Abstract
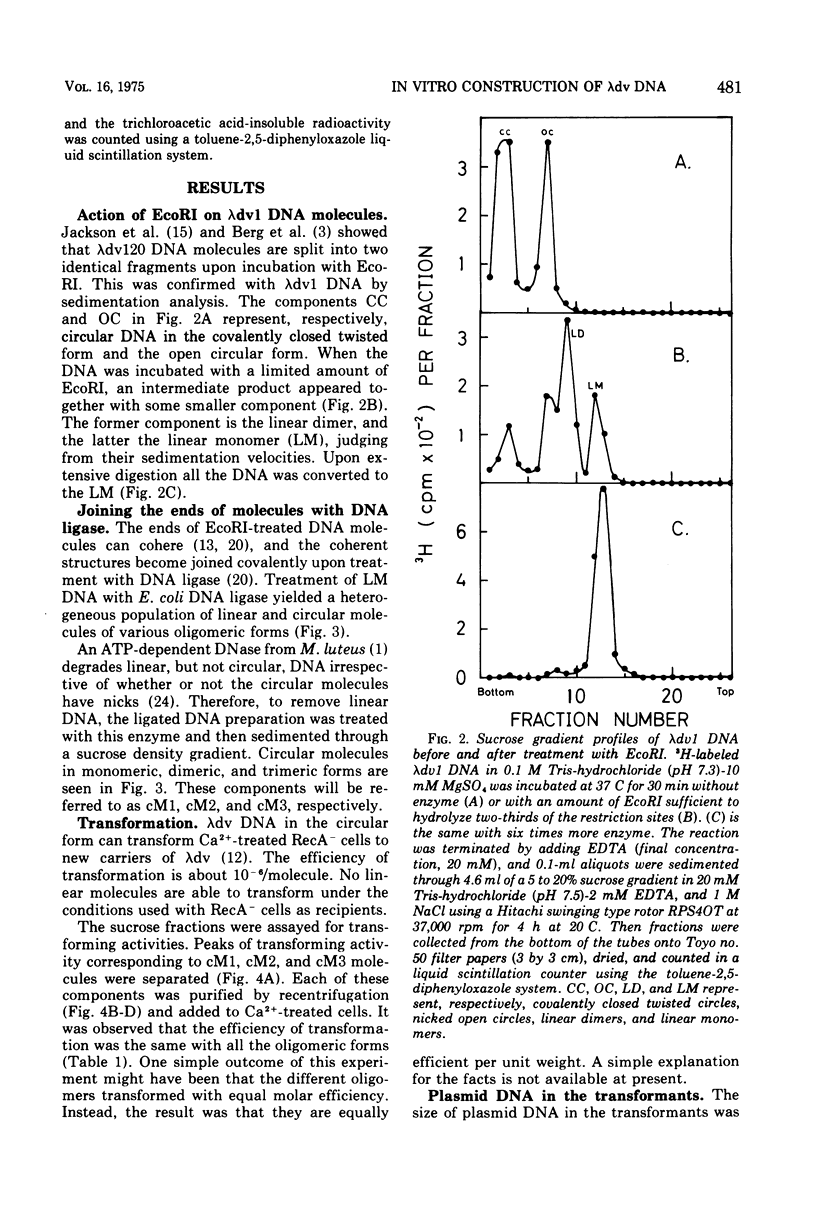
Plasmid lambdadv1, which is in a dimeric form, was converted to a linear monomer duplex by the action of EcoRI restriction endonuclease that incises at a unique site in this plasmid genome. The resulting products were then joined by Escherichia coli DNA ligase to produce molecules with various oligomeric forms, and from these monomeric, dimeric, or trimeric circular molecules were purified. By transformation of cells with these DNAs, clones were obtained that carried lambdadv1 in a monomeric or dimeric form. The former type of clones have not been generated in vivo, except for one in a different host strain, and carriers of timeric or tetrameric lambdadv1's have not been obtained so far. It was observed that a considerable fraction of these oligomeric circular DNAs were converted to lower oligomers (e.g., from trimer to dimer) during transformation. The characteristics of the monomeric lambdadv1 carriers obtained were compared with those of dimeric lambdadv1 carriers. The stabilities of the plasmids of the two forms were the same. However, the monomeric plasmid carriers were less tolerant to lambdavir phage infection and perpetuated about 30% less plasmid genomes in monomer units. Furthermore, dimeric plasmid carriers appeared spontaneously and accumulated in cultures of the monomeric lambdadv1 carriers.
