous aggregations of loricariids can significantly elevate dissolved nutrient concentrations via excretion relative to ambient nitrogen and phosphorus concentrations during daylight hours, creating biogeochemical hotspots and potentially altering nutrient dynamics in invaded systems.
Introduction
Mobile organisms can generate areas of enhanced nutrient recycling rates, or biogeochemical hotspots, that may influence primary productivity in both terrestrial and aquatic ecosystems [1]–[3]. McClain et al. [2] defined biogeochemical hotspots as small areas within a landscape matrix that show comparably high reaction rates relative to the surrounding areas. For organisms to generate biogeochemical hotspots within an ecosystem, their population densities must vary through space and/or time and the contribution of the species to nutrient remineralization rates must be significant relative to ecosystem demand [3]. Therefore, spatially or temporally heterogeneous aggregations of organisms can potentially generate hotspots of biogeochemical activity that influence patterns of nutrient remineralization and alter ecosystem nutrient dynamics.
In aquatic ecosystems, consumer-driven nutrient remineralization can be significant relative to ecosystem nutrient demand and can enhance periphyton biomass and productivity [4]–[6]. Fishes can play important roles in nutrient dynamics in freshwater ecosystems through nutrient sequestration and nutrient remineralization [3], [7], [8] and this may influence primary producer biomass and productivity [9]. For example in a study examining the influence of a native fish assemblage on nitrogen (N) and phosphorus (P) cycling in a tropical river, McIntyre et al. [3] found that the aggregate excretion of fishes was sufficient to turn over the entire ambient pool of N in the water column, the nutrient limiting primary productivity, in less than 0.3km. Though previous investigations have linked ecosystem processes such as biogeochemical cycling with native fish assemblages [3], [10]–[12], few studies have demonstrated how the effects of fish invasion alter these processes [13], [14], [15].
Armored catfishes (Loricariidae) are native to Central and South America [16], [17], and have been introduced to tropical and subtropical freshwater ecosystems throughout the globe [16], [18]–[26]. In invaded ecosystems, loricariids attain high population densities and they are thought to compete with native organisms for food resources and space [27], [28]. Natural resource managers have noted that populations of non-native loricariids create large aggregations [29]; however, the potential influence for this behavior to alter nutrient concentrations in space and time has not been explored. The purpose of this study was to document the diel changes in behavior of non-native populations of armored catfish and investigate the potential of these fishes to generate biogeochemical hotspots, or localized areas of increased nutrient concentrations, in invaded river systems. We predicted that nutrient remineralization by loricariids would represent a substantial flux of N and P. Moreover, we posited daytime aggregations of loricariids would generate biogeochemical hotspots, resulting in discontinuities of stream nutrient availability in space and time.
Methods
Study Site
The field work for this study was conducted in the Chacamax River (N17°29′047′′ W91°58′430′′) in Chiapas, Mexico during the dry season months of March-May 2008–2010. The benthos in the study reach was characterized by large cobble and gravel substrate. During the study, stream discharge averaged ∼1,600 L s−1 and water temperature in the river ranged from 21 to 28°C. Ambient nutrient concentrations, collected from sites without aggregations of armored catfish, in the study reaches were low to moderate (average values: NH4 +-N, 10 µg L−1; NO3 −-N, 353 µg L−1; total dissolved nitrogen, 387 µg L−1; soluble reactive phosphorus, <2 µg L−1; total dissolved phosphorus, 3 µg L−1). Nutrient diffusing substrates, constructed after methods outlined in Capps et al. [30], indicated primary producers in the stream were limited by P (Figure S1). Loricariid density was 2.3±3.4 m−2 (mean ± SD) and areal biomass was 225±45 g m−2 (mean ± SD), two orders of magnitude greater than the native fish biomass in the study reach in 2010 [31].
Non-native loricariids were first documented in the Chacamax River in 2004, and include Pterygoplichthys pardalis (Castelnau, 1855), Pterygoplichthys disjunctivis (Weber, 1991) and Pterygoplichthys that do not adhere to type specimens (Figure S2). The wide variations in pattern suggest the Pterygoplichthys population in the Chacamax may be comprised of hybrids of the two species; hence, we refer to the fish as Pterygoplichthys.
Diel Patterns in Fish Behavior and Ambient Nutrient Concentrations
To describe diel patterns in Pterygoplichthys behavior, we counted the number of fish found in five 1 m×1 m quadrats along the edge of a 100 m reach of stream during daytime and nighttime hours. These measurements were collected to document loricariids were spreading out from their daytime aggregations to graze the entire stream bed. We counted Pterygoplichthys every four hours over a period of three days in March 2010. To document diel fluctuations in water chemistry, we collected duplicate water samples from the thalweg of the stream in single a run habitat for NH4 + and PO4 −3 analysis every four hours on three dates in 2010.
To estimate the influence of time of day (day/night) on loricariid feeding behavior, 77 fish were harvested and weighed during daytime (22 fish; 1200–1730 hrs) or nighttime (55 fish; 1900–0400 hrs) hours, euthanized using an overdose of MS-222, and their gut contents were collected, dried, and weighed according to methods outlined in German and Bittong (2009) under IACUC protocol number: 2006–0169, Cornell University. All values were expressed as the ratio of gut content dry mass (g) to fish wet mass (g) to account for size variation in the fishes we sampled. Again, these measurements were taken to document pronounced, diel changes in loricariid behavior and their nocturnal foraging activities.
