Abstract
Necrotic enteritis (NE) is one of the most important enteric diseases in poultry and is a high cost to the industry worldwide. It is caused by avian-specific, Necrotic Enteritis Beta toxin (NetB)-producing, strains of Clostridium perfringens that also possess in common other virulence-associated genes. In Europe the disease incidence has increased since the ban on in-feed “growth promoting” antibiotics. Because of this, many recent studies of NE have focused on finding different ways to control the disease, and on understanding its pathogenesis. Frustratingly, reproduction of the disease has proven impossible for some researchers. This review describes and discusses factors known to be important in reproducing the disease experimentally, as well as other considerations in reproducing the disease. The critical bacterial factor is the use of virulent, netB-positive, strains; virulence can be enhanced by using tpeL- positive strains and by the use of young rather than old broth cultures to increase toxin expression. Intestinal damaging factors, notably the use of concurrent or preceding coccidial infection, or administration of coccidial vaccines, combined with netB-positive C. perfringens administration, can also be used to induce NE. Nutritional factors, particularly feeding high percentage of cereals containing non-starch polysaccharides (NSP) (wheat, rye, and barley) enhance disease by increasing digesta viscosity, mucus production and bacterial growth. Animal proteins, especially fish meal, enhance C. perfringens proliferation and toxin production. Other factors are discussed that may affect outcome but for which evidence of their importance is lacking. The review compares the different challenge approaches; depending on the aim of particular studies, the different critical factors can be adjusted to affect the severity of the lesions induced. A standardized scoring system is proposed for international adoption based on gross rather than histopathological lesions; if universally adopted this will allow better comparison between studies done by different researchers. Also a scoring system is provided to assist decisions on humane euthanasia of sick birds.
Table of contents
1. Introduction
2. Different reasons to reproduce necrotic enteritis
3. Points to be considered in successful reproduction of necrotic enteritis
3.1. Nutritional factors
3.1.1. Feeding indigestible non-starch polysaccharides
3.1.2. Feeding large amounts of animal protein (fish meal)
3.2. Role of coccidia
3.3. The role of immunosuppression in experimental necrotic enteritis
3.4. Bacteriological aspects
3.4.1. Critical virulence features of C. Perfringens strains involved in necrotic enteritis in chickens, and in reproducing the disease
3.4.2. Preparation of C. perfringens for challenge
3.4.2.1. Type of culture media
3.4.2.2. Incubation time
3.4.2.3. Amount of bacteria for challenge
3.5. Challenge methods
4. Other considerations
5. Lesion scoring systems
5.1. Gross lesions of necrotic enteritis
5.2. Different scoring systems
6. Reproduction of clinical and subclinical necrotic enteritis
7. Determining performance parameters (Weight gain, Feed intake, feed conversion ratio = FCR)
8. Welfare considerations
9. Conclusions
10. Competing interests
11. Authors’ contributions
12. Acknowledgements
13. References
1. Introduction
Necrotic enteritis (NE) in chickens, first reported by Parish [1], is an enteric disease caused by C. perfringens, a Gram-positive anaerobic spore-forming, rod-shaped bacterium [2]. According to the current classification, C. perfringens has five toxinogenic types (A, B, C, D, E), which are differentiated according to the production of four different major toxins (Alpha, Beta, Epsilon, Iota) [3]. The discovery in recent years of new toxins (Beta2, NetB, TpeL) in C. perfringens shows the need for an enhanced classification scheme. NE is caused by type A isolates [3] and rarely by type C isolates [4,5]. The recently discovered new toxin, NetB, is crucial for development of the disease [6,7]. Keyburn et al.’s [6] seminal discovery of the crucial role of the pore-forming toxin NetB led to the subsequent characterization of three pathogenicity loci (PAL) that are characteristic of NE isolates [8]. Two PAL (NELoc1, NELoc 3) are plasmid-encoded, usually on different plasmids [8]. Two plasmids on which these PAL are found have recently been fully sequenced [9]. NE isolates belong to two major lineages or clones [10], suggesting that these lineages have adapted to cause NE in chickens.
The intestinal number of C. perfringens in healthy and in NE-affected birds are different. The C. perfringens population is found to be normally less than l02 to 104 colony-forming units (CFU) per g of the intestinal contents in the small intestine of healthy chickens compared to 107 - 109 CFU/g in diseased birds [11].
NE occurs in broilers aged between two and six weeks [4,12]. Mortality can reach 1% per day with a total mortality of 10-40% [13]. Clinical signs include depression, dehydration, diarrhea, ruffled feathers and lower feed intake [4]. The gross lesions of the small intestine range from thin and friable walls to frank and extensive necrotic lesions [12]. Two forms of the disease are described, clinical and subclinical [4,7,14]. The clinical form appears with the clinical signs and mortality noted above. The subclinical form presents as poor performance (reduced growth, reduced feed efficiency) without mortality. This form of the disease can be diagnosed by reduced feed conversion, by gross lesions in the small intestine and by bacteriology [14]. Most of the economic losses due to NE are related to the subclinical form and the high cost of preventing the disease with antibiotics.
Antibacterial drugs are commonly used to prevent or control the disease. In recent years, the European Union has banned the use of in-feed antimicrobials or growth promoters, leading to an increase in disease outbreaks in broiler flocks in European countries [15,16]. Globally, the economical impact of the disease is estimated at US$ 2 billion year through mortalities and poor performance and the cost of prevention and treatment [15,17]. In EU countries, the profit of severely NE affected broiler flocks has been 33% less than flocks with low level of the disease [17]. The variables affected the significant economic cost of subclinical NE have been estimated [18].
In recent years there has been an explosion of interest in understanding the pathogenesis of NE and investigating how it can be prevented by approaches other than the use of antibiotics. Many of these studies necessitate experimental induction of the disease. However, published and anecdotal reports attest to the difficulty that some workers have experienced in reproduction of the disease [16,19,20]. It is the purpose of this review to summarize the different approaches used to reproduce NE, to identify factors that are critical to successful production of experimental disease, and to highlight areas of uncertainty that require further investigation. The purpose of the review is to assist researchers to understand NE so that their experimental procedures are based on an evidence-based and logical approach.
Necrotic enteritis is a complex and multi-factorial disease. The factors affecting development of the disease in the field were well summarized by Williams [21], unfortunately just before the discovery of NetB. Figure 1 is a simplified diagram, extending that of William’s earlier figure, which summarizes some of the critical factors influencing the development of the disease.
Figure 1.
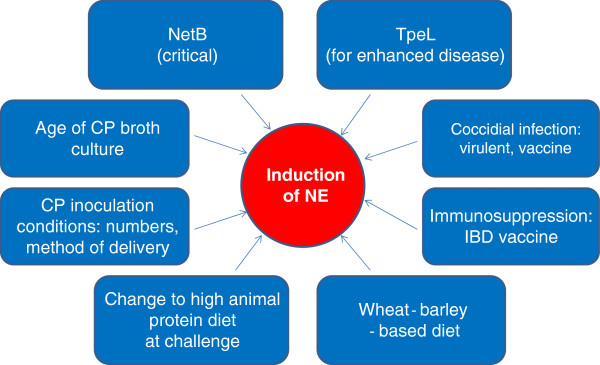
Critical factors influencing the development of necrotic enteritis. Summary of different factors important for the successful reproduction of necrotic enteritis; the most critical factor is presence of netB and the netB plasmid, but other factors summarized in the figure all affect the outcome of experimental infection. They can be manipulated by researchers to vary the severity of the disease produced and the outcome desired.
