Abstract
Numerous human and animal studies indirectly implicate neurons in the anterior cingulate cortex (ACC) in the encoding of the affective consequences of nociceptor stimulation. No causal evidence, however, has been put forth linking the ACC specifically to this function. Using a rodent pain assay that combines the hind-paw formalin model with the place-conditioning paradigm, we measured a learned behavior that directly reflects the affective component of pain in the rat (formalin-induced conditioned place avoidance) concomitantly with “acute” formalin-induced nociceptive behaviors (paw lifting, licking, and flinching) that reflect the intensity and localization of the nociceptive stimulus. Destruction of neurons originating from the rostral, but not caudal, ACC reduced formalin-induced conditioned place avoidance without reducing acute pain-related behaviors. These results provide evidence indicating that neurons in the ACC are necessary for the “aversiveness” of nociceptor stimulation.
Current conceptualizations of pain in humans recognize several interrelated components that are identifiable as distinct constructs (1–4). One of these components involves the encoding and perception of stimulus parameters (e.g., stimulus localization, intensity, and quality). Another component involves the encoding of the affective salience or unpleasantness of the noxious stimulus. It is possible that these components are mediated by separate neural systems (but see ref. 4 for an alternate view). A projection from the spinal dorsal horn through the lateral thalamus to somatosensory cortex is thought to transmit information regarding noxious stimulus parameters (5). Another system originating in the spinal dorsal horn and projecting through the medial/intralaminar thalamic nuclei to the anterior cingulate cortex (ACC) has been proposed to process information relating to pain-related unpleasantness (6). In addition to contributing to the immediate affective consequences of noxious stimulation, the “ACC system” may contribute to the avoidance learning that sometimes follows as a secondary reaction to pain (7).
Much of the data implicating the ACC in pain processing come from studies performed in humans. The human ACC contains neurons that respond to noxious, but not innocuous, thermal and mechanical stimulation (8). Early anecdotal reports indicated that surgical ablation of the ACC and surrounding cortical tissue decreases pain-related unpleasantness/dysphoria without affecting the human subject's ability to discriminate the intensity or localization of the noxious stimulus (9, 10). In more recent years, studies using functional neuroimaging techniques have further implicated the ACC in pain processing. Positron emission tomography and functional magnetic resonance imaging have demonstrated increases in neural activity in the ACC (as inferred from changes in regional cerebral blood flow) after noxious stimulation (11). Importantly, these indirect measures of neural activity in the ACC have been correlated specifically with the affective component of pain in humans. Using hypnotic suggestion, Rainville and colleagues (12) were able to increase or decrease the subject's reported unpleasantness of a noxious stimulus without affecting their intensity rating of the same stimulus. Modulations of perceived unpleasantness were associated with corresponding modulations in neural activity in the ACC but not in the somatosensory cortex. This evidence supports the notion of parallel neural pathways relating to the processing of distinct components of the pain experience and implicates the ACC specifically in the processing of pain-related unpleasantness.
A number of animal studies also support a role for the ACC in nociceptive processing. In the rabbit and rat, neurons within Brodmann's area 24b of the ACC have been characterized as noci-responsive (13, 14). These cells have large or whole-body receptive fields and bilateral nociceptor innervation—properties that are not inconsistent with a role in affective processing. Additionally, prolonged noxious stimulation induces expression of the c-fos protooncogene bilaterally in the ACC (15). Finally, the interconnections of the ACC with other limbic regions (e.g., amygdala, ventral striatum, and hypothalamus) (16–19) and regions involved in pain modulation (e.g., medial thalamus and periaqueductal gray matter) (20, 21) suggest ways in which the ACC can influence other brain areas that are involved in “emotional” information processing and reactions to noxious stimulation (e.g., aversion learning). Thus, anatomical and functional studies lend indirect support for ACC involvement in pain processing and encoding of negative affect.
