Abstract
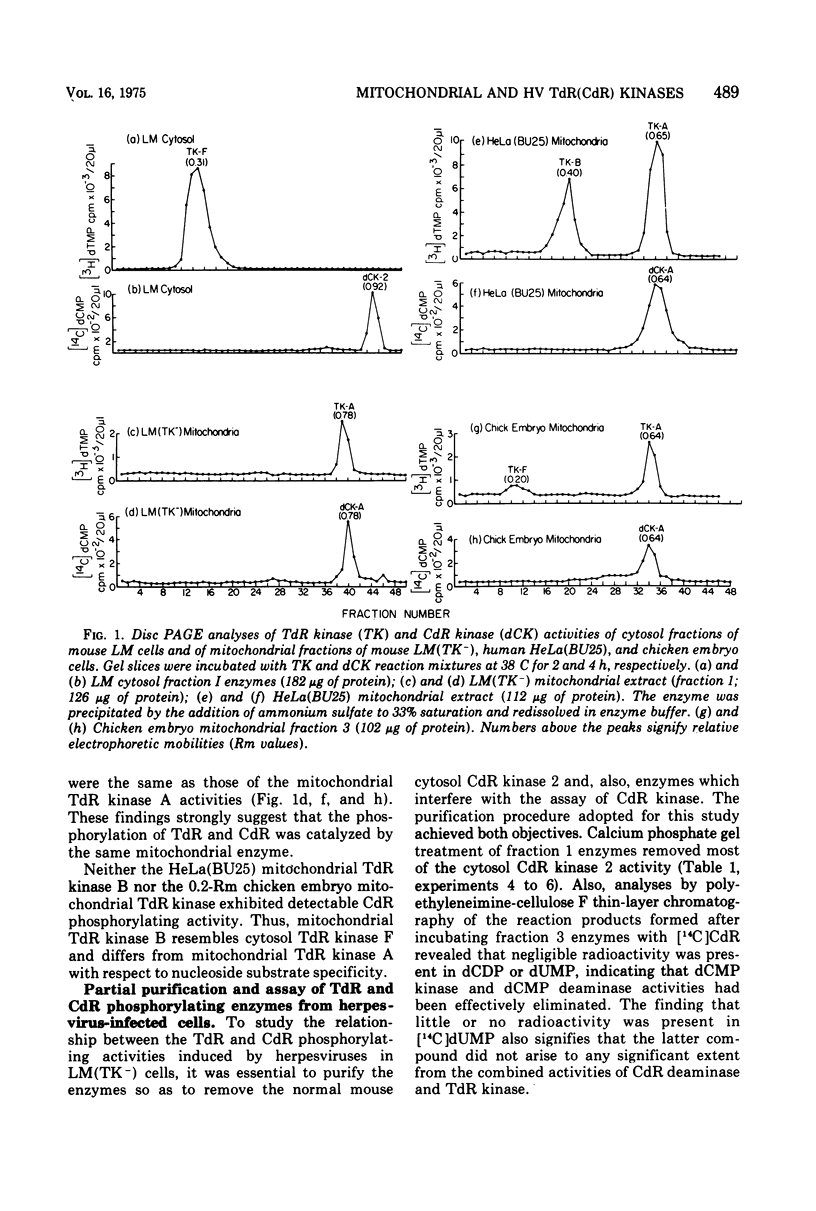
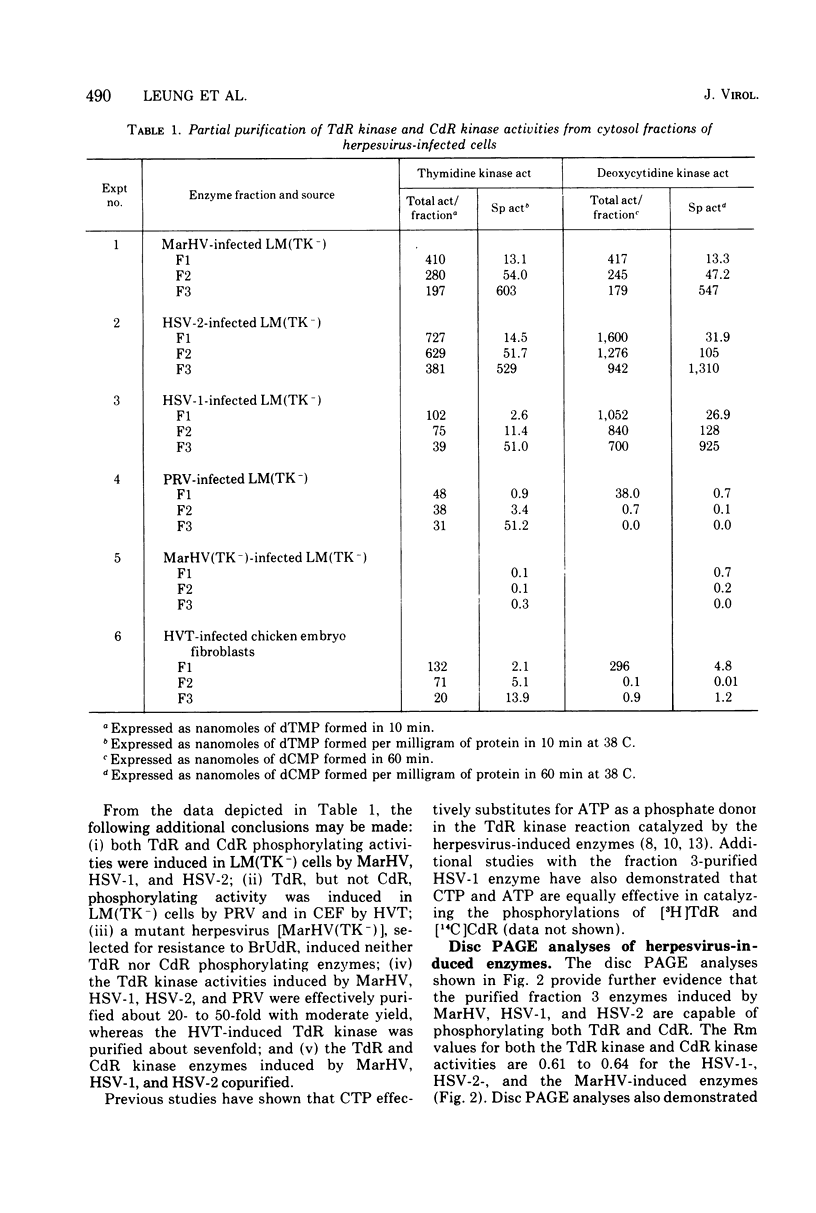
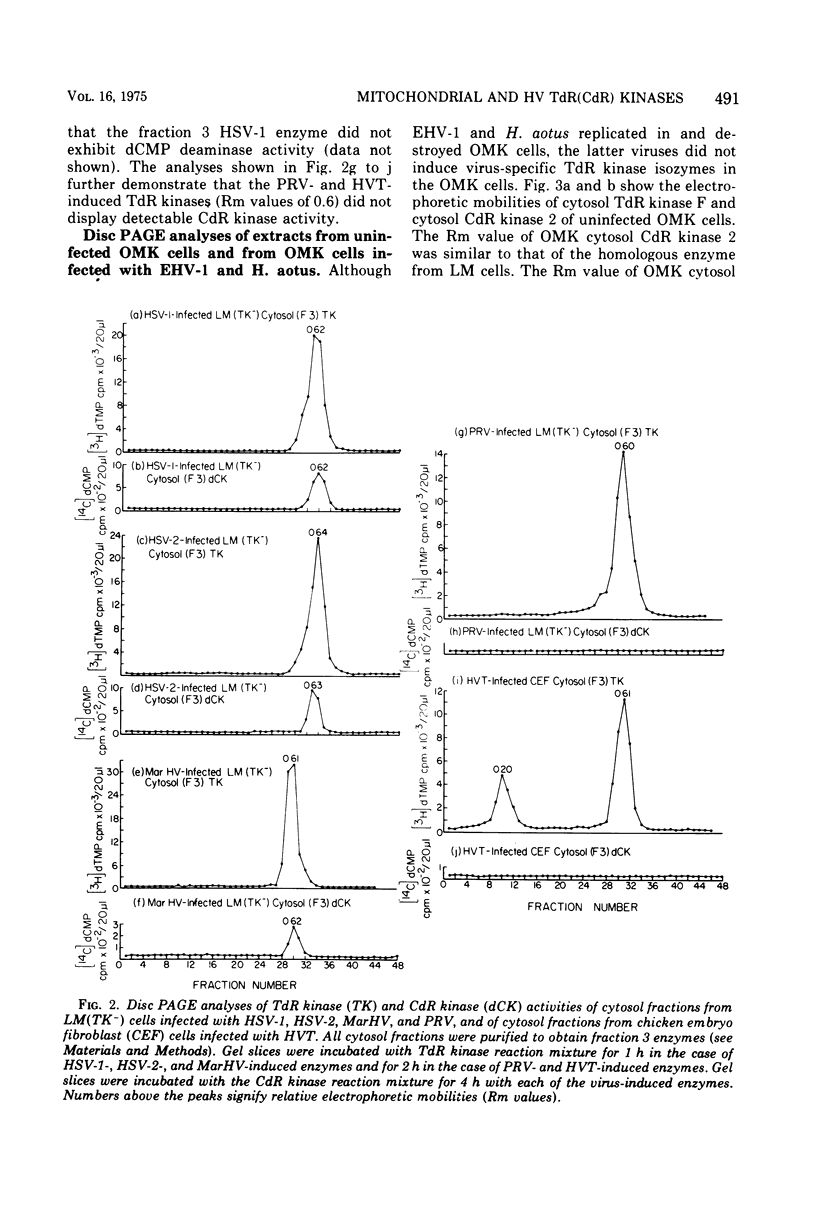
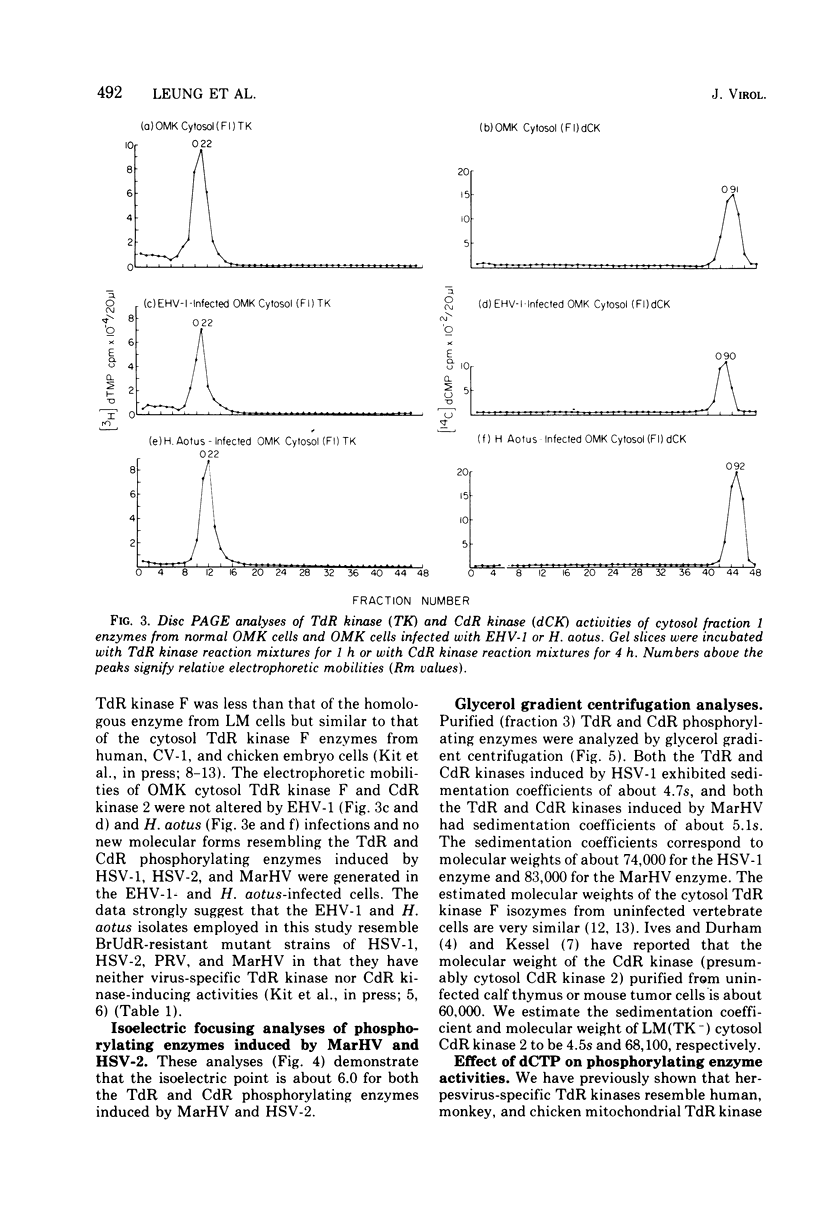
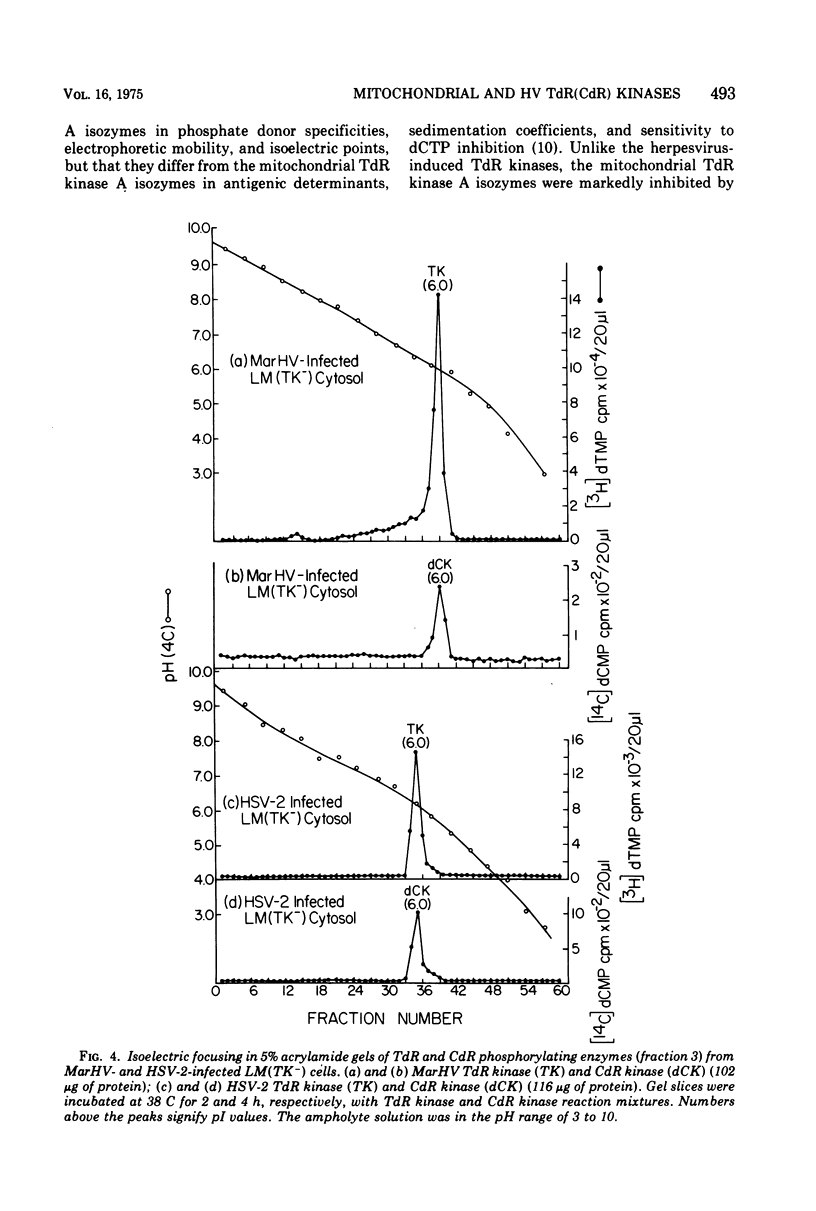
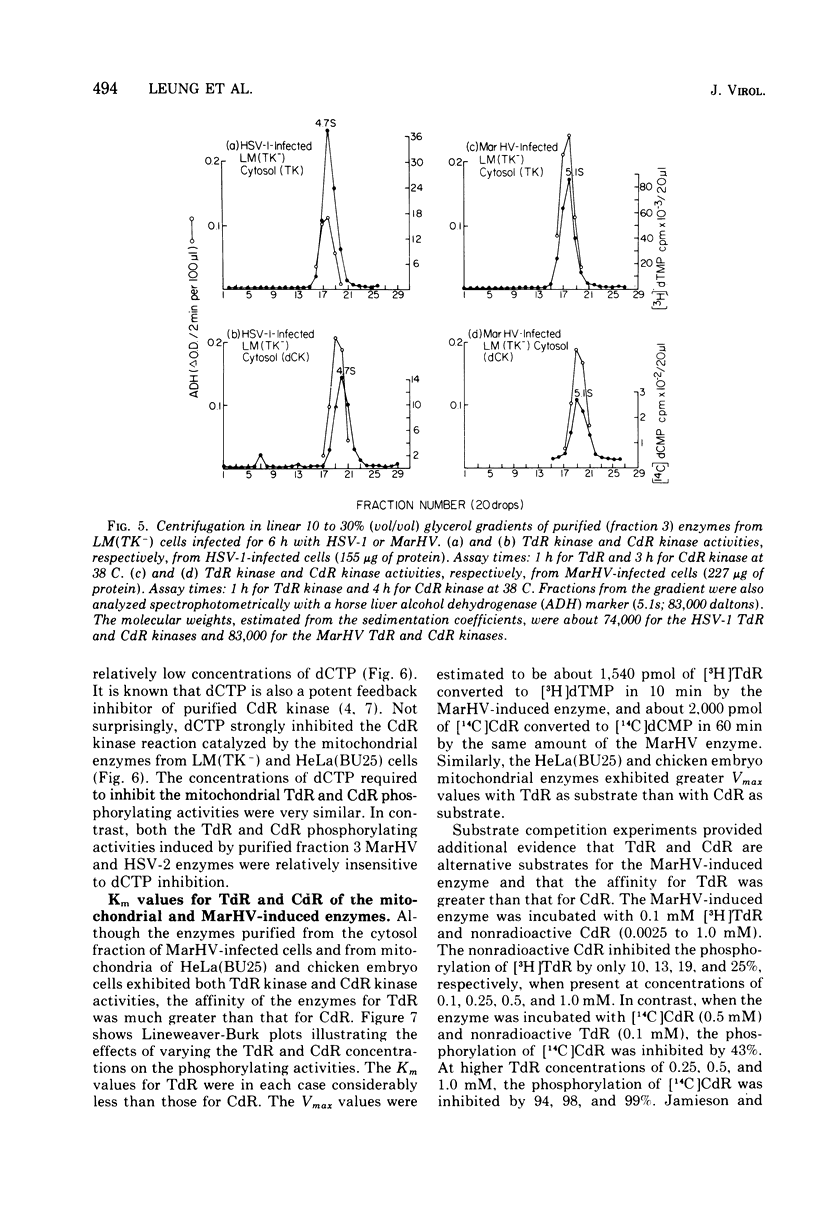
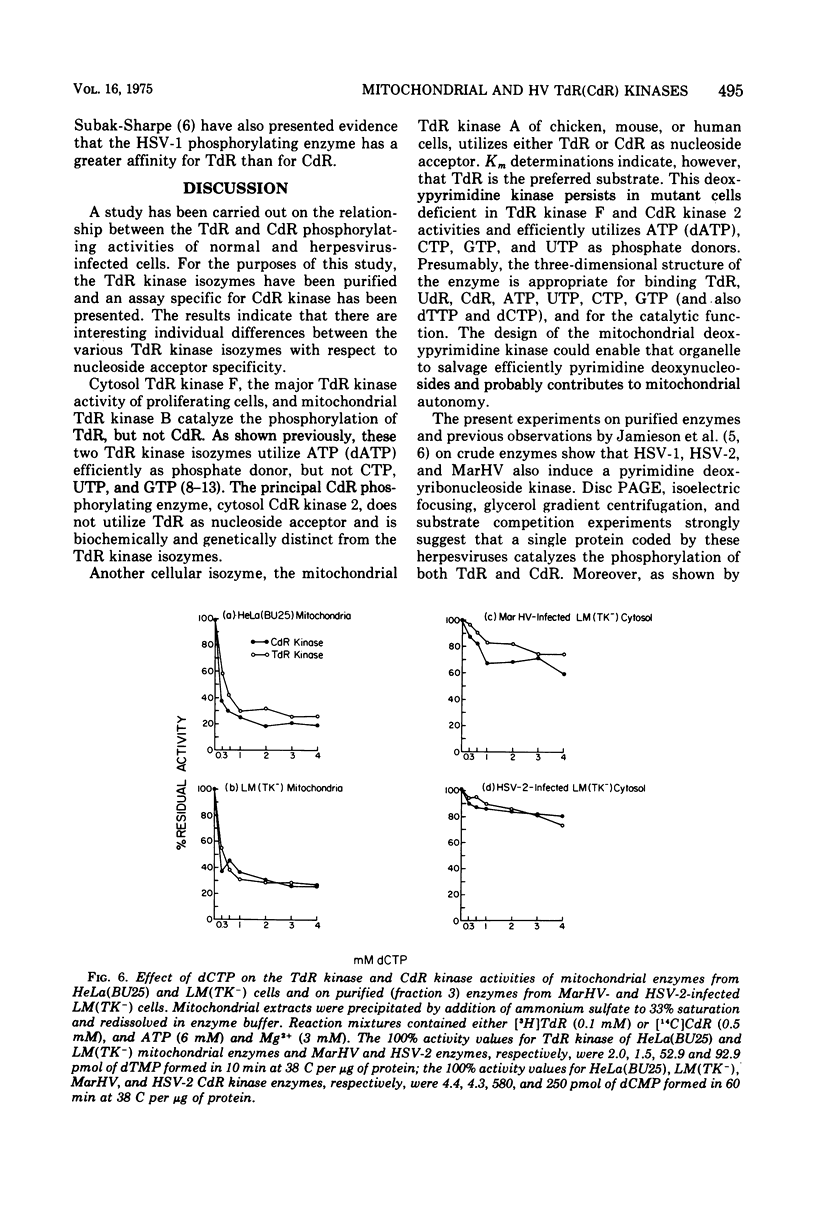
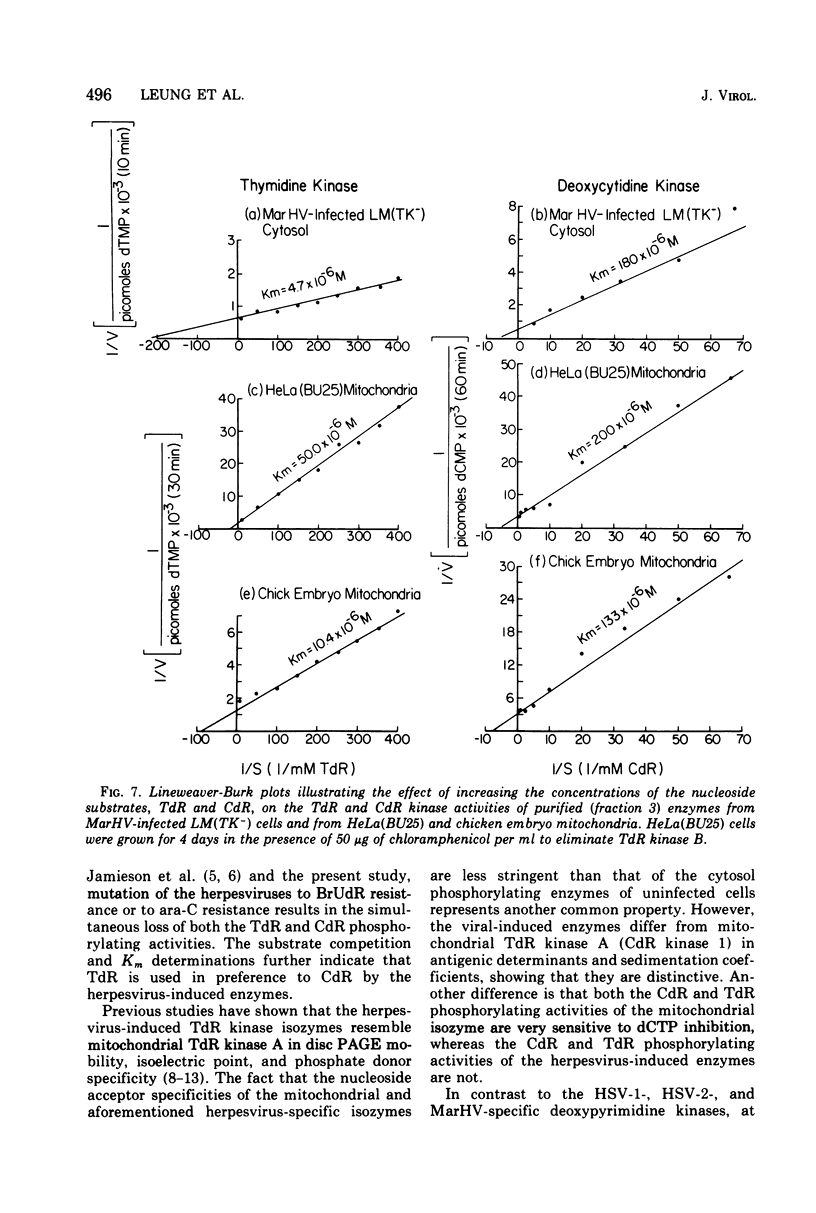
To characterize and compare the thymidine (TdR) and deoxycytidine (CdR) kinase isozymes of uninfected and herpesvirus-infected cells: (i) the subcellular distribution of the isozymes has been studied; (ii) a specific assay for CdR kinase has been devised; (iii) the TdR kinase isozymes have been partially purified; and (iv) the purified