Full text
PDF

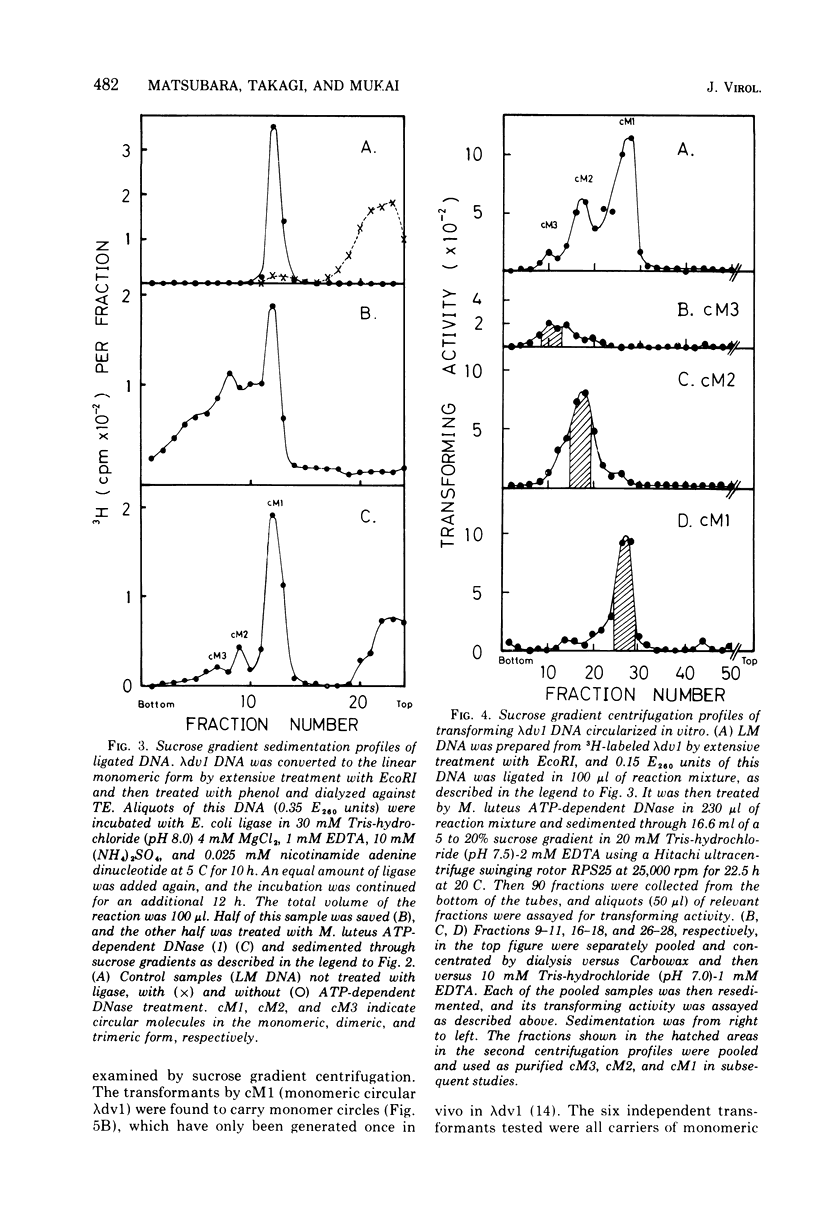


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
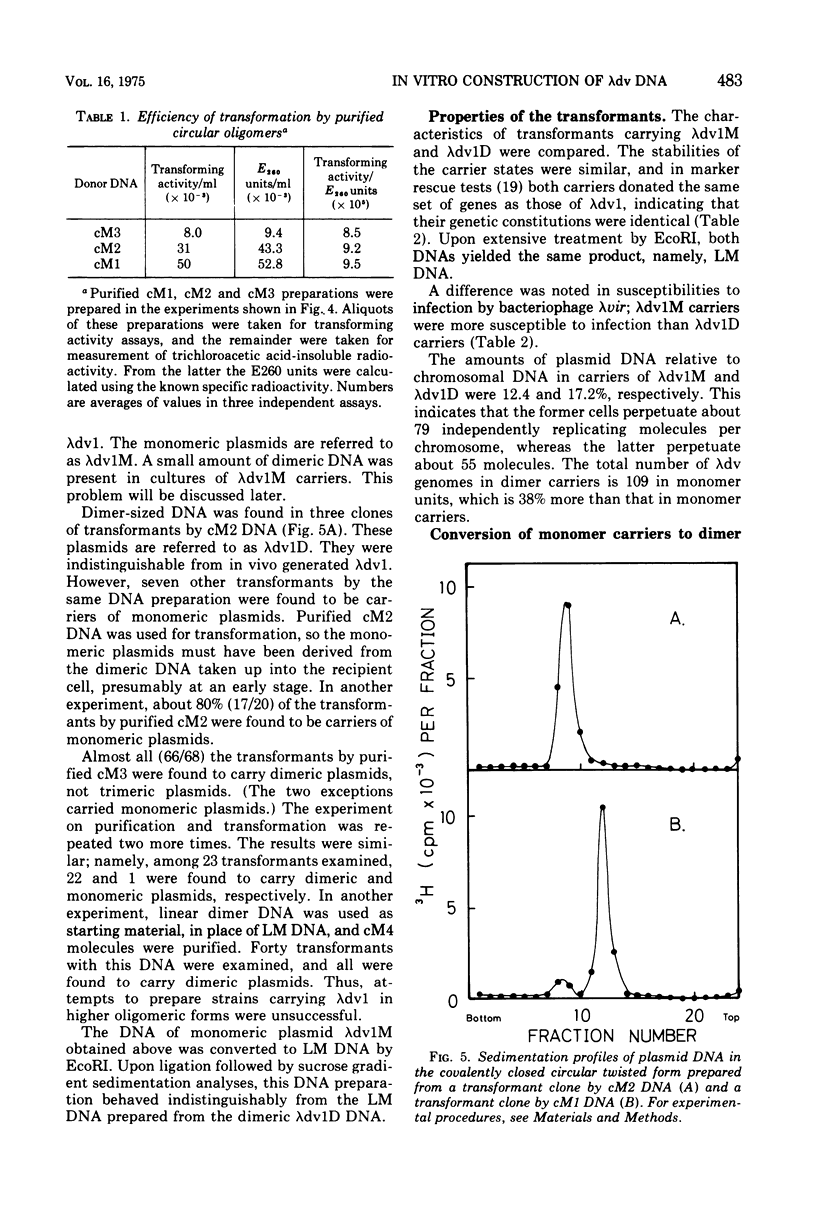
- Anai M., Hirahashi T., Takagi Y. A deoxyribonuclease which requires nucleoside triphosphate from Micrococcus lysodeikticus. I. Purification and characterization of the deoxyribonuclease activity. J Biol Chem. 1970 Feb 25;245(4):767–774. [PubMed] [Google Scholar]
- Berg D. E. Genes of phage lambda essential for lambda dv plasmids. Virology. 1974 Nov;62(1):224–233. doi: 10.1016/0042-6822(74)90317-1. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Jackson D. A., Mertz J. E. Isolation of a lambda dv plasmid carrying the bacterial gal operon. J Virol. 1974 Nov;14(5):1063–1069. doi: 10.1128/jvi.14.5.1063-1069.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope study of the structures of lambdadv DNAs. J Mol Biol. 1974 Jun 15;86(1):69–89. doi: 10.1016/s0022-2836(74)80008-2. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Complex mitochondrial DNA in leukemic and normal human myeloid cells. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1077–1084. doi: 10.1073/pnas.62.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court D., Sato K. Studies of novel transducing variants of lambda: dispensability of genes N and Q. Virology. 1969 Oct;39(2):348–352. doi: 10.1016/0042-6822(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Vogt M., Dieckmann M., Berg P. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. II. Formation of oligometric polyoma DNA molecules. J Mol Biol. 1970 Feb 14;47(3):317–333. doi: 10.1016/0022-2836(70)90305-0. [DOI] [PubMed] [Google Scholar]