Although the human and animal literature described above is strongly suggestive of a role for the ACC in pain-related affect, the evidence therein is indirect and/or correlative. The literature is conspicuously devoid of a definitive study, demonstrating the necessity of neural activity in the ACC for processing the affective consequences of nociceptor stimulation. This deficiency is due partly to the limitations of animal pain models. Most animal pain models are notable for their lack of a behavioral index relating to the affective component of pain. In the current study, we have used a pain model that allows simultaneous measurement of behaviors in the rat that reflect both the affective component of pain and the processing of noxious stimulus parameters (intensity, localization) (22). The model combines a widely used tonic pain model, the formalin test, with the place-conditioning paradigm. Specifically, in addition to eliciting “acute” nociceptive behaviors (lifting, licking, and flinching of the injected paw), hind-paw injection of dilute formalin induces conditioned place avoidance (F-CPA) when paired with a distinct environmental context. Because it is reasonable to assume that F-CPA directly reflects a negative affective state produced by the nociceptive stimulus, this approach makes it possible to address the contribution of the ACC to pain-related affect in the rat.
The present experiments were designed to examine the contribution of the ACC to pain-related affective processing in the rat by using the F-CPA model. We studied the effects of excitotoxin-induced lesions of the ACC on the expression of both acute formalin-induced nociceptive behaviors and F-CPA. Specifically, we hypothesized that lesions of the ACC would reduce F-CPA without affecting acute formalin-induced nociceptive behaviors. Our results support previous studies of ACC function and provide direct evidence for the necessity of ACC neurons in the encoding of pain-related affect.
Materials and Methods
Subjects.
Subjects were male Long Evans rats (Simonsen Laboratories, Gilroy, CA) weighing 290–320 g at the start of the experiment. Rats were group-housed on a 12-h light-dark schedule with food and water available ad libitum. All experiments were carried out with the approval of the Institutional Animal Care and Use Committee at the University of California, San Francisco. All efforts were made to minimize animal suffering and reduce the number of animals used.
Drugs.
Ibotenic acid (0.5 mg/ml) was dissolved in 0.1 M PBS and adjusted to pH 7.2–7.4 by using 0.1 M NaOH. Stock formaldehyde solution (37% formaldehyde or 100% formalin) was diluted to 2.5% formalin in isotonic saline. U69,593 (0.16 mg/ml) was dissolved in 20% propylene glycol/saline and stirred overnight.
Apparatus.
In the present experiments, the effects of hind-paw formalin injection or s.c. U69,593 (a κ-opioid receptor agonist) were paired with a distinct compartment in a place-conditioning apparatus. The ability of each treatment to produce CPA under different experimental conditions was assessed. A standard place-conditioning apparatus, with one major modification (see below), was used. The apparatus consisted of three wooden compartments (45 × 45 cm each). Two compartments were “conditioning” compartments (i.e., formalin or U69,593 treatments were paired with one or the other of these compartments), and the third was a “neutral” compartment. Each of the three compartments was characterized by distinct visual and olfactory stimuli. One conditioning compartment had horizontal stripes on the walls and an odor of 1.0% acetic acid, whereas the other had vertical stripes and a standardized cinnamon scent associated with it. Walls of uniform color and no distinctive odor characterized the neutral compartment. Glass, rather than distinct tactile coverings, characterized the floors of the “conditioning” compartments so that a mirror could be angled at 45° under them and formalin behaviors scored. The neutral compartment had two doors, consisting of an opening to each conditioning compartment. These openings could be closed during conditioning to constrain the animal within a single conditioning compartment. All three compartments were equipped with eight IR photo beams aimed at IR detectors. The photo beams were positioned horizontally 1.5 cm above the floor. On the test day (i.e., after the conditioning procedure; see below), signals from the photo beams were fed into a computer programmed to record the total time spent in each compartment. In addition, the number of photo beams broken by an animal during conditioning sessions provided an index of horizontal locomotor activity on these days.
Surgery.
Animals were anesthetized with an i.p. injection of sodium pentobarbital (50 mg/kg). Surgery was performed by using a Kopf stereotaxic apparatus. An injection cannula (30-gauge stainless steel tubing) filled with ibotenic acid or 0.1 M PBS was connected to a microinfusion pump (Razel Scientific Instruments, Stamford, CT) via PE 10 tubing. The cannula was lowered into the ACC by using coordinates obtained from the atlas of Paxinos and Watson (23) (rostral ACC: AP + 2.6 from bregma, DV −2.5, ML ± 0.6; caudal ACC: AP + 0.2 from bregma, DV −2.6, ML ± 0.6). Six minutes after lowering the cannulae to the target coordinates, 0.6 μl of ibotenic acid or PBS was slowly microinjected over a period of 6 min. The cannulae remained in place for 7 min to allow diffusion of the injectate. This procedure was then repeated in the opposite hemisphere. A recovery period of 6 days was allowed before commencement of behavioral testing.