enzymes have been analyzed by disc polyacrylamide gel electrophoresis, isoelectric focusing, and glycerol gradient centrifugation and by substrate competition and dCTP inhibition studies. The results indicate that there are interesting individual differences with respect to nucleoside acceptor specificity between the cytosol and mitochondrial pyrimidine deoxyribonucleoside kinases of uninfected cells and between the enzymes induced by different herpesviruses. In the cytosol of uninfected mouse, chicken, and owl monkey kidney cells, two different proteins, TdR kinase F and CdR kinase 2, catalyze the phosphorylations of TdR and CdR, respectively. TdR kinase F does not phosphorylate CdR, nor does CdR kinase 2 phosphorylate TdR. A second TdR kinase isozyme present in HeLa(BU25) mitochondria (TdR kinase B) also lacks CdR phosphorylating activity. In contrast, a genetically distinctive deoxypyrimidine kinase (TdR kinase A) of mouse, human, and chick mitochondria catalyzes the phosphorylation of both TdR and CdT. Three herpesviruses, marmoset herpesvirus and herpes simplex virus types 1 and 2, induce in the cytosol fraction of LM(TK-) mouse cells isozymes which share common properties with mitochondrial TdR kinase A, including the ability to catalyze the phosphorylation of both TdR and CdR. However, the herpesvirus-induced deoxypyrimidine