Nutrient Remineralization and Hotspot Sampling
To determine nutrient remineralization rates of loricariids and estimate the effects of their remineralization on ambient water chemistry, we conducted fish excretion incubations and sampled water within and outside of aggregations of loricariids in the Chacamax River. We predicted that aggregations of loricariids would generate biogeochemical hotspots, evidenced by increased ambient nutrient concentrations from samples collected in the aggregations relative to samples collected outside of the aggregations. Twenty Pterygoplichthys (Standard length 23.5 cm ±5 cm(mean ± SD)) were collected using hand nets and immediately, individually incubated in 15L plastic tubs in 10L of filtered stream water for approximately 1 h. Ten incubations were conducted between 1200 and 1500 hrs (daytime) and 10 incubations occurred between 1900 and 2100 hours (nighttime). Two additional fish-free tubs were maintained as controls during each incubation period. Tubs were filled with 10L of filtered stream water and placed in the shade for the duration of the incubation. At the end of the incubation, we collected filtered water samples for NH4 + and total dissolved phosphorus (TDP) analysis. Fish nutrient recycling rates were estimated based on the difference in dissolved N and P concentrations between plastic tubs incubated with and without Pterygoplichthys [3], [8]. At the end of the incubation period, water samples were filtered through glass-fiber filters (Gelman A/E) and were either acidified and shipped to the USA for P analysis, or were analyzed in the field for NH4 +. We used standard colorimetric methods to analyze TDP and soluble reactive phosphorus (SRP) samples (APHA 1998) using a Lachat QuickChem 8000 (Lachat Instruments, Loveland, Colorado). All NH4 +samples were refrigerated and analyzed in the field using the flurometric methods outlined by Taylor et al. [32].
To ascertain if Pterygoplichthys generated hotspots of nutrient remineralization (within aggregations) relative to ambient water chemistry (outside of aggregations), we collected paired stream water samples within (i.e. within the white boundary in Figure 1A) and outside of aggregations (i.e. outside of the white boundary in Figure 1A) of loricariids in the Chacamax River in 2008 and 2010. We collected water samples from aggregations of Pterygoplichthys with minimum areas of 3 m2 with at least 50 Pterygoplichthys m−2 (Figure 1A). Paired sites were located parallel to one another along a transect extending between river banks. They were matched for similar depth (min = 0.5 m, max = 1.5 m, mean = 0.9 m) and water velocity (min = 0.04 m s−1, max = 0.09 m s−1, mean = 0.07 m s−1). Water samples were collected and analyzed for NH4 + and SRP using the aforementioned methods.
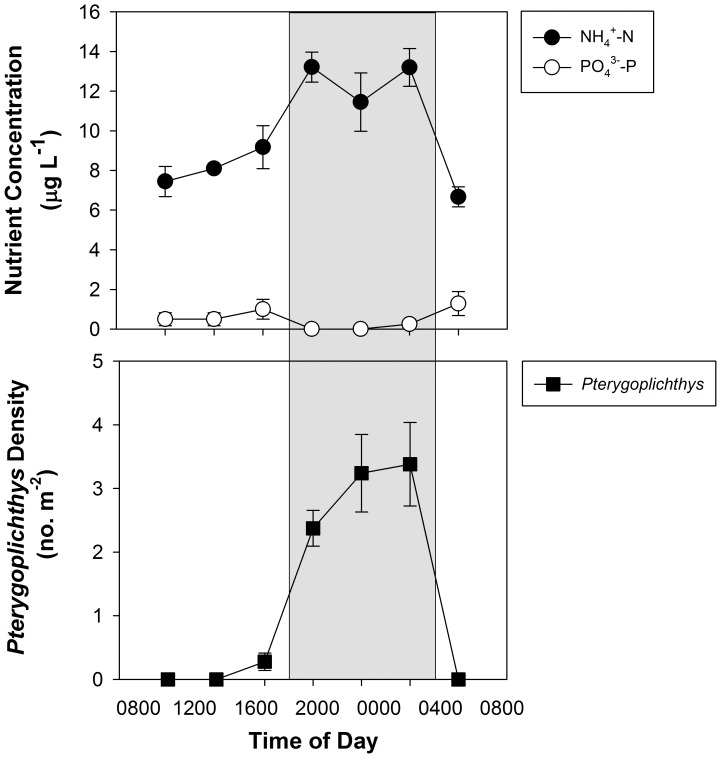
Figure 1. Pterygoplichthys in the Chacamax River (N17°29’047” W91°58’430”).
(A) Daytime aggregation of loricariids. The white line outlines the aggregation boundary. (B) Underwater photo of loricariid aggregation. Individual fish are marked with white numbers (1–35). (C) Loricariids spreading out from aggregation to begin evening feeding. Each dark spot (C) is at least one Pterygoplichthys. A small group of fishes (1–10) have been marked with individual numbers to demonstrate fish abundance. Photo credits: K. A. Capps.