All animals in the rostral ACC lesion and sham groups recovered well from surgery, as evidenced by a weight gain on the pretest day (see below) compared with the surgery day. Additionally, no differences were detected between sham and lesion groups in locomotor activity on the conditioning days (see below), thereby ruling out motor impairments because of rostral ACC lesions.
General Method of Training and Testing in the Place-Conditioning Apparatus.
All experiments were done by using a counterbalanced, unbiased CPA design as described in Swerdlow et al. (24). No initial preferences for any of the compartments in the place-conditioning apparatus were detected on the pretest days, indicating that rats did not prefer any one compartment to the others before conditioning. Experiments lasted 6 days. Animals were handled by the experimenter for habituation purposes on each of 2 days before behavioral testing.
Preconditioning Day (Day 1).
On day 1, the entrance connected to each compartment was opened. Each rat was allowed to move freely throughout the entire apparatus (i.e., all three compartments) for 20 min. The time spent by the rat in each compartment was recorded.
Conditioning Days (Days 2–5).
The conditioning phase of all experiments consisted of 4 days, with 2 days of treatment being paired with each of the conditioning compartments (A and B) in the place-conditioning apparatus. Rats (lesion or sham lesion) received an injection of an aversive chemical stimulus (hind-paw injection of formalin or s.c. injection of U69,593) or a control treatment (formalin experiments, no treatment; U69,593 experiments, s.c. vehicle injection) and then were allowed to freely explore one of the conditioning compartments for a certain length of time (formalin experiments, 50 min; U69,593 experiments, 60 min). Both the day on which the animals received treatment (formalin or U69,593) and the room (context) in which they received treatment were counterbalanced. During the formalin experiments, acute formalin-induced nociceptive behaviors were quantified on the conditioning days. A trained rater scored formalin-induced nociceptive behaviors simultaneously using both the rating scale and flinch frequency methods (25). Scoring was carried out over the entire duration of the conditioning session (50 min) by using time bins of 5 min each.
Postconditioning Day (Day 6, Test Day).
On the day after the conditioning phase, each rat was allowed to move freely throughout the three compartments for 20 min with no aversive stimulus present. The time spent in each compartment was recorded.
Histology.
After completion of the experiments, the animals were overdosed with a lethal dose of sodium pentobarbital and perfused transcardially with isotonic saline followed by 10% formalin. The brains were then removed and fixed first in formalin for 24 h, then in 30% sucrose 24–72 h before slicing. The brains were cut on a sledge microtome at a thickness of 50 μm, stained with cresyl violet, and analyzed to assess the extent of the lesion. Using a projecting microscope (Bausch & Lomb), lesions were traced and analyzed by using an unbiased stereological method (26). Intra-ACC microinjection of ibotenic acid produced lesions with clearly definable borders of neuronal cell loss and gliosis as compared with intra-ACC microinjection of PBS. Based on past studies (14, 27, 28), areas of the rodent ACC rich in nociceptive input were targeted. For lesion criterion analyses, rostral ACC corresponded to perigenual Brodmann's areas 24b, portions of perigenual 24a, and caudodorsal area 32 as defined by Vogt and Peters (29) and corresponded to the following coordinates (23) from bregma, the lambda–bregma plane and midline, respectively: AP, +3.2 to +2.2; DV, −1.0 to −3.2; ML, 0.0 ± 1.4. Caudal ACC included portions of postgenual Brodmann's areas 24a and 24b (29) and corresponded to the following coordinates (23) from bregma, the lambda–bregma plane and midline, respectively: AP, +0.50 to −0.3; DV, −0.8 to −3.2; ML, 0.0 ± 1.4. Although most damage to the caudal ACC was confined to these coordinates, three lesion animals in the caudal ACC group had damage extending to AP + 1.0. Lesions meeting inclusion criteria in the rostral ACC and caudal ACC groups had a minimum percent bilateral damage of 50% and at least 30% damage in the least damaged hemisphere. Unfortunately, histological processing produced unavoidable tearing in the lesioned area in some sections across all experimental groups, as has been reported for ACC lesions by other investigators (30).