2. Different reasons to reproduce necrotic enteritis
Different researchers have different reasons for reproducing necrotic enteritis, which will impact the design of studies as well as the severity of disease that they wish to produce. Reasons to reproduce the disease have included comparison of antimicrobial drugs [22-27], vaccines [28-31], assessment of virulence determinants [6,32], or assessment of pathological processes [19]. Others have studied the effect of different predisposing factors including nutritional components on the severity of NE [2,33-37], the effects of probiotics and prebiotics [23], or different experimental models of disease [38-41]. Design considerations will also include the need for control groups and the need for isolation facilities to ensure lack of spread of infection between control and infected birds. The novice researcher is advised to perform a small scale pilot study both to confirm their ability to reproduce the disease and to identify the different effects of variables discussed below that affect the severity of the disease that they wish to produce. It would be anticipated that production of severe experimental disease would mask some of the beneficial effects of interventions such as feed components or immunization.
3. Points to be considered in successful reproduction of necrotic enteritis
3.1. Nutritional factors
3.1.1. Feeding indigestible non-starch polysaccharides
Several studies have shown that feeds (barley, rye, wheat) containing high amount of water-soluble indigestible non-starch polysaccharide (NSP), such as β-glucans or arabinoxylans, increase the viscosity of the digesta and predispose chickens to NE [13,14,42,43]. The higher viscosity of digesta in diets containing wheat or barley leads to prolonged transit time in the intestine, which may be responsible for the direct correlation between intestinal viscosity and clostridial counts [42]. However, equally or more importantly, NSP also interact with glycoproteins on the epithelial surface to increase mucin production [44]. C. perfringens has an “arsenal” of up to 56 glycoside hydrolases directed at the muco-oligosacharides prominent in the mucosal layers of the intestine [45,46]. The two prominent chitinase genes present on the major pathogenicity locus (NELoc1) of NE isolates are speculated to have mucin degradation functions [8].
In a C. perfringens challenge model, mortalities in birds fed a supernatant of digested maize ranged from 0-12%, whereas birds that received barley, rye or wheat showed mortality of 26-35% [47]. Mortality due to NE in coccidiosis-challenged chicks with a corn-based diet was also less than mortality in a wheat-based diet [48]. The number of C. perfringens in the intestine in corn-based diet fed broilers were 1.2-1.5 log10 colony-forming units/g lower than in the birds fed 50% rye in their feed [49]. Others have also reported the increased incidence of NE associated with wheat or barley diets [14,50]. Since NSPs are not well digested they reach the lower intestinal tract and alter its environment [51,52].
The size of the feed particles has been shown to affect the number of C. perfringens in the intestine. As expected, highly ground feed allows C. perfringens to proliferate faster and to larger numbers than coarse ground feed [48,53], and can predispose birds to NE [48,54].
Other nutritional factors can impact the severity of NE. For example, trypsin inhibitors in non-toasted soya bean based ration increased the severity of NE lesions, in direct proportion to the quantity of soya bean in the feed [55]. Trypsin inhibition is well established as a predisposing factor to enteric disease caused by C. perfringens, since trypsin in the small intestine destroys C. perfringens toxins [4].
Researchers should ensure that feed of experimental birds contains neither antibiotics nor anti-coccidials, recognizing that the latter may also have antibacterial properties.
3.1.2. Feeding large amounts of animal protein (fish meal)
A characteristic of C. perfringens identified through genome sequencing is the inability of this “anaerobic flesh eater” to synthesize the majority of amino acids; C. perfringens rapidly breakdown tissues through the extraordinary array of hydrolytic enzymes that it produces [56]. Large amounts of animal-origin protein in the diet predispose poultry to NE [21,50,57]. The presence of high crude protein concentration and some amino acids are related to commencement of C. perfringens overgrowth and production of alpha toxin [58]. Glycine is among the amino acids that stimulate growth and production of alpha toxin [59,60] and is positively correlated with the number of C. perfringens in the intestine [61]. Therefore the glycine content of the diet may be important to predispose birds to NE. The level of C. perfringens has been found highest with the greater amount of animal protein (40% crude protein/feed) and lowest in plant-source protein diets fed to chicks [19,62]. Large amounts of fish meal have been associated with C. perfringens proliferation and the occurrence of NE [63]. The amounts of glycine and methionine, which increase C. perfringens proliferation and alpha toxin concentrations in vitro, are higher than other amino acids in fish meal protein-based diets [60]. Although there has been no study of the effect of diet specifically on NetB toxin production, both the netB gene as well as the internalin gene adjacent to it on NELoc1 have VirR boxes [8,64], suggesting that, like alpha toxin, their expression is under control of the VirR-VirS regulatory system that controls virulence gene expression in this organism. It is therefore likely that conditions that increase alpha toxin also increase the critically important NetB.
Having a diet with, or changing the diet to one with high protein before the time of challenge seems to enhance the severity of NE although this effect has not been critically evaluated in detail, and comparisons are difficult because of use of different scoring systems. Changing the diet to a high protein diet before C. perfringens challenge increased the severity of induced NE, but mixing the diet with 30-50% fish meal has been done either on the same day [65], one day after [41], or seven [24,26,65,66] days before C. perfringens challenge. In one study, turkey feed (28% protein) was mixed with 50% fish meal and given to chicks from 1–13 days of age, which resulted in 12% mortality and 65% of the birds showing NE lesions when the chicks were challenged with C. perfringens at 14 days of age [22]. Consumption of diets containing lower energy: protein ratios not only leads to increased feed intake and higher nitrogen content of the digesta and feces [13] but can also lead to an enhanced substrate for C. perfringens[67,68]. It seems that feeding birds with a high protein diet for a longer period of time is better in reproducing more severe NE, but details of timing have not been determined. The high protein ration should be present at the time of challenge.
3.2. Role of coccidia
Coccidiosis is an enteric parasitic disease caused in poultry by various Eimeria spp. [21]. Some (E. brunetti, E. maxima, E. necatrix, E. tenella) produce more severe disease than others (E. acervulina, E. mitis, E. praecox) [21]. Damage to the epithelium caused by coccidia is a major predisposing factor for NE, allowing C. perfringens to replicate rapidly and produce toxin [69], probably because leakage of proteins including plasma into the lumen of the gut during Eimeria infection provides the protein-rich nutrient substrates favorable to C. perfringens proliferation and toxin production [16]. The mucogenesis induced by coccidial infection may also be important [70].
For these reasons, Eimeria spp. have often been used in conjunction with C. perfringens to induce NE experimentally. Since NE is a disease of small intestine, E. acervulina, E. maxima or E. necatrix are the most suitable species [2,15,23,40,71]. There are differences in opinion as to whether the more virulent Eimeria, such as E. necatrix are better than the less virulent E. acervulina choices for this purpose [2,72]. To obtain the effect of coccidial challenge, damage to the epithelium should occur before challenge with C. perfringens[43]. Table 1 summarizes the timing and the effect of different Eimeria spp. in conjunction with the timing of C. perfringens challenge. Eimeria vaccines have also been used to enhance the effect of C. perfringens challenge [20,41].
Table 1.