Statistical Analysis
Nutrient recycling rates and gut content time comparisons (daytime/nighttime) were made using one-way ANOVAs, where time was the fixed factor. We used a mixed model to estimate the effects of fish size on nutrient recycling rates, where wet mass was considered the fixed factor and time and wet mass×time were considered random factors. Aggregation/ambient comparisons were made using a two-way ANOVA where sample site, year, and the interaction term were considered fixed factors and sample pair (aggregation/ambient) was considered a random factor. All data were log10 transformed to meet the assumptions of the models and analyzed using JMP 9 statistical software (SAS Institute, 2010).
Results
Diel Changes in Fish Behavior and Nutrient Concentrations
Loricariids formed large aggregations in the main channel of the Chacamax River during the day, but spread out to graze the entire riverbed at night (Figures 1, 2). The aggregations occurred in same relative area each day unless there was a large discharge event that moved large, woody debris and larger cobbles. Concurrent increases in ambient NH4 + occurred at night when loricariids were broadly dispersed and actively feeding; however, there was no similar increase in ambient PO4 3− (Figure 2). Importantly, all of the PO4 3− samples were at or near detection; thus, any change in ambient levels would have been difficult to detect.
Figure 2. Diel changes in ambient water chemistry and Pterygoplichthys behavior.
Data were collected in 2008 and 2010 (±1 SE). (A) NH4+-N and PO4-3-P concentrations over time; (B) number of Pterygoplichthys counted in 1 m2 quadrats near the stream bank (within 24 cm) over time. The shaded areas represent nighttime sampling hours.
Nutrient Recycling and Hotspot Observations
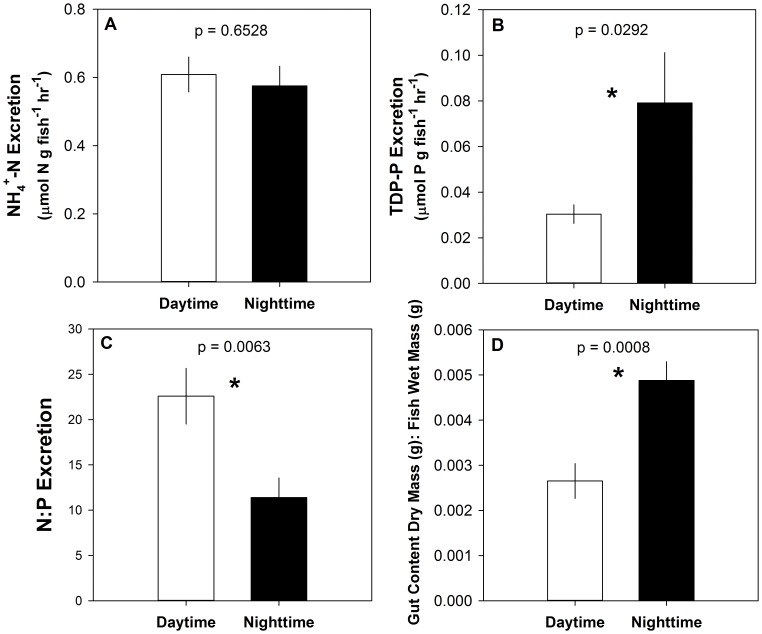
Average N excretion was approximately 0.6 µmol NH4 +-N g wet mass −1 hr−1 and did not differ between the afternoon and evening sample periods (F(1, 18) = 0.209, p = 0.6528; Figure 3A). In contrast, average P excretion was twice as high in samples collected during nighttime sampling periods (approximately 0.077 µmol TDP-P g wet mass −1 hr−1) than those collected in the daytime (approximately 0.031 µmol TDP-P g wet mass −1 hr−1; F(1, 18) = 5.61, p = 0.0292; Figure 3C). This resulted in a significant decrease in the N:P ratio of excretion from an average of 23 in the daytime to approximately 12 in the nighttime (F(1, 75) = 9.57, p = 0.006; Figure 3C). This pattern may have been driven by nocturnal loricariid feeding behavior, evidenced by more amorphous detritus found in loricariid guts during nighttime sampling hours (F(1, 75) = 12.09, p = 0.0008; Figure 3D). Loricariid size also influenced excretion rates, as larger fishes tended to excrete less N and P per gram of fish than smaller loricariids (F(1,18) = 7.52, p = 0.013 and F(1, 18) = 6.67, p = 0.019, respectively). However, loricariid size did not influence excretion stoichiometry (F(1, 18) = 0.633, p = 0.6966). By multiplying loricariid excretion rates by their average areal biomass, we estimate that loricariids remineralize approximately 7 µmol P m −2 hr−1 during daylight hours, 18 µmol P m −2 hr−1 during nighttime hours, and 135 µmol N m −2 hr−1 during both time periods.
Figure 3. Diel changes in Pterygoplichthys excretion and gut content mass.
Average Pterygoplichthys excretion and gut content mass during daytime (1000–1500 h) and nighttime hours (1900–0400 h). (A) Pterygoplichthys NH4 +-N excretion rates; (B) Pterygoplichthys total dissolved phosphorus excretion rates; (C) N:P of Pterygoplichthys excretion; (D) gut content dry mass per wet mass of Pterygoplichthys. Error bars represent ±1 SE.