Statistical Analyses.
Rating scale nociceptive scores and flinch-frequency nociceptive scores collected from formalin-treated rats during each 5-min time bin were averaged across the two conditioning sessions. The data then were analyzed in separate two-factor ANOVAs (intracerebral treatment × time), with time analyzed as a repeated measure. Multiple pairwise comparisons were made by using Tukey's honestly significant difference tests. The accepted level of statistical significance was P < 0.05.
For the CPA data, the amount of time spent in the conditioning compartment (i.e., compartment paired with formalin or U69,593) on the postconditioning day (i.e., final test day) was subtracted from the amount of time spent in the same compartment on the preconditioning day. This process resulted in a magnitude of CPA score for each rat. Magnitude of CPA scores for sham lesion vs. lesion animals were compared by using a Student's t test. In addition, the absolute amount of time spent in the conditioning compartment on the preconditioning day vs. the postconditioning day was compared in both sham lesion and lesion animals by using Student's t tests. Again, the accepted level of statistical significance was P < 0.05. In experiments 1–3 below, a Bonferonni correction was applied to the α level for significance to correct for the performance of multiple Student's t tests.
Results
Histology.
For all experiments, bilateral infusions of ibotenic acid made into rostral or caudal ACC produced neuronal cell loss and proliferation of small glial cells with clearly definable borders between lesioned and healthy areas (Fig. 1). All animals included in analyses met lesion inclusion criterion as described in Materials and Methods.
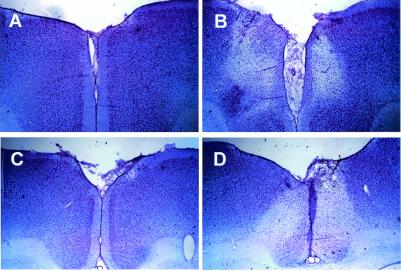
Figure 1.
Photomicrographs of representative coronal sections through the rostral (A and B) and caudal (C and D) ACC. Sections were stained with cresyl violet. (A) Section from a rostral ACC sham lesion animal as compared with a section taken from a rostral ACC lesion animal (B) at the same antero-posterior level. Sections taken from the same antero-posterior level of caudal ACC sham lesion and caudal ACC lesion animals are compared in C and D, respectively. Clear lesion areas are evidenced by neuronal cell loss and by the proliferation of smaller glial cells (especially apparent around the borders of the lesions).
Experiment 1.
The purpose of this experiment was to examine the effect of excitotoxin-induced lesions of the ACC on both F-CPA and acute nociceptive behaviors. A selective reduction in F-CPA was hypothesized. Importantly, the rodent ACC has been divided into a perigenual region that preferentially receives nociceptive input (14, 27, 28) and a postgenual region that receives comparatively little nociceptive input. For the purposes of this study, we named these regions the rostral ACC and the caudal ACC, respectively (see Materials and Methods for more details). The rostral ACC was the target of our lesioning procedure in experiment 1.
Acute formalin-induced nociceptive behaviors.
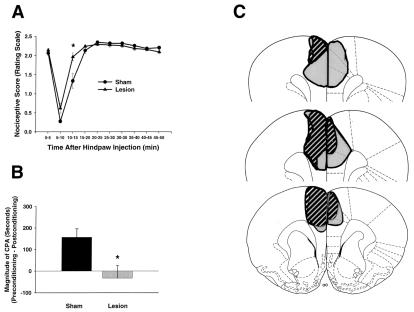
Rating scale nociceptive scores of rats in experiment 1 are shown in Fig. 2A. An ANOVA performed on these scores found no significant main effect of intracerebral treatment but did reveal a significant interaction between intracerebral treatment and time [F(9,153) = 5.43; P < 0.05]. Further analysis of the interaction revealed only one time point at which nociceptive scores were different between ACC lesion rats and ACC sham lesion rats; during the interval 10–15 min after formalin injection, ACC lesion rats showed significantly higher rating scale scores than did ACC sham lesion rats (Fig. 2A; Tukey's honestly significant difference test; P < 0.05). At all other time points, rating scale nociceptive scores of ACC lesion rats were not significantly different from those of ACC sham lesion rats (Fig. 2A; Tukey's honestly significant difference test; P > 0.05).