- Goebel W., Helinski D. R. Generation of higher multiple circular DNA forms in bacteria. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1406–1413. doi: 10.1073/pnas.61.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobom G., Hogness D. S. The role of recombination in the formation of circular oligomers of the lambda plasmid. J Mol Biol. 1974 Sep 5;88(1):65–87. doi: 10.1016/0022-2836(74)90295-2. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Symons R. H., Berg P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2904–2909. doi: 10.1073/pnas.69.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
- Matsubara K. Plasmid formation from bacteriophage lambda as a result of interference by resident plasmid lambda dv. Virology. 1972 Mar;47(3):618–627. doi: 10.1016/0042-6822(72)90551-x. [DOI] [PubMed] [Google Scholar]
- Matsubara K. Preparation of plasmid lambda dv from bacteriophage lambda: role of promoter-operator in the plasmid replicon. J Virol. 1974 Mar;13(3):596–602. doi: 10.1128/jvi.13.3.596-602.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Hopkins N. The operators controlled by the lambda phage repressor. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1282–1287. doi: 10.1073/pnas.60.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer E. R. Plasmid formation: a new mode of lysogeny by phase lambda. Nature. 1969 Jul 12;223(5202):158–160. doi: 10.1038/223158a0. [DOI] [PubMed] [Google Scholar]
- Takagi Y., Matsubara K., Anai M. A deoxyribonuclease which requires nucleoside triphosphate from Micrococcus lysodeikticus. IV. The mode of DNA hydrolysis. Biochim Biophys Acta. 1972 May 29;269(3):347–353. doi: 10.1016/0005-2787(72)90121-9. [DOI] [PubMed] [Google Scholar]