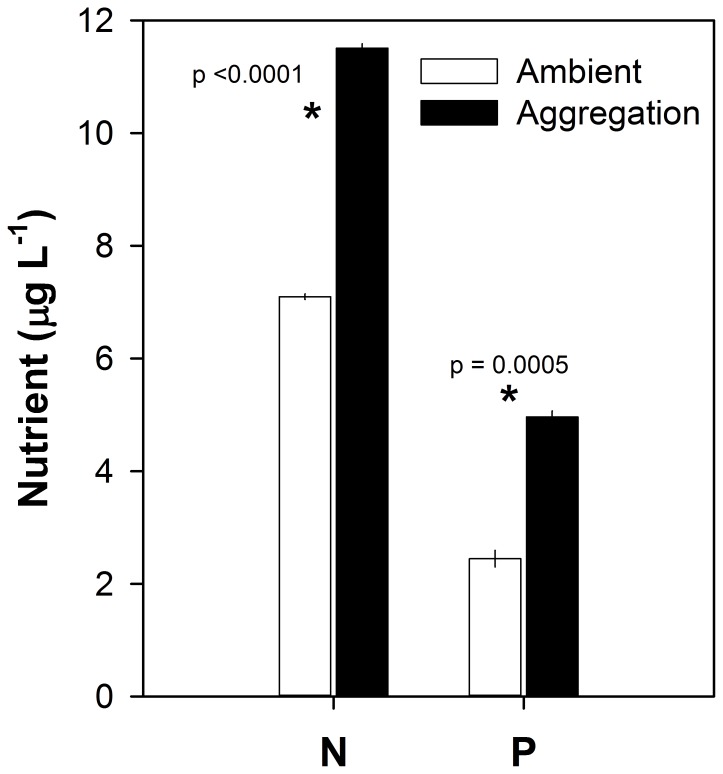
Aggregations of loricariids (Figures 1, 4) generated hotspots of nutrient recycling relative to ambient water chemistry in paired river sites. Water samples collected within aggregations of loricariids had 41% higher concentrations of NH4 +-N (p<0.0001, F(3, 60) = 9.63, Figure 4) and 66% higher concentrations of SRP (p = 0.0005, F(3, 60) = 11.7, Figure 4) relative to paired ambient chemistry sites.
Figure 4. Biogeochemical hotspots created by aggregations of Pterygoplichthys.
Means of NH4 +-N and PO4 −3-P (±1 SE) samples taken from paired sites within and outside of loricariid aggregations in the Chacamax River in 2008 (n = 16 ambient sites, n = 16 aggregation sites) and 2010 (n = 16 ambient sites, n = 16 aggregation sites). Aggregations were defined as water samples taken within groups of Pterygoplichthys that had an area of at least 5 m2 with at least 40 Pterygoplichthys per m2. Ambient samples were collected from sites parallel to the aggregations without immediate upstream aggregations of loricariids.
Discussion
The results from this study support findings from other studies documenting the important role consumer nutrient recycling can play in stream ecosystems [3], [11], [33]. For example, fish excretion of the limiting nutrient N exceeded N demand in a Venezuelan stream [3]. Similarly, freshwater shrimp excretion was equivalent to approximately 20% of the N uptake and 5% of the P uptake in Puerto Rican streams [33]. In our study, excretion of N and P by high densities of loricariids appears to be a large and potentially important flux of nutrients in the Chacamax River that is both spatially and temporally heterogeneous throughout the stream reach. This is one of the first studies to measure novel biogeochemical hotspots generated by an aquarium invader.
Similar to observations reported for loricariids in their native ranges [34] and introduced populations of Pterygoplichthys in Florida [35], Pterygoplichthys in the Chacamax River were nocturnally active (Figure 2). Gut content mass was greater during evening hours, indicating loricariids are primarily active and feeding at night in the study site (Figure 3D). Loricariid behavior may have evolved to minimize predation from day-active predators, as diel changes in behavior has been attributed to predator avoidance in native populations of loricariids [36]. Unfortunately, due to the limited number of potential predators remaining in the Chacamax River and the potential large body size of Pterygoplichthys (up to at least 70cm SL [36]), it is unlikely that predation will control the loricariid population in the region.
For organisms to generate areas of enriched nutrient recycling rates in space, or hotspots, across a landscape, their distribution must change through space and/or time [2], [3]. In this study, we observed diurnal aggregating behavior of loricariids (Figure 1) and temporal variation in fish excretion rates (Figure 3), which created a mechanism for armored catfish to generate biogeochemical hotspots in the Chacamax River. Water samples collected within loricariid aggregations had roughly double the concentration of N and P than water collected from outside the aggregations (Figure 4), suggesting that loricariids generate localized pulses of nutrients downstream from aggregations during daylight hours when primary producers are photosynthesizing. Primary producers in the study site were nutrient limited (Figure S1) [31]. Hence, elevated ambient nutrient availability may locally alleviate some nutrient limitation and enhance algal growth and primary productivity immediately downstream of loricariid aggregations. However, loricariid aggregations disperse daily; therefore, any increase in algal biomass driven by proximity to an aggregation of loricariids is likely removed quickly by nocturnal loricariid grazing and would be difficult to detect. Future work should attempt to separate the grazing and nutrient remineralization effects of loricariids to estimate the net effects of these fishes on algal biomass and gross primary production in invaded systems.