Effect of coccidial challenge on severity of experimental necrotic enteritis
| Researchers | Eimeria spp. | Age of coccidia challenge (day) | Age of C. perfringens challenge (day) |
% Mortality |
|
|---|---|---|---|---|---|
| With coccidia | Without coccidia | ||||
| [71] |
E. necatrix |
11 |
15 |
28.3 |
16 |
| [71] |
E. acervulina |
13 |
15 |
53.3 |
16 |
| [70] |
E. acervulina E. maxima |
14 |
18, 19, 20 |
7.6 |
2.8 |
| [2] |
E. necatrix |
4 |
5, 6, 7, 8, 9 |
22.5 |
0 |
| [41] |
Paracox-8 (Vaccine) |
19 |
18, 19, 20, 21 |
01 |
0 |
| [41] |
E. maxima |
20 |
19, 20, 21, 22 |
01 |
0 |
| [41] |
Paracox-8 (Vaccine) |
16 |
19, 20, 21, 22 |
01 |
0 |
| [20] |
Paracox-5 (vaccine) |
20 |
19, 20, 21, 22 |
01 |
0 |
| [84] |
Paracox-8 (Vaccine) |
10 |
9, 10, 11, 12 |
01 |
01 |
| [84] | Paracox-8 (Vaccine) | 18 | 17, 18, 19, 20 | 01 | 01 |
1Sub-clinical necrotic enteritis (No mortality).
The time of administration of the coccidial vaccine or of virulent Eimeria is critical, and should be not more than 4–5 days before the C. perfringens challenge so that the coccidia-induced intestinal damage coincides with bacterial challenge [21,40]. When the C. perfringens challenge lasted for 4 days, no significant difference was observed in the severity of lesions in birds receiving coccidial vaccine 3 days before or one day after the onset of C. perfringens challenge [20,41].
The dose of Eimeria is also important; successful NE disease has been induced by 2 × 104E. necatrix or 2–5 × 104E. maxima[2,41,70]. Numbers above these may be fatal. For less pathogenic species such as E. acervulina, higher doses (7.5 × 104 up to 5 × 105) have been used [70,71]. Attenuated coccidial vaccines, which as commercial products may be more accessible for some researchers, have been used at 10-times recommended vaccination doses [20,41], since lower doses of the vaccine may not be as efficacious.
When reproducing NE, the purpose of the study will determine which combination of the C. perfringens and Eimeria spp. or Eimeria vaccines should be used, for example whether the intention is to produce severe or mild disease, or whether the intention is to test C. perfringens vaccines. The studies should be designed to include controls of Eimeria alone, C. perfringens alone, and combined Eimeria and C. perfringens. The dose and species or type of coccidal challenge should be chosen with care, depending on the purpose of reproducing NE, since use of coccidia can readily result in severe disease. For example, when testing a vaccine, or a specific drug against NE, it is probably better to reproduce the NE without the help of virulent coccidial challenge, or to use attenuated coccidial vaccines.
An unresolved question is whether, if coccidia are used to enhance the disease process, it is a requirement for the C. perfringens to contain netB; it seems highly likely, but requires to be demonstrated.
3.3. The role of immunosuppression in experimental necrotic enteritis
Immunosuppressed chickens are more likely to develop necrotic enteritis, so that some researchers have used methods to induce immunosuppression. These methods mostly use Infectious Bursal Disease vaccine (IBD) [20,33,34,41]. It has resulted in a significant increase in NE lesions [33]. This has been done by administering a usual dose of IBD vaccine with a medium pathogenicity (intermediate class) [20,41], or 10 times the dose of an IBD vaccine with relatively higher pathogenicity (intermediate plus) [73]. The lesion scores are higher when IBD vaccine is used, even with a normal dose of an intermediate class IBD vaccine [41].
Stressful conditions may decrease immunity and predispose to NE [13]. Increasing stocking density, as a “stressful” measure, combined with IBD vaccine, was used in an experimental model of NE [73], but was not evaluated independently of IBD. Welfare considerations preclude deliberately “stressing” chickens. As evident from this review, there are numerous other factors that can be used to induce disease reliably. Use of immunosuppression as part of the induction of NE is inappropriate if vaccines are being evaluated.
3.4. Bacteriological aspects
3.4.1. Critical virulence features of C. perfringens strains involved in NE in chickens, and in reproducing the disease
Clostridum perfringens is notorious for its ability to produce a wide variety of toxins. Although for many years alpha toxin, a chromosomally-encoded zinc metalloenzyme with lecithinase and sphingomyelinase activity [43], was thought to be the major virulence factor in NE [72,74], the early histological changes in NE are not consistent with the sphingomyelinase or phospholipase C activities of alpha-toxin [19]. A critical study by Keyburn et al. [32], showed that a cpa mutant, which was unable to produce alpha toxin, was still able to produce the disease. The final breakthrough in understanding NE was the demonstration by the same workers of a novel pore-forming toxin, NetB, which was shown to be essential in producing disease [6]. NetB has homology to the pore-forming beta toxin of C. perfringens, as well as to the alpha hemolysin and gamma toxin of Staphylococcus aureus[7]. The netB gene is mostly found in isolates from NE outbreaks, and is relatively uncommon in isolates from healthy birds [7,75-78]. In a chicken experimental model assessing the virulence of 10 C. perfringens isolates from several sources, including cattle, normal chickens, humans, soil and swine, Cooper et al. [79] found that only the netB-positive isolate from a field case of NE was able to cause disease.
The netB gene is associated with a 42 kb pathogenicity locus (NELoc-1), which is on a 85 kb plasmid, as well as with another possibly less important plasmid-associated locus, NELoc3, present on a separate plasmid, and with a chromosomally-located pathogenicity locus, NELoc2 [8,9]. It seems very likely that the difficulty that many workers have experienced in reproducing disease can be attributed to the loss of the entire virulence plasmid containing the netB pathogenicity locus. It is however insufficient simply to use C. perfringens isolated from NE lesions to try to reproduce NE, since not all these may contain netB[76]. Recent multilocus sequence typing (MLST) studies have also identified two prevalent clonal groups among NE-associated isolates [10,75], suggesting that not just the virulence plasmids but also specific chromosomal genes (i.e., the bacterial host background) may also be important in NE pathogenesis or possibly for maintenance of the plasmids.
TpeL, a member of Large Clostridial Toxins (LCT) family [80], is present in some type A NE isolates [75]. There is evidence that netB-positive strains that are also tpeL-positive cause more severe disease than strains that lack tpeL[81].
In summary, emerging understanding of NE is that the strain and particularly the virulence plasmids of NE isolates are critical in producing disease, with netB being the only virulence factor to date shown to be essential in producing disease. Severity of disease may vary with the presence of other virulence determinants, of which the best implicated accessory toxin is tpeL. Because of the complexity of virulence regulation in C. perfringens, spontaneous mutations in VirR-VirS or other regulatory genes may affect virulence. It would therefore be prudent before embarking on the expense of large-scale NE studies for researchers to compare and confirm the virulence of potential netB-positive challenge strains in a pilot study. If more severe disease is desired, it is recommended to obtain a strain possessing tpeL. Because of the danger of loss of virulence plasmids, researchers attempting to reproduce NE should continuously maintain a “paranoid” approach to confirming throughout their work (using PCR) that the experimental strain has not lost the netB gene (and therefore the netB plasmid). The PCR should be done on individual colonies, not as a “sweep” of a blood plate, since PCR is so sensitive that researchers may not recognize through “sweeps” of a plate that the strain is gradually losing plasmids as it is subcultured. Maintaining a frozen stock of the virulent strain will ensure against loss of plasmid during subculture. A specific strain designation should always be given to the challenge strain, which should be made available to other researchers on request.
3.4.2. Preparation of clostridium perfringens for challenge
3.4.2.1. Type of culture media
Several different anaerobic culture media and different incubation times have been used to reproduce NE. The choice depends on both convenience and cost, so that fluid thioglycolate medium (with dextrose) (FTG) is the most common culture medium used for challenge purposes [19,33-35,38,39,47]. Adding peptone and starch to this medium increased the level of alpha toxin production [72], although as noted it is NetB not alpha toxin that is critically important in NE. Others have used cooked meat medium (CMM) [36,60] or brain heart infusion broth (BHI) [82-84], but CMM is expensive and BHI does not have reducing agents in it, so that it needs to be cultured anaerobically which will likely be inconvenient. FTG broth has the advantage that it can be cultured aerobically. Many researchers cultured the C. perfringens challenge strain initially in fluid CMM and then subsequently inoculate this CMM “stock” into FTG [12,22,65,66,79,85-87]. This system is mostly used for convenience but partly for historical reasons. The FTG is used as the challenge inoculum.
3.4.2.2. Incubation time
Younger (15 h) FTG cultures produce more severe disease than 24 h cultures [28,38] probably because of greater production of toxin(s) at this time. For example, 15 h broth culture increased lesion scores by over 50% compared to 24 h cultures [28]. It is also likely that older, stationary, phase cultures will produce proteases that degrade NetB and other toxins. Logistically, however, for researchers who challenge birds twice a day with broth, it may be difficult to prepare a 15 h culture for the afternoon challenge since they would need to start FTG incubation of their challenge strain at midnight. If researchers want to produce less severe disease, then an afternoon feeding with a 24-h culture can be used.
Successful challenge using C. perfringens alone depends on initiating intestinal damage by preformed toxin rather than by toxin produced in the intestine [72]. Therefore whole broth cultures (which contain pre formed toxins) and not just vegetative C. perfringens cells should always be used.
3.4.2.3. Amount of bacteria for challenge
The amount of C. perfringens used for challenge is important for successful challenge. It is normally between 107 to 109 CFU/mL [33,34,60]. A 15-h overnight FTG broth culture inoculated with a 3% (v/v) overnight CMM culture and incubated at 37°C will contain over 108C. perfringens/mL.
3.5. Challenge methods
One successful approach that has commonly been used is to present the challenge strain to birds through feed, inoculated at a ratio of 1.25-1.5 fluid FTG: feed (v/w). Birds are usually initially starved overnight so that they will eat the first batch of infected feed ravenously. Feed is usually prepared fresh twice daily (morning and evening). The period of feeding contaminated feed has varied from 1–5 days, with birds being euthanized on the day of or after the final feed [22,79,86]. On average, researchers challenging birds through the feed will challenge for 3 or 4 days and euthanize on day 4 or 5 (the first day after the last challenge). The time of exposure is important, since 5 days of challenge induced more than twice the mortality of one day challenge [38]. Infected feed should always be available to birds. The advantage of challenging birds through feed is that it does not involve handling birds individually. The disadvantage is that it involves handling and incubating large volumes of media, which can smell unpleasant because of production of hydrogen sulfide, requires often large number of flasks which logistically may be difficult to autoclave, and is expensive.
An alternative to infecting birds through large volumes of feed is to infect birds individually by crop gavage with broth cultures. This has involved inoculation of 3 mL of 105-107 colony-forming units twice daily for three consecutive days [33-35] or 1 mL of 1–4 × 108 CFU/mL for 4 days or 7 days [19,20,41]. The method is combined with some NE risk factors such as coccidial challenge or IBD vaccination. For example, no lesions were detected using oral C. perfringens challenge without coccidial challenge [41]. McReynolds et al. [33] used IBD vaccine as an immunosuppressant followed by oral C. perfringens challenge, and this resulted to a significant increase in NE lesions comparing to the birds that just received C. perfringens challenge alone. However, as noted earlier, numerous studies have successfully used broth culture mixed with feed without using coccidial or immunosuppressing predisposition. In conclusion, researchers need to decide on the approach (C. perfringens alone in feed; coccidial or IBD immunosuppression, followed by C. perfringens given by gavage or in feed) that best fits their facilities, ability to handle birds, the severity of the disease that they wish to produce, and other considerations. Small-scale pilot studies using different variables should establish the conditions best fitted for their study.
4. Other considerations
Most studies use broiler chickens, usually of mixed sex. It is possible that male birds, which eat more, may be more susceptible, as are male turkeys in field cases [62]. Immunization of the parent flock has shown to reduce the NE lesions and mortality [88] so chicks should not be from hens vaccinated with C. perfringens vaccines. Challenge at 2–3 weeks of age should ensure low maternally-derived antibodies in chicks from unvaccinated hens.
5. Lesion scoring systems
5.1. Gross lesions of necrotic enteritis
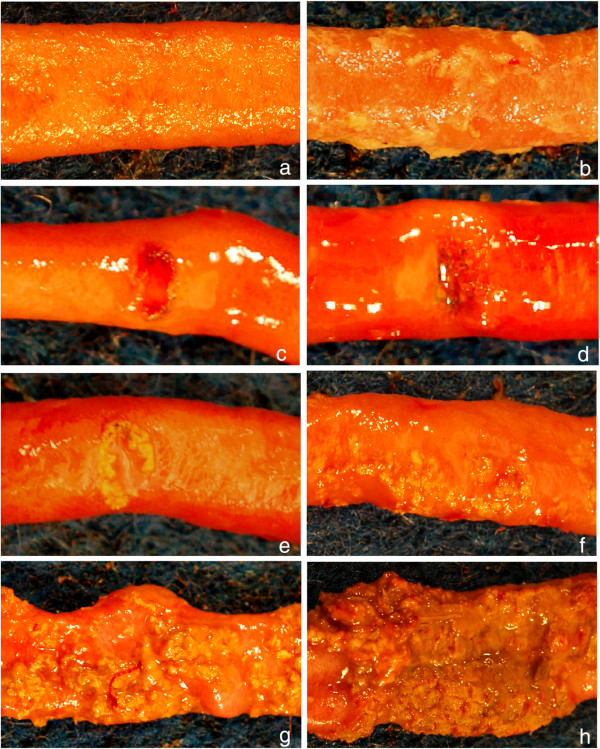
The gross small intestinal lesions of necrotic enteritis comprise a spectrum of mucosal erosion-to-ulceration that vary in both extent and in individual character and severity. In many animals, individual lesions of various characters may be identified. Normally, the mucosal surface of the intestinal tract should be smooth and shiny, reflecting an intact mucosal epithelial barrier (Figure 2a). With erosion and ulceration one may see a complex of subtle mucosal dullness (reflecting fibrin exudation; Figure 2b), cavitation/ulceration (Figures 2c-e), acute hemorrhage (seen as localized intense reddening, often co-localized to areas of cavitation/ulceration, occasionally with frank blood clots in the intestinal lumen; Figure 2c), subacute hemorrhage (seen as localized green-black mucosal pigmentation, often co-localized to areas of cavitation/ulceration; Figure 2d), and the formation of individual mucosal fibrin plaques that cannot be removed with a finger (Figure 2f). The disease process progresses, with or without obvious cavitation, to produce the large-scale accumulation of fibrin, necrotic tissue debris, and inflammatory cells that coalesce to form the suffusive extensive mat of tenacious exudate on the mucosal surface (Figures 2g-h) that is typical of field cases of birds dying of necrotic enteritis.
Figure 2.
Different lesions of necrotic enteritis in chickens, used to illustrate the scoring system (Table 2 ).a: Necrotic enteritis score 0, everted jejunal segment. No gross lesions are present. b: Necrotic enteritis score 1, everted jejunal segment. There are no obvious ulcers in the mucosa, but the entire mucosal surface is covered with a layer of loosely adherent fibrin. c: Necrotic enteritis score 2–4, everted jejunal segment. There is an excavated ulcer of the mucosa with acute, bright red hemorrhage within the ulcer bed and scant crusting of fibrin around the periphery. d: Necrotic enteritis score 2–4, everted jejunal segment. There is an excavated ulcer of the mucosa with dark green-black pigment within the ulcer bed and scant crusting of fibrin over the surface. e-f: Necrotic enteritis score 2–4, everted jejunal segments. There are excavated ulcers of the mucosae, the periphery of which are covered by thick, tightly-adherent layers of fibrin, necrotic tissue, and inflammatory cells. g-h: Necrotic enteritis score 5–6, everted jejunal segment. The mucosae are covered by large, confluent plaques of fibrin, necrotic tissue, and inflammatory cells (g) to the point where they extend over broad regions of the intestinal mucosa (h).
5.2. Different scoring systems
Following humane euthanasia, for example by overdose of carbon dioxide, birds are necropsied and the small intestine examined for gross pathological lesions. It is important to assess the entire length of the small intestine, since lesions may be present in the upper duo denum that are not found elsewhere. Most lesions will however be in the jejunum. A variety of systems for scoring NE gross lesions have been used, using scales that have varied from 0–3 [31,41,70], 0–4 [12,22,26,33,79,87] to 0 – 6 [6,20,32,65]. A serious drawback of this variability, and of the different criteria used even within these scoring systems, is that it is impossible to compare studies that use different scoring systems. International adoption of a common standard system will considerably ease comparison between studies, including comparison of different control methods. One difficulty however of adopting a common scoring system is that there is variation in the gross lesions of NE, described above, which anecdotally may be exacerbated by the effects of different interventions, such as for example immunization of birds with different antigens.
An ideal scoring system should encompass the severity of the disease produced experimentally, have a reasonably wide range for purposes of statistical analysis, be simple so that large numbers of birds can be examined in a reasonable time, and be reproducible between observers. The scoring system that best approximates these criteria is the six-point system of Keyburn et al. [32]. We therefore propose that this system, with slight modification (Table 2), becomes the international standard for experimental NE studies. The modification proposed is that the “1” includes the presence of non-adherent fibrin in affected intestines (Figure 2b) as an additional alternative to “thin walled and friable intestine”. It is recommended that researchers include an experienced pathologist in evaluating lesions of NE, that the lesions be scored “blind”, and that at least two people work together to score the lesions. Figure 2 shows some of the variability in the gross lesions associated with NE, and the scores to which they would be assigned.
Table 2.
Scoring system for experimental necrotic enteritis lesions (slightly modified from[32]) proposed for international adoption
| Score | Lesion | Number of lesions |
|---|---|---|
| 0 |
No gross lesions |
- |
| 1 |
Thin or friable walls, or diffuse superficial but removable fibrin |
- |
| 2 |
Focal necrosis or ulceration, or non-removable fibrin deposit |
1 to 5 foci |
| 3 |
Focal necrosis or ulceration, or non-removable fibrin deposit |
6 to 15 foci |
| 4 |
Focal necrosis or ulceration, or non-removable fibrin deposit |
16 or more foci |
| 5 |
Patches of necrosis 2 to 3 cm long |
Variable |
| 6 | Diffuse necrosis typical of field cases | Variable, but extensive |
Although some researchers have included histopathology as part of the scoring of lesions [19,41], this is both expensive and has considerable potential for bias, since lesions are usually pre-selected for histopathological examination. Our recommendation is therefore not to include histopathology as part of a routine scoring system, unless there are special reasons to do so.
6. Reproduction of clinical and subclinical necrotic enteritis
Necrotic enteritis, can occur in two clinically different forms; clinical and subclinical [15]. The clinical form of the disease is associated with signs such as depression, ruffled feathers, diarrhea and even mortalities, while no clinical signs or mortality occur in the subclinical form [7,14,69,89,90]. Therefore if researchers are seeking to produce a mild form of the disease, for example to study the effect of interventions including prebiotics on production parameters over time, they may be interested to reproduce the less severe, subclinical, form of NE [41,90,91].
Factors determining outcome of the clinical form of NE with severe lesions and mortalities have been discussed; as noted, it is possible to vary parameters to determine the severity of the disease. To reproduce subclinical NE, the approach is to use approaches outlined to reduce the severity of the disease produced. For example, using even relatively large numbers of C. perfringens (108-1010 CFU/mL) when challenged by oral route, just once or twice or even 3 times a day for 4–5 days), when not combined with feed factors (high protein, NSP-rich grains) or use of coccidia or IBD vaccines, produced a subclinical form of the disease with no clinical signs [19,89,90]. Using C. perfringens strains that lack tpeL, or feeding birds 24-h cultures of FTG, will predictably reduce disease severity. Nevertheless, using strains that lack netB will almost certainly never produce subclinical NE [79].
Figure 1 summarizes important factors affecting successful induction of NE.
7. Determining performance parameters (weight gain, feed intake, feed conversion ratio)
Since there is a correlation between performance parameters and lesion scores [87], measuring the performance of the birds, such as weight gain, feed intake and food conversion efficiency (FCR), can be used to evaluate the severity of the disease or the effect of interventions on disease [24-26,60]. This evaluation can be important in subclinical NE, when no clinical signs or mortalities occur. For this purpose, birds can be weighed every week, and at the time of interventions. Measuring feed consumption enables the researcher to determine the FCR. Groups will need to be appropriately large and randomized for performance parameters to be assessed for statistical significance.
8. Welfare considerations
Proposed studies on the reproduction of NE will require approval under national and local animal care legislation and regulations. It is important that the welfare of experimental birds be of the highest concern to researchers. An example of a score sheet and the criteria we use for humane euthanasia is given in Table 3. It should be noted that birds with NE often appear normal until shortly before they die. We euthanize birds humanely using immersion in 100% CO2 gas.
Table 3.
An example of a scoring sheet used in a necrotic enteritis reproduction study
| Date and Time dd/mm/yy and am-pm | Cage # or Group # as appears on the cage | Number of birds in the cage / group | General behaviour*: Score | Comments | |
|---|---|---|---|---|---|
| |
|
|
0 |
bright and alert; |
|
| |
|
|
1 |
reduced spontaneous activity |
|
| |
|
|
2 |
socially isolated but moves when approached |
|
| 3 | pronounced lethargy, only moves when stimulated | ||||
*Actions to be taken.
During experimental infection the birds will be observed every 4 h and the last observation will be made late evening around 8 pm. Birds will be examined around 2 AM, twice on day 3 and 4 of infection (approx. 12:30 AM, 4:30 AM).