Figure 2.
Formalin-induced pain behaviors and brain lesion maps associated with rats with rostral ACC lesions (n = 8) and rostral ACC sham lesions (n = 10). Data are represented as mean ± SEM. Acute formalin-induced nociceptive scores (rating scale) are shown in A. Magnitude of CPA scores are shown in B. Representations of the largest and smallest lesions in the rostral ACC lesion group are shown in C. Mean percent damage calculations for each hemisphere and a bilateral mean for each experimental group are as follows: left hemisphere, 71% ± 4%; right hemisphere, 51% ± 6%; mean, 62% ± 4%. Rostral ACC lesions completely abolished F-CPA (B) without causing a concomitant reduction in acute formalin-induced nociceptive behaviors (A). (A) *, P < 0.05, Tukey's honestly significant difference test, as compared with rats with sham lesions of the rostral ACC. (B) *, P < 0.05, Student's t test, as compared with rats with sham lesions of the rostral ACC.
The flinch frequency method also was used to quantify acute nociception. Analysis of flinch scores revealed a similar pattern of results to that obtained with the rating scale method (data not shown). An ANOVA performed on the flinch scores did not reveal a significant main effect of intracerebral treatment [F(1,17) = 0.799; P > 0.05] or a significant interaction between intracerebral treatment and time [F(9,153) = 1.601; P > 0.05]. Overall, flinch nociceptive scores were not different between rostral ACC lesion rats and rostral ACC sham lesion rats.
F-CPA.
F-CPA scores of rats in experiment 1 are shown in Fig. 2B. When hind-paw formalin (2.5%) injection was paired with a particular compartment in the place-conditioning apparatus, ACC sham lesion rats spent significantly less time in this compartment on the postconditioning test day as compared with the preconditioning test day (i.e., CPA was produced; 417.2 ± 34.2 s vs. 267 ± 18.9 s; Student's t test, P < 0.05). By contrast, the same procedure did not result in the production of CPA in rostral ACC lesion rats (355.8 ± 29.9 s vs. 388.6 ± 39.9 s; Student's t test, P > 0.05). Fig. 2B expresses these data as difference scores or magnitude of CPA scores (see Materials and Methods). It is clear that rostral ACC lesion rats displayed significantly lower magnitude of CPA scores than did rostral ACC sham lesion rats (Fig. 2B; Student's t test, P < 0.05).
Experiment 2.
The results of experiment 1 demonstrate that excitotoxin-induced lesions of the portion of the ACC that receives nociceptive input (i.e., the rostral ACC) reduces F-CPA without reducing acute formalin-induced nociceptive behaviors. It was of interest, however, to determine whether this effect is specific to the rostral ACC or whether a similar pattern of results can be obtained with lesions centered on another part of the ACC. Experiment 2 was conducted, therefore, to assess whether similarly sized lesions of the caudal ACC (as defined in Materials and Methods) also would attenuate F-CPA. Because the caudal ACC receives comparatively little nociceptive input as compared with the rostral ACC (14, 27, 28), we predicted that lesions of caudal ACC would have no effect on either F-CPA or acute formalin-induced nociceptive behaviors.
Acute formalin-induced nociceptive behaviors.