Species-specific characteristics and areal biomass are important factors to consider when predicting if a consumer could be a significant driver of nutrient recycling or if they have the potential to increase or relax nutrient limitation [3], [12]. For example, the elemental composition, or stoichiometry, of an invader may influence the impact of changing nutrient dynamics in an ecosystem [37]. Phosphorus excretion rates are highly variable among fish species [8], [12]; thus, P-cycling in P-limited systems, such as the Chacamax River, may be strongly influenced by changes in fish communities [12]. In such a system, the introduction of a P-rich invader such as loricariids [38] may actually intensify P-limitation of primary producers and microbial heterotrophs, and influence nutrient cycling rates in streams. For example, in our site, nocturnal feeding activity (Figure 3D) may have generated increased nocturnal P excretion by loricariids (Figure 3B) and significantly altered the N:P ratio of excretion between daytime and nighttime hours (Figure 3C). Notably, the N:P ratio of excretion during daylight hours (Figure 3C) was greater than the Redfield ratio [39]; therefore, diurnal loricariid excretion may have actually enhanced P-limitation.
It is important to mention, increased loricariid activity occurred simultaneously with increases in stream water NH4-N concentrations, but there was no measureable change in P concentrations in the water column (Figure 2). Though we documented diel changes in P excretion by loricariids that we attributed to feeding behavior, we did not see the same pattern in N excretion (Figure 3). Most likely, increased nighttime NH4-N concentrations in the stream water were due to diel patterns in autotrophic ammonium demand [40] rather than differences in ammonium excretion by loricariids. Though a similar pattern may have been evident with autotrophic uptake of P, all of the P concentrations measured outside of loricariid aggregations were at or very near the level of detection. Thus, stoichiometric changes at the ecosystem level were most likely being driven by large changes in ambient ammonium concentrations. This also suggests pulses of soluble P generated by loricariid aggregations may be locally significant.
Well-mixed rivers like the Chacamax add complexity to studying the formation of biogeochemical hotspots, for unlike terrestrial environments, hotspots may not have discrete boundaries. Hence, it is important to note that loricariid remineralization contributed to the N and P concentrations of samples we collected within and outside of fish aggregations. Moreover, there was likely an array of influences loricariid invasion had on nutrient dynamics in addition to remineralization, such as bioturbation. However, we focused this study on the direct influence of invaders on nutrient cycling via remineralization. Importantly, ambient water chemistry data on the Chacamax were not collected prior to loricariid invasion, so we cannot determine the potential additive effects of loricariid nutrient recycling to total ambient solute concentrations. Moreover, P concentrations in the Chacamax were near the level of detection; thus changes in ambient P after invasion may be difficult to detect.
To be important drivers of nutrient cycling, the contribution of nutrient remineralization by organisms must be significant at the ecosystem-level [2], [3]. Our data suggest that loricariids strongly influence nutrient remineralization rates in the Chacamax River. The volumetric excretion estimates for loricariids were greater than ambient water chemistry values indicating that remineralization by loricariids may be an important flux of nutrients within the river. Moreover, loricariid biomass was two orders of magnitude greater than native fish biomass [31]. Consequently, loricariid invasion may have shifted the Chacamax River from a system where fishes were not central drivers of biogeochemical processes to a system where remineralization of nutrients by fishes is a large and important flux of nutrients.
Recent work has suggested invasive species management and eradication efforts should target species that present serious environmental risks and alter the function of ecosystems [41]. When coupled with work demonstrating non-native loricariids are: competing with native species for food resources [42], [43], altering the structure of aquatic and riparian ecosystems [44], [45], and negatively affecting populations of threatened and endangered species [35], [46]; the results from this study indicate high-densities of invading loricariids threaten the structure and function of ecosystems. Moreover, the results from this investigation provide additional evidence [47] that aggregations of non-native organisms have the potential to alter nutrient dynamics and generate biogeochemical hotspots. This work highlights the potential threat invasive, aquarium species present to ecosystem function in freshwater ecosystems.
Supporting Information
Nutrient limitation of periphyton in the Chacamax River. Mean (±1 SE) algal biomass collected from nutrient diffusing substrates (NDS) for each of four nutrient treatments (control (CON), nitrogen (N), phosphorus (P), nitrogen and phosphorus (N+P)). Bars with different letters have significantly different (p<0.005) algal biomasses according to Tukey’s Honestly Significant Difference test. Nutrient diffusing substrates were constructed using methods outlined in Capps et al. [1]. They were deployed for a total of 14 days. Nutrient diffusion rate estimates were made for each nutrient treatment on day 0 and day14 by subtracting the diffusion rate of nutrient amended NDS from the rate of control NDS [1]. Nutrient diffusion rate estimates were made for each nutrient treatment by subtracting the diffusion rate of nutrient amended NDS from the rate of control NDS. On day 14 (N: 7.2×10−5±3.8×10−6; P: 7.1×10−4±3.1×10−5; N+P: (N) 9.5×10−6±2.1×10−6, (P) 8.1×10−5±1.5×10−6, mean ± SE (mol m−2 hr−1)), all treatments were diffusing less than on day 0 (N: 2.1×10−2±3.1×10−4; P: 4.9×10−3±4.3×10−5; N+P: (N) 3.9×10−2±3.1×10−4, (P) 9.1×10−3±5.8×10-5, mean ± SE (mol m−2 hr−1)). The results from NDS indicated that primary producers in the Chacamax River were P-limited (p<0.0001, F(3, 43) = 13.6). References Cited: 1. Capps KA, Booth MT, Collins SM, Davison MA, Moslemi JM, et al. (2011) Nutrient diffusing substrata: a field comparison of commonly used methods to assess nutrient limitation. Journal of the North American Benthological Society 30∶522–532.