Birds showing scores of 1 will be head-marked with a marker pen. If the signs still persist 4 h later or if birds have or develop a score of 2 (or 3), such birds will be euthanized immediately using CO2.
9. Conclusions
This review has critically evaluated factors that affect the reproduction of NE in chickens. The reproduction of many infections experimentally is never guaranteed, but understanding the many variables that affect outcome and careful attention to these should allow researchers to reliably produce the disease. The use of small scale pilot studies should also identify the optimal way to produce the disease of desired severity. Best of luck!
Competing interests
The authors declare that there is no competing interest for this review paper.
Authors’ contributions
BS: Gathering information and papers, writing the outlines, writing the paper; JP: Proposing the subject, reviewing and editing the paper; AV: Preparation and interpretation of the pictures for the scoring system. All authors agreed on outlines and the final version of the paper.
Contributor Information
Bahram Shojadoost, Email: bshojae@ut.ac.ir.
Andrew R Vince, Email: avince@uoguelph.ca.
John F Prescott, Email: prescott@uoguelph.ca.
Acknowledgements
The authors thanks the Ontario Ministry of Agriculture, Food and Rural Affairs, the Canadian Poultry Research Council, Agriculture and Agri-Food Canada, the Poultry Industry Council and the Saskatchewan Chicken Industry Development Fund for support of research into necrotic enteritis.
References
- Parish WE. Necrotic enteritis in the fowl Gallus gallus domesticus. I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J Comp Patho. 1961;71:377–393. [PubMed] [Google Scholar]
- Baba E, Ikemoto T, Fukata T, Sasai K, Arakawa A, McDougald LR. Clostridial population and the intestinal lesions in chickens infected with Clostridium perfringens and Eimeria necatrix. Vet Microbiol. 1997;54:301–308. doi: 10.1016/S0378-1135(96)01289-8. [DOI] [PubMed] [Google Scholar]
- Songer JG, Meer RR. Genotyping of Clostridium perfringens by polymerase chain reaction is a useful adjunct to diagnosis of clostridial enteric disease in animals. Anaerobe. 1996;2:197–203. doi: 10.1006/anae.1996.0027. [DOI] [Google Scholar]
- Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom BE, Fermer C, Lindberg A, Saarinen E, Baverud V, Gunnarsson A. Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet Microbiol. 2003;94:225–235. doi: 10.1016/S0378-1135(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Rubbo AD, Rood JI, Moore RJ. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn AL, Bannam TL, Moore RJ, Rood JI. NetB, a pore forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins. 2010;2:1913–1927. doi: 10.3390/toxins2071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepp D, Roxas B, Parreira VR, Marri PR, Rosey EL, Gong J, Songer JG, Vedantam G, Prescott JF. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One. 2010;5:e10795. doi: 10.1371/journal.pone.0010795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannam TL, Yan XX, Harrison PF, Seeman T, Keyburn AL, Stubenrauch C, Weeramatri LH, Cheung JK, McClane BA, Boyce JD, Moore RJ, Rood JI. Necrotic enteritis-derived Clostridium perfringens strain with three closely related independently conjugative toxin and antibiotic resistance plasmids. MBio. 2011;2:e00190–e00211. doi: 10.1128/mBio.00190-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd MC, Neumann AP, Rehberger TG, Siragusa GR. Multilocus sequence typing subtypes of poultry Clostridium perfringens isolates demonstrate disease niche partitioning. J Clin Microbiol. 2011;49:1556–1567. doi: 10.1128/JCM.01884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo F. In vitro lecithinase activity and sensitivity to 22 antimicrobial agents of Clostridium perfringens isolated from necrotic enteritis of broiler chickens. Res Vet Sci. 1988;45:337–340. [PubMed] [Google Scholar]
- Cooper KK, Songer JG. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet Microbiol. 2010;142:323–328. doi: 10.1016/j.vetmic.2009.09.065. [DOI] [PubMed] [Google Scholar]
- McDevit RM, Brooker JD, Acamovic T, Sparks NHC. Necrotic enteritis; a continuing challenge for the poultry industry. World’s Poult Sci J. 2006;62:221–247. doi: 10.1079/WPS200593. [DOI] [Google Scholar]
- Kaldhusdal M, Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult Sci. 1992;71:1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Van der Sluis W. Clostridial enteritis is an often underestimated problem. World Poult. 2000;16:42–43. [Google Scholar]
- Lovland A, Kaldhusdal M. Severely impaired production performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis. Avian Pathol. 2001;30:73–81. doi: 10.1080/03079450020023230. [DOI] [PubMed] [Google Scholar]
- Skinner JT, Bauer S, Young Young V, Pauling G, Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010;54:1237–1240. doi: 10.1637/9399-052110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Olkowski AA, Wojnarowicz C, Chirino-Trejo M, Drew MD. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res Vet Sci. 2006;81:99–108. doi: 10.1016/j.rvsc.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Timbermont L, Lanckriet A, Gholamiandehkordi AR, Pasmans F, Martel A, Haesebrouck F, Ducatelle R, Van Immerseel F. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comp Immunol Microbiol Infect Dis. 2009;32:503–512. doi: 10.1016/j.cimid.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Williams RB. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Prescott JF, Sivendra R, Barnum DA. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in chickens. Can Vet J. 1978;19:181–183. [PMC free article] [PubMed] [Google Scholar]
- Hofacre CL, Froyman R, George B, Goodwin MA, Brown J. Use of Aviguard, virginiamycin, or bacitracin MD against Clostridium perfringens-associated necrotizing enteritis. J App Poult Res. 1998;7:412–418. [PubMed] [Google Scholar]
- Brennan J, Moore G, Poe SE, Zimmermann A, Vessie G, Barnum DA, Wilson J. Effect of in-feed tylosin phosphate for the treatment of necrotic enteritis in broiler chickens. Poult Sci. 2001;80:1451–1454. doi: 10.1093/ps/80.10.1451. [DOI] [PubMed] [Google Scholar]
- Brennan J, Bagg R, Barnum D, Wilson J, Dick P. Effect of narasin in the prevention of necrotic enteritis in broiler chickens. Avian Dis. 2001;45:210–214. doi: 10.2307/1593030. [DOI] [PubMed] [Google Scholar]
- Brennan J, Skinner J, Barnum DA, Wilson J. The efficacy of bacitracin methylene disalicylate when fed in combination with narasin in the management of necrotic enteritis in broiler chickens. Poult Sci. 2003;82:360–363. doi: 10.1093/ps/82.3.360. [DOI] [PubMed] [Google Scholar]
- Lanckriet A, Timbermont L, De Gussem M, Marien M, Vancraeynest D, Haesebrouck F, Ducatelle R, Van Immerseel F. The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model. Avian Pathol. 2010;39:63–68. doi: 10.1080/03079450903505771. [DOI] [PubMed] [Google Scholar]
- Thompson DR, Parreira VR, Kulkarni RR, Prescott JF. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet Microbiol. 2005;113:25–34. doi: 10.1016/j.vetmic.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Kulkarni RR, Parreira VR, Sharif S, Prescott JF. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin Vaccine Immunol. 2007;14:1070–1077. doi: 10.1128/CVI.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RR, Parreira VR, Sharif S, Prescott JF. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine. 2008;26:4194–4203. doi: 10.1016/j.vaccine.2008.05.079. [DOI] [PubMed] [Google Scholar]
- Lovland A, Kaldhusdal M, Redhead K, Skjerve E, Lillehaug A. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. 2004;33:81–90. doi: 10.1080/0379450310001636255. [DOI] [PubMed] [Google Scholar]
- Keyburn AL, Sheedy SA, Ford ME, Williamson MM, Awad MM, Rood JI, Moore RJ. Alpha toxin of Chlostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds L, Byrd JA, Anderson RC, Moore RW, Edrington TS, Genovese KJ, Poole TL, Kubena LF, Nisbet DJ. Evaluation of Immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poult Sci. 2004;83:1948–1952. doi: 10.1093/ps/83.12.1948. [DOI] [PubMed] [Google Scholar]
- McReynolds JL, Byrd JA, Genovese KJ, Poole TL, Duke SE, Farnell MB, Nisbet DJ. Dietary lactose and its effect on the disease condition of necrotic enteritis. Poult Sci. 2007;86:1656–1661. doi: 10.1093/ps/86.8.1656. [DOI] [PubMed] [Google Scholar]
- McReynolds J, Waneck C, Byrd J, Genovese K, Duke S, Nisbet D. Efficacy of multistrain direct–fed microbial and phytogenetic products in reducing necrotic enteritis in commercial broilers. Poult Sci. 2009;88:2075–2080. doi: 10.3382/ps.2009-00106. [DOI] [PubMed] [Google Scholar]
- Baba E, Fuller AL, Gilbert JM, Thayer SG, McDougald LR. Effects of Eimeria brunetti infection and dietary zinc on experimental induction of necrotic enteritis in broiler chickens. Avian Dis. 1992;36:59–62. doi: 10.2307/1591716. [DOI] [PubMed] [Google Scholar]
- Branton SL, Lott BD, Deaton JW, Maslin WR, Austin FW, Pote LM, Keirs RW, Latour MA, Day EJ. The effect of added complex carbohydrates or added dietary fiber on necrotic enteritis lesions in broiler chickens. Poult Sci. 1997;76:24–28. doi: 10.1093/ps/76.1.24. [DOI] [PubMed] [Google Scholar]
- Long JR, Truscott RB. Necrotic enteritis in broiler chickens. III. Reproduction of the disease. Can J Comp Med. 1976;40:53–59. [PMC free article] [PubMed] [Google Scholar]
- Cowen BS, Schwartz LD, Wilson RA, Ambrus SI. Experimentally induced necrotic enteritis in chickens. Avian Dis. 1987;31:904–906. doi: 10.2307/1591050. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marshal RN, La Ragione RM, Catchpole J. A new model for the experimental production of necrotic enteritis and its use for studies on the relationship between necrotic enteritis and anticoccidial vaccination of chickens. Parasitol Res. 2003;90:19–26. doi: 10.1007/s00436-002-0803-4. [DOI] [PubMed] [Google Scholar]
- Gholamiandehkordi AR, Timbermont L, Lanckriet A, Van Den Broeck W, Pedersen K, Dewulf J, Pasmans F, Haeesbrouck F, Ducatelle R, Van Immerseel F. Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathol. 2007;36:375–382. doi: 10.1080/03079450701589118. [DOI] [PubMed] [Google Scholar]
- Annett CB, Viste JR, Chirino-Trejo M, Classen HL, Middleton DM, Simko E. Necrotic enteritis: effect of barley, wheat and corn diet on proliferation of Clostridium perfringens Type A. Avian Pathol. 2002;31:599–602. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Dahiya JP, Wilkie DC, Van Kessel AG, Drew MD. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim Feed Sci Tech. 2006;129:60–88. doi: 10.1016/j.anifeedsci.2005.12.003. [DOI] [Google Scholar]
- Kleesen B, Hartmann L, Blaut M. Fructans in the diet cause alterations of intestinal mucosal architecture, released mucins and mucosa-associated bifidobacteria in gnotobiotic rats. Brit J Nutr. 2003;89:597–606. doi: 10.1079/BJN2002827. [DOI] [PubMed] [Google Scholar]
- Ficko-Blean E, Stuart CP, Boraston AB. Structural analysis of CPF_2247, a novel a-amylase from Clostridium perfringens. Proteins. 2011;79:2771–2777. doi: 10.1002/prot.23116. [DOI] [PubMed] [Google Scholar]
- Ficko-Blean E, Stuart CP, Suits MD, Cid M, Tessier M, Woods RJ, Boraston AB. Carbohydrate recognition by an architecturally complex α-N-Acetylglucosaminidase from Clostridium perfringens. PLoS One. 2012;7:e33524. doi: 10.1371/journal.pone.0033524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell C, Kong XM. The influence of diet on necrotic enteritis in broiler chicks. Avian Dis. 1992;36:499–503. doi: 10.2307/1591740. [DOI] [PubMed] [Google Scholar]
- Branton SL, Reece FN, Hagler WM Jr. Influence of a wheat diet on mortality of broiler chicks associated with necrotic enteritis. Poultry Sci. 1987;66:1326–1330. doi: 10.3382/ps.0661326. [DOI] [PubMed] [Google Scholar]
- Craven SE. Colonization of the intestinal tract by Clostridium perfringens and fecal shedding in diet stressed and unstressed broiler chickens. Poult Sci. 2000;79:843–849. doi: 10.1093/ps/79.6.843. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M, Skjerve E. Association between cereal contents in the diet and incidence of necrotic enteritis in broiler chickens in Norway. Prev Vet Med. 1996;28:1–16. doi: 10.1016/0167-5877(96)01021-5. [DOI] [Google Scholar]
- Reid CA, Hillman K. The effects of retrogradation and amylose/amylopectin ratio of starches on carbohydrate fermentation and microbial populations in the porcine colon. Anim Sci. 1999;68:503–510. [Google Scholar]
- Weurding RE, Veldman A, Veen WAG, Van Der Aar PJ, Verstegen MWA. Starch digestion in the small intestine of chickens differs among feedstuffs. J Nutr. 2001;131:2329–2335. doi: 10.1093/jn/131.9.2329. [DOI] [PubMed] [Google Scholar]
- Engberg RM, Hedemann MS, Jensen BB. The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens. Brit Poult Sci. 2002;43:569–579. doi: 10.1080/0007166022000004480. [DOI] [PubMed] [Google Scholar]
- Branton SL, Reece FN, Hagler WM Jr. Influence of a wheat diet on mortality of broiler chickens associated with necrotic enteritis. Poult Sci. 1987;66:1326–1330. doi: 10.3382/ps.0661326. [DOI] [PubMed] [Google Scholar]
- Palliyeguru MWCD, Rose SP, Mackenzie AM. Effect of trypsin inhibitor activity in soya bean on growth performance, protein digestibility and incidence of sub-clinical necrotic enteritis in broiler chicken flocks. Brit Poult Sci. 2011;52:359–367. doi: 10.1080/00071668.2011.577054. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldhusdal MI. Necrotic enteritis as affected by dietary ingredients. World Poult. 2000;16:42–43. [Google Scholar]
- Titball RW, Naylor CE, Basak AK. The Clostridium perfringens alpha toxin. Anaerobe. 1999;5:51–64. doi: 10.1006/anae.1999.0191. [DOI] [PubMed] [Google Scholar]
- Dahiya JP, Hoehler D, Wilkie DC, Van Kessel AG, Drew MD. Dietary glycine concentration affects intestinal Clostridium perfringens and Lactobacilli population in broiler chickens. Poult Sci. 2005;84:1875–1885. doi: 10.1093/ps/84.12.1875. [DOI] [PubMed] [Google Scholar]
- Dahiya JP, Hoehler D, Van Kessel AG, Drew MD. Dietary encapsulated glycine influences Clostridium perfringens and Lactobacilli growth in the gastrointestinal tract of broiler chickens. J Nutr. 2007;137:1408–1414. doi: 10.1093/jn/137.6.1408. [DOI] [PubMed] [Google Scholar]
- Wilkie DC, Van Kessel AG, White LJ, Laarveld B, Drew MD. Dietary amino acids affect intestinal Clostridium perfringens populations in broiler chickens. Can J Anim Sci. 2005;85:185–193. doi: 10.4141/A04-070. [DOI] [Google Scholar]
- Drew MD, Syed NA, Goldade BG, Van Larveld B, Kessel AG. Effect of dietary protein source and level on intestinal population of Clostridium perfringens in broiler chickens. Poult Sci. 2004;83:414–420. doi: 10.1093/ps/83.3.414. [DOI] [PubMed] [Google Scholar]
- Kocher A. Nutritional manipulation of necrotic enteritis outbreak in broilers. Recent Adv Anim Nutr. 2003;14:111–116. [Google Scholar]
- Cheung JK, Keyburn AL, Carter GP, Lanckriet AL, Van Immerseel F, Moore RJ, Rood JI. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect Immun. 2010;78:3064–3072. doi: 10.1128/IAI.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L, Lanckriet A, Dewulf J, Nollet N, Schwarzer K, Haesebrouk F, Ducattele R, Van Immerseel F. Control of Clostridium perfringence- induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010;39:117–121. doi: 10.1080/03079451003610586. [DOI] [PubMed] [Google Scholar]
- Cooper KK, Songer JG. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2009;15:55–60. doi: 10.1016/j.anaerobe.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Williams BA, Martin WA, Verstegen ST. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr Res Rev. 2001;14:207–227. doi: 10.1079/NRR200127. [DOI] [PubMed] [Google Scholar]
- Lan Y, Verstegen MWA, Tamminga S, Williams BA. The role of the commensal gut microbial community in broiler chickens. World’s Poult Sci J. 2005;61:95–104. doi: 10.1079/WPS200445. [DOI] [Google Scholar]
- Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2008;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, Gaskins HR. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Al-Sheikhly F, Al-Saieg A. Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 1980;24:324–333. doi: 10.2307/1589700. [DOI] [PubMed] [Google Scholar]
- Al-Sheikhly F, Truscott RB. The interaction of Clostridium perfringens and its toxins in the production of necrotic enteritis in chickens. Avian Dis. 1977;21:256–263. doi: 10.2307/1589345. [DOI] [PubMed] [Google Scholar]
- Nikpiran H, Shojadoost B, Peighambari SM. Experimental induction of necrotic enteritis in broiler chickens by Clostridium perfringens isolates from the outbreaks in Iran. J Vet Res. 2008;63:127–132. [Google Scholar]
- Fukata T, Hadate Y, Baba E, Uemura T, Arakawa A. Influence of Clostridium perfringens and its toxin in germ-free chickens. Res Vet Sci. 1988;44:68–70. [PubMed] [Google Scholar]
- Chalmers G, Bruce HL, Hunter DB, Parreira VR, Kulkarni RR, Jiang YF, Prescott JF, Boerlin P. Multi-locus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chickens populations. J Clin Microbiol. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TG, Smyth JA. Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States. Vet Microbiol. 2009;136:202–205. doi: 10.1016/j.vetmic.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Keyburn AL, Yan XX, Bannam TL, Van Immerseel F, Rood JI, Moore RJ. Association between avian necrotic enteritis and Clostridium perfringens expressing NetB. Vet Res. 2010;41:21. doi: 10.1051/vetres/2009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolooe A, Shojadoost B, Peighambari SM, Tamaddon Y. Prevalence of netB gene among Clostridium perfringens isolates obtained from healthy and diseased chicks. J Anim Vet Adv. 2011;10:106–110. [Google Scholar]
- Cooper KK, Theoret JR, Stewart A, Trinh HT, Glock RD, Songer JG. Virulence for chickens of Clostridium perfringens isolated from poultry and other sources. Anaerobe. 2010;16:289–292. doi: 10.1016/j.anaerobe.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Amimoto K, Noro T, Oishi E, Shimizu M. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiol. 2007;153:1198–1206. doi: 10.1099/mic.0.2006/002287-0. [DOI] [PubMed] [Google Scholar]
- Coursodon CF, Glock RD, Moore KL, Cooper KK, Songer JG. TpeL-producing strains of Clostridium perfringens type A are highly virulent for broiler chicks. Anaerobe. 2012;18:117–121. doi: 10.1016/j.anaerobe.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M, Hofshagen M, Lovland A, Langstrand H, Redhead K. Necrotic enteritis challenge models with broiler chickens raised on litter: evaluation of preconditions, Clostridium perfringens strains and outcome variables. Immun Med Microbiol. 1999;24:337–343. doi: 10.1111/j.1574-695X.1999.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Pedersen K, Bjerrum L, Nauerby B, Madsen M. Experimental infection with rifampicin-resistant Clostridium perfringens strains in broiler chickens using isolator facilities. Avian Pathol. 2003;32:401–408. doi: 10.1080/0307945031000121158. [DOI] [PubMed] [Google Scholar]
- Pedersen K, Bjerrum L, Eske Heuer O, Lo Fo Wong DMA, Nauerby B. Reproducible infection model for Clostridium perfringens in Broiler chickens. Avian Dis. 2008;52:34–39. doi: 10.1637/7955-022307-Reg. [DOI] [PubMed] [Google Scholar]
- Prescott JF. The prevention of experimentally induced necrotic enteritis in chickens by avoparcin. Avian Dis. 1979;23:1072–1074. doi: 10.2307/1589625. [DOI] [PubMed] [Google Scholar]
- Chalmers G, Bruce HL, Toole DL, Barnum DA, Boerlin P. Necrotic enteritis potential in a model system using Clostridium perfringens isolated from field outbreaks. Avian Dis. 2007;51:834–839. doi: 10.1637/7959-022807-REGR.1. [DOI] [PubMed] [Google Scholar]
- Cooper KK, Trinh HT, Songer G. Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens. Vet Microbiol. 2009;133:92–97. doi: 10.1016/j.vetmic.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Crouch CF, Withanage GSK, de Haas V, Etore F, Francis MJ. Safety and efficacy of a maternal vaccine for the passive protection of broiler chicks against necrotic enteritis. Avian Pathol. 2010;39:489–497. doi: 10.1080/03079457.2010.517513. [DOI] [PubMed] [Google Scholar]
- Wilson J, Tice G, Brash ML, Hilaire SST. Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens. World's Poult Sci J. 2005;61:435–449. doi: 10.1079/WPS200566. [DOI] [Google Scholar]
- Olkowski AA, Wojnarowicz C, Chirino-Trejo M, Laarveld B, Sawicki G. Sub-clinical necrotic enteritis in broiler chickens: Novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Res Vet Sci. 2008;85:543–553. doi: 10.1016/j.rvsc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Wu SB, Rodgers N, Choct M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010;54:1058–1065. doi: 10.1637/9338-032910-Reg.1. [DOI] [PubMed] [Google Scholar]