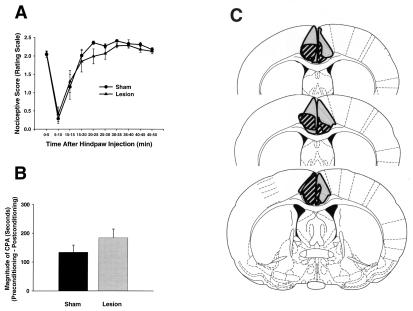
Rating scale nociceptive scores of rats in experiment 2 are shown in Fig. 3A. An ANOVA performed on these scores found neither a significant main effect of intracerebral treatment [F(1,13) = 0.439; P > 0.05] nor a significant interaction between intracerebral treatment and time [F(9,117) = 0.58; P > 0.05]. The flinch frequency method also was used to quantify acute nociception. Analysis of flinch scores revealed a similar pattern of results to that obtained with the rating scale method (data not shown). An ANOVA performed on the flinch scores did not reveal a significant main effect of intracerebral treatment [F(1,12) = 0.138; P > 0.05] or a significant interaction between intracerebral treatment and time [F(9,108) = 0.369; P > 0.05]. Overall, neither rating scale scores nor flinch nociceptive scores were different between caudal ACC lesion rats and caudal ACC sham lesion rats.
Figure 3.
Formalin-induced pain behaviors and brain lesion maps associated with rats with caudal ACC lesions (n = 8) and caudal ACC sham lesions (n = 11). Data are represented as mean ± SEM. Acute formalin-induced nociceptive scores are shown in A. Magnitude of CPA scores are shown in B. Representations of the largest and smallest lesions in the caudal ACC lesion group are shown in C. Mean “percent damage” calculations for each hemisphere and a bilateral mean for each experimental group are as follows: left hemisphere, 82% ± 5%; right hemisphere, 67% ± 5%; mean, 74% ± 4%. Caudal ACC lesions had no effect on either acute formalin-induced nociceptive behaviors (A) or F-CPA (B).
F-CPA.
F-CPA scores of rats in experiment 2 are shown in Fig. 3B. When the noxious effects of hind-paw formalin (2.5%) were paired with a particular compartment in the place-conditioning apparatus, caudal ACC sham lesion rats spent significantly less time in this compartment on the postconditioning test day as compared with the preconditioning test day (i.e., CPA was produced; 414.6 ± 31.8 s vs. 281.1 ± 43.5 s; Student's t test, P < 0.05). Unlike the results obtained in rostral ACC lesion rats (Fig. 2B), hind-paw formalin did produce CPA in caudal ACC lesion rats (391.6 ± 13.5 s vs. 208.1 ± 37.6 s; Student's t test, P < 0.05). Fig. 3B expresses these data as difference scores or magnitude of CPA scores (see Materials and Methods). It is clear that caudal ACC lesion rats displayed magnitude of CPA scores that were not significantly different from those of ACC sham lesion rats (Fig. 3B; Student's t test, P > 0.05). Thus, unlike the effect observed in rats with lesions of the rostral ACC (Fig. 2), rats with lesions of the caudal ACC displayed F-CPA that was not significantly different from that displayed by sham lesion rats.
Experiment 3.
The results of experiments 1 and 2 indicate that excitotoxin-induced lesions of the rostral, but not caudal, ACC reduce F-CPA without reducing acute formalin-induced nociceptive behaviors. One interpretation of these results is that the rostral ACC encodes, at least in part, the affective component of pain in rats. A lesion of this area would reduce the aversive aspect of the response to a nociceptor-activating stimulus, thereby reducing the animal's motivation to avoid the compartment in which the stimulus was applied (see Discussion). Along these lines, it is important to know whether neurons in the rostral ACC are associated specifically with aversiveness of nociceptor-activating stimuli, or whether this region is associated with aversive stimuli in general (i.e., nociceptor activating or non-nociceptor activating). To address this issue, the effect of excitotoxin-induced lesions of the rostral ACC on CPA elicited by an aversive, but non-nociceptor-activating, stimulus was assessed. For this purpose, the κ-opioid receptor agonist U69,593, which is known to be aversive when injected systemically (31), was administered s.c. and paired with a distinct compartment in the place-conditioning apparatus. The conditioning procedure was similar to that used to elicit F-CPA in experiments 1 and 2. The ability of U69,593 to produce CPA was then assessed in rats with lesions or sham lesions of the rostral ACC.
Histology.
Lesions of the rostral ACC in this experiment were similar in location and size to those described in experiment 1. There were no significant differences in percent damage of the rostral ACC between lesion animals in experiment 1 vs. experiment 3 (Student's t tests; P > 0.05; for percent damage data, see the legends for Figs. 2 and 4).