(TIF)
Range of ventral patterns of Pterygoplichthy s collected in the Chacamax River (N17°29′047′′ W91°58′430′′). Pterygoplichthys pardalis is characterized by a ventral pattern of dark spots (A). Pterygoplichthys disjunctivus is characterized by dark, vermiculated lines (G) (Armbruster & Page 2006). The wide variations in pattern suggest the Pterygoplichthys population in the Chacamax may be comprised of hybrids of the two species. Photo credit: K. A. Capps.
(TIF)
Acknowledgments
We thank Christy Goodale, Nelson Hairston Jr., and Stuart Findlay for their comments on earlier versions of this manuscript. We also thank Rocío Rodiles-Hernández, Jessica Strickland, and Daniel Rodríguez for help with fieldwork in Mexico. Finally, we would like to thank the personnel of the Hotel Nututun for their support in Mexico. Organisms were harvested in Mexico using Mexican collection permit number DGOPA.07525.25706.3233 and fishes were handled using methods outlined in the IACUC protocol 2006–0169 from Cornell University.
Funding Statement
This work was funded by the National Science Foundation (Doctoral Dissertation Enhancement Program Grant (183-8371); Integrated Graduate Education and Research in Biogeochemistry and Environmental Biocomplexity (0221658) Small Grant Program), the Fulbright-Hays Doctoral Dissertation Research Abroad Program, Sigma Xi, the Tinker Field Research Grant, the Andrew and Margaret Paul Graduate Fellowship in the Life Sciences, and the American Cichlid Association Paul V. Loiselle Conservation Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Meyer JL, Schultz ET, Helfman GS (1983) Fish schools-an asset to corals. Science 220: 1047–1049. [DOI] [PubMed] [Google Scholar]
- 2. McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, et al. (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6: 301–312. [Google Scholar]
- 3. McIntyre PB, Flecker AS, Vanni MJ, Hood JM, Taylor BW, et al. (2008) Fish distributions and nutrient cycling in streams: Can fish create biogeochemical hotspots? Ecology 89: 2335–2346. [DOI] [PubMed] [Google Scholar]
- 4. Liess A, Hillebrand H (2006) Role of nutrient supply in grazer-periphyton interactions: reciprocal influences of periphyton and grazer nutrient stoichiometry. Journal of the North American Benthological Society 25: 632–642. [Google Scholar]
- 5. Evans-White MA, Lamberti GA (2006) Stoichiometry of consumer-driven nutrient recycling across nutrient regimes in streams. Ecology Letters 9: 1186–1197. [DOI] [PubMed] [Google Scholar]
- 6. Knoll LB, McIntyre PB, Vanni MJ, Flecker AS (2009) Feedbacks of consumer nutrient recycling on producer biomass and stoichiometry: separating direct and indirect effects. Oikos 118: 1732–1742. [Google Scholar]
- 7. Vander Zanden MJ, Vadeboncoeur Y (2002) Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83: 2152–2161. [Google Scholar]
- 8. Vanni MJ, Flecker AS, Hood JM, Headworth JL (2002) Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecology Letters 5: 285–293. [Google Scholar]
- 9. McIntyre PB, Michel E, Olsgard M (2006) Top-down and bottom-up controls on periphyton biomass and productivity in Lake Tanganyika. Limnology and Oceanography 51: 1514–1523. [Google Scholar]
- 10. McIntyre PB, Jones LE, Flecker AS, Vanni MJ (2007) Fish extinctions alter nutrient recycling in tropical freshwaters. Proceedings of the National Academy of Sciences of the United States of America 104: 4461–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reisinger AJ, Presuma DL, Gido KB, Dodds WK (2011) Direct and indirect effects of central stoneroller (Campostoma anomalum) on mesocosm recovery following a flood: can macroconsumers affect denitrification? Journal of the North American Benthological Society 30: 840–852. [Google Scholar]
- 12. Small GE, Pringle CM, Pyron M, Duff JH (2011) Role of the fish Astyanax aeneus (Characidae) as a keystone nutrient recycler in low-nutrient Neotropical streams. Ecology 92: 386–397. [DOI] [PubMed] [Google Scholar]
- 13. Kitchell JF, Koonce JF, Tennis PS (1975) Phosphorus flux through fishes. Verhandlungen International Verein Limnologie 19: 2478–2484. [Google Scholar]
- 14. Kraft CE (1993) Phosphorus regeneration by Lake Michigan alewives in the mid-1970s. Transactions of the American Fisheries Society 122: 749–755. [Google Scholar]
- 15. Bunnell DB, Johnson TB, Knight CT (2005) The impact of introduced round gobies (Neogobius melanostomus) on phosphorus cycling in central Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 62: 15–29. [Google Scholar]
- 16. Nico LG, Martin RT (2001) The South American suckermouth armored catfish, Pterygoplichthys anisitsi (Pisces : Loricariidae), in Texas, with comments on foreign fish introductions in the American southwest. Southwestern Naturalist 46: 98–104. [Google Scholar]
- 17. Weber C (1991) New taxa in Pterygoplichthys S I (Pisces, Siluriformes, Loricariidae). Revue Suisse De Zoologie 98: 637–643. [Google Scholar]
- 18. Capps KA, Nico LG, Mendoza-Carranza M, Arevalo-Frias W, Ropicki AJ, et al. (2011) Salinity tolerance of non-native suckermouth armoured catfish (Loricariidae: Pterygoplichthys) in south-eastern Mexico: implications for invasion and dispersal. Aquatic Conservation-Marine and Freshwater Ecosystems 21: 528–540. [Google Scholar]
- 19.Courtenay WR, Hensley DA, Taylor JN, McCann JA (1986) Distribution of exotic fishes in North America. In: Hocutt CH, Wiley EO, editors. The zoogeography of North American freshwater fishes. New York: John Wiley and Sons. 675–698. [Google Scholar]
- 20.Arthington AH, Kailola PJ, Woodland DJ, Zalucki JM (1999) Baseline environmental data relevant to an evaluation of quarantine risk potentially associated with the importation to Australia of ornamental finfish. Canberra: Department of Agriculture, Fisheries, and Forestry. [Google Scholar]
- 21.Bomford M, Glover J (2004) Risk assessment model for the import and keeping of exotic freshwater and estuarine finfish. In: Sciences BoR, editor. Canberra: Australian Government. [Google Scholar]
- 22. Liang SH, Wu HP, Shieh BS (2005) Size structure, reproductive phenology, and sex ratio of an exotic armored catfish (Liposarcus multiradiatus) in the Kaoping River of southern Taiwan. Zoological Studies 44: 252–259. [Google Scholar]
- 23.Chavez JM, De La Paz RM, Manohar SK, Pagulayan RC, Vi JRC (2006) New Philippine record of South American sailfin catfishes (Pisces : Loricariidae). Zootaxa: 57–68. [Google Scholar]
- 24.Kailola PJ (2007) Risk assessment of ten species of ornamental fish under the Environment Protection and Biodiversity Conservation Act 1999. Sydney: Australian Government. [Google Scholar]
- 25. Ozdilek SY (2007) Possible threat for Middle East inland water: an exotic adn invasive species, Pterygoplichthys disjuntivis (Weber, 1991) in Asi river, Turkey (Pices: Loricariidae). Journal of Fisheries and Aquatic Sciences 24: 303–306. [Google Scholar]
- 26. Sinha RK, Sinha RK, Sarkar UK, Lakra WS (2010) First record of the southern sailfin catfish, Pterygoplichthys anisitsi, Eigenmann and Kennedy, 1903 (Teleostei: Loricariidae), in India. Journal of Applied Ichthyology 26: 606–608. [Google Scholar]
- 27.Devick WS (1989) Disturbances and fluctuations in the Wahiawa Reservoir ecosystem. Honolulu: Hawaii Department of Land and Natural Resources, Division of Aquatic Resources. 30 p. [Google Scholar]
- 28.Hoover JH, Kilgore KJ, Cofrancesco AF (2004) Suckermouth catfishes: Threats to aquatic ecosystems of the United States. Vicksburg: U.S. Army Corps of Engineers. [Google Scholar]
- 29.Mendoza RE, B C, Orr R, Balderas SC, Courtenay WR, et al.. (2009) Trinational risk assessment guidelines for aquatic alien invasive species. Montréal: Commission for Environmental Cooperation. [Google Scholar]
- 30. Capps KA, Booth MT, Collins SM, Davison MA, Moslemi JM, et al. (2011) Nutrient diffusing substrata: a field comparison of commonly used methods to assess nutrient limitation. Journal of the North American Benthological Society 30: 522–532. [Google Scholar]
- 31.Capps KA (2012) Changes in community structure and ecosystem processes in response to armored catfish (Silurformes: Loricariidae) invasion. Ithaca: Cornell University. 142 p. [Google Scholar]
- 32. Taylor BW, Keep CF, Hall RO, Koch BJ, Tronstad LM, et al. (2007) Improving the fluorometric ammonium method: matrix effects, background fluorescence, and standard additions. Journal of the North American Benthological Society 26: 167–177. [Google Scholar]
- 33. Benstead JP, Cross WF, March JG, McDowell WH, Ramirez A, et al. (2010) Biotic and abiotic controls on the ecosystem significance of consumer excretion in two contrasting tropical streams. Freshwater Biology 55: 2047–2061. [Google Scholar]
- 34. Casatti L, Castro RMC (2006) Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio Sao Francisco, southeastern Brazil. Neotropical Ichthyology 4: 203–214. [Google Scholar]
- 35. Nico LG (2010) Nocturnal and diurnal activity of armored suckermouth catfish (Loricariidae: Pterygoplichthys) associated with wintering Florida manatees (Trichechus manatus latirostris). Neotropical Ichthyology 8: 893–898. [Google Scholar]
- 36.Froese R, Pauly D (2011) FishBase. 10/2011 ed: World Wide Web. [Google Scholar]
- 37. Gonzalez AL, Kominoski JS, Danger M, Ishida S, Iwai N, et al. (2010) Can ecological stoichiometry help explain patterns of biological invasions? Oikos 119: 779–790. [Google Scholar]
- 38. Hood JM, Vanni MJ, Flecker AS (2005) Nutrient recycling by two phosphorus-rich grazing catfish: the potential for phosphorus-limitation of fish growth. Oecologia 146: 247–257. [DOI] [PubMed] [Google Scholar]
- 39. Redfield AC (1958) The biological control of chemical factors in the environment. American Scientist 64: 205–221. [PubMed] [Google Scholar]
- 40. Johnson LT, Tank JL (2009) Diurnal variations in dissolved organic matter and ammonium uptake in six open-canopy streams. Journal of the North American Benthological Society 28: 694–708. [Google Scholar]
- 41. Davis MA, Chew MK, Hobbs RJ, Lugo AE, Ewel JJ, et al. (2011) Don't judge species on their origins. Nature 474: 153–154. [DOI] [PubMed] [Google Scholar]
- 42. Pound KL, Nowlin WH, Huffman DG, Bonner TH (2011) Trophic ecology of a nonnative population of suckermouth catfish (Hypostomus plecostomus) in a central Texas spring-fed stream. Environmental Biology of Fishes 90: 277–285. [Google Scholar]
- 43. Mendoza-Carranza M, Hoeinghaus DJ, Garcia AM, Romero-Rodriguez A (2010) Aquatic food webs in mangrove and seagrass habitats of Centla Wetland, a Biosphere Reserve in Southeastern Mexico. Neotropical Ichthyology 8: 171–178. [Google Scholar]
- 44.Lienart GDH, Rodiles-Hernandez R, Capps KA (2012) Nest burrows and nesting behavior of non-native catfishes (Silurformes: Loricariidae) in the Usumacinta watershed, Mexico. Southwestern Naturalist In press. [Google Scholar]
- 45. Nico LG, Jelks HL, Tuten T (2009) Non-native suckermouth armored catfishes in Florida: description of nest burrows and burrow colonies with assessment of shoreline conditions. Aquatic Nuisance Species Research Program 09: 1–30. [Google Scholar]
- 46. Bunkley-Williams L, Williams EH, Lilystrom CG, Corujo-Flores I, Zerbi AJ, et al. (1994) The South American sailfin armored catfish Liposarcus multiradiatus (Hancock), a new exotic established in Puerto Rican fresh waters. Caribbean Journal of Science 30: 90–94. [Google Scholar]
- 47. Bouletreau S, Cucherousset J, Villeger S, Masson R, Santoul F (2011) Colossal Aggregations of Giant Alien Freshwater Fish as a Potential Biogeochemical Hotspot. Plos One 6: 10.1371/journal.pone.0025732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nutrient limitation of periphyton in the Chacamax River. Mean (±1 SE) algal biomass collected from nutrient diffusing substrates (NDS) for each of four nutrient treatments (control (CON), nitrogen (N), phosphorus (P), nitrogen and phosphorus (N+P)). Bars with different letters have significantly different (p<0.005) algal biomasses according to Tukey’s Honestly Significant Difference test. Nutrient diffusing substrates were constructed using methods outlined in Capps et al. [1]. They were deployed for a total of 14 days. Nutrient diffusion rate estimates were made for each nutrient treatment on day 0 and day14 by subtracting the diffusion rate of nutrient amended NDS from the rate of control NDS [1]. Nutrient diffusion rate estimates were made for each nutrient treatment by subtracting the diffusion rate of nutrient amended NDS from the rate of control NDS. On day 14 (N: 7.2×10−5±3.8×10−6; P: 7.1×10−4±3.1×10−5; N+P: (N) 9.5×10−6±2.1×10−6, (P) 8.1×10−5±1.5×10−6, mean ± SE (mol m−2 hr−1)), all treatments were diffusing less than on day 0 (N: 2.1×10−2±3.1×10−4; P: 4.9×10−3±4.3×10−5; N+P: (N) 3.9×10−2±3.1×10−4, (P) 9.1×10−3±5.8×10-5, mean ± SE (mol m−2 hr−1)). The results from NDS indicated that primary producers in the Chacamax River were P-limited (p<0.0001, F(3, 43) = 13.6). References Cited: 1. Capps KA, Booth MT, Collins SM, Davison MA, Moslemi JM, et al. (2011) Nutrient diffusing substrata: a field comparison of commonly used methods to assess nutrient limitation. Journal of the North American Benthological Society 30∶522–532.
(TIF)
Range of ventral patterns of Pterygoplichthy s collected in the Chacamax River (N17°29′047′′ W91°58′430′′). Pterygoplichthys pardalis is characterized by a ventral pattern of dark spots (A). Pterygoplichthys disjunctivus is characterized by dark, vermiculated lines (G) (Armbruster & Page 2006). The wide variations in pattern suggest the Pterygoplichthys population in the Chacamax may be comprised of hybrids of the two species. Photo credit: K. A. Capps.
(TIF)