kinases differ from mitochondrial TdR kinase A with respect to sedimentation coefficient, sensitivity to dCTP inhibition, and antigenic determinants. The herpesvirus-specific and the mitochondrial deoxypyrimidine kinases exhibit a preference for TdR over CdR as nucleoside acceptor. Pseudorabies virus and herpesvirus of turkeys induce cytosol TdR kinases resembling the other herpesvirus-induced TdR kinases in several properties, but like cellular TdR kinase F, the pseudorabies virus and herpesvirus of turkeys TdR kinases lack detectable CdR phosphorylating activities. Finally, a marmoset herpesvirus nutant resistant to bromodeoxyuridine, equine herpesvirus type 1, and Herpesvirus aotus induces neither TdR nor CdR phosphorylating enzymes during productive infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper G. M., Greer S. The effect of inhibition of cytidine deaminase by tetrahydrouridine on the utilization of deoxycytidine and 5-bromodeoxycytidine for deoxyribonucleic acid synthesis. Mol Pharmacol. 1973 Nov;9(6):698–703. [PubMed] [Google Scholar]
- Cooper G. M. Phosphorylation of 5-bromodeoxycytidine in cells infected with herpes simplex virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3788–3792. doi: 10.1073/pnas.70.12.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives D. H., Durham J. P. Deoxycytidine kinase. 3. Kinetics and allosteric regulation of the calf thymus enzyme. J Biol Chem. 1970 May 10;245(9):2285–2294. [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Kessel D. Properties of deoxycytidine kinase partially purified from L1210 cells. J Biol Chem. 1968 Sep 25;243(18):4739–4744. [PubMed] [Google Scholar]
- Kit S., Leung W. C. Genetic control of mitochondrial thymidine kinase in human-mouse and monkey-mouse somatic cell hybrids. J Cell Biol. 1974 Apr;61(1):35–44. doi: 10.1083/jcb.61.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Dubbs D. R. Distinctive properties of thymidine kinase isozymes induced by human and avian hepresviruses. Int J Cancer. 1974 Nov 15;14(5):598–610. doi: 10.1002/ijc.2910140506. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Trkula D. Distinctive properties of mitochondrial thymidine(dT)kinase from bromodeoxyuridine(dBU)-resistant mouse lines. Biochem Biophys Res Commun. 1973 Sep 5;54(1):455–461. doi: 10.1016/0006-291x(73)90943-1. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Trkula D., Jorgensen G. Gel electrophoresis and isoelectric focusing of mitochondrial and viral-induced thymidine kinases. Int J Cancer. 1974 Feb 15;13(2):203–218. doi: 10.1002/ijc.2910130208. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Trkula D. Properties of mitochondrial thymidine kinases of parental and enzyme-deficient HeLa cells. Arch Biochem Biophys. 1973 Oct;158(2):503–513. doi: 10.1016/0003-9861(73)90542-0. [DOI] [PubMed] [Google Scholar]
- Maley F., Maley G. F. Tetrahydrodeoxyuridylate: a potent inhibitor of deoxycytidylate deaminase. Arch Biochem Biophys. 1971 Jun;144(2):723–729. doi: 10.1016/0003-9861(71)90379-1. [DOI] [PubMed] [Google Scholar]
- de Saint-Vincent B. R., Buttin G. Studies on 1-beta-D-arabinofuranosyl-cytosine-resistant mutants of Chinese-hamster fibroblasts. A mitochondrial deoxycytidine kinase devoid of activity on arabino-cytosine. Eur J Biochem. 1973 Sep 3;37(3):481–488. doi: 10.1111/j.1432-1033.1973.tb03009.x. [DOI] [PubMed] [Google Scholar]