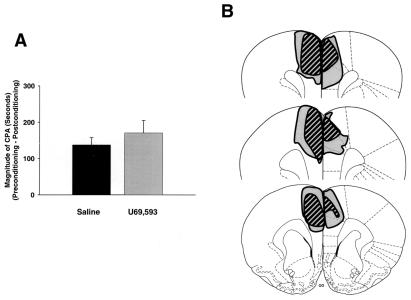
Figure 4.
CPA produced by a non-nociceptive stimulus (the κ-opioid receptor agonist U69,593) in rats with rostral ACC lesions (n = 9) and rostral ACC sham lesions (n = 8). Data are represented as mean ± SEM. U69,593-induced magnitude of CPA scores are shown in A. Representations of the largest and smallest lesions in the rostral ACC lesion group are shown in B. Mean percent damage calculations for each hemisphere and a bilateral mean for each experimental group are as follows: left hemisphere, 84% ± 4%; right hemisphere, 60% ± 3%; mean, 69% ± 3%. Rostral ACC lesions had no effect on U69,593-induced CPA.
U69,593-induced CPA.
CPA scores of rats in experiment 3 are shown in Fig. 4A. When the effects of systemically administered U69,593 were paired with a particular compartment in the place-conditioning apparatus, rostral ACC sham lesion rats spent significantly less time in this compartment on the postconditioning test day as compared with the preconditioning test day (i.e., CPA was produced; 343.0 ± 11.4 s vs. 197.6 ± 15.1 s; Student's t test, P < 0.05). Unlike the F-CPA results obtained in experiment 1 (Fig. 2B), systemic U69,593 did produce CPA in rostral ACC lesion rats (359.0 ± 37.4 s vs. 201.0 ± 17.3 s; Student's t test, P < 0.05). Fig. 4A expresses these data as difference scores or magnitude of CPA scores (see Materials and Methods). It is clear that rostral ACC lesion rats displayed magnitude of CPA scores that were not significantly different from those of rostral ACC sham lesion rats (Fig. 4A; Student's t test, P > 0.05). Thus, whereas lesions of the rostral ACC reduced CPA elicited by a nociceptive stimulus (Fig. 2B), lesions of the rostral ACC did not reduce CPA elicited by a non-nociceptive stimulus (Fig. 4A).
Discussion
It is clear that dilute formalin activates nociceptive primary afferent fibers (Aδ and c fibers) (32) and that s.c. formalin injection is painful to humans (33). This evidence has helped to establish the formalin test as a valid animal model of persistent pain. Despite such evidence, however, it is never possible to know with certainty how closely an animal “pain” model reflects pain as experienced by humans. The fact that hind-paw formalin injection produces CPA in addition to other nociceptive behaviors indicates that formalin is aversive to the animal in a manner resembling the response to noxious stimuli in humans.
Excitotoxin-induced lesions of the rostral (Fig. 2), but not caudal (Fig. 3), ACC reduced F-CPA without reducing acute formalin-induced nociceptive behaviors (e.g., lifting, licking, or flinching of the injected paw). When a non-nociceptive stimulus (in this case U69,593) was used to elicit CPA, rostral ACC lesions were ineffective in reducing CPA (Fig. 4). These results represent direct evidence demonstrating that neurons in the ACC are necessary for the acquisition or expression of CPA elicited by a nociceptive stimulus. Reduction in F-CPA by rostral ACC lesions may reflect a reduction in the aversiveness or perceived unpleasantness of the nociceptive stimulus (for other interpretations, see below). The concomitant sparing of acute formalin-induced nociceptive behaviors by rostral ACC lesions provides evidence indicating that, in the rodent, the neural substrates underlying the affective-motivational component of pain can be dissociated from the neural substrates encoding other features of the sensory experience elicited by nociceptor stimulation, such as stimulus localization.
The present results confirm and extend electrophysiological and anatomical studies implicating the ACC in nociceptive processing. Noci-responsive neurons in the rat are found in more rostral ACC areas that include both rostral area 24b and dorsal area 32 (14). These electrophysiological findings are supported by anatomical studies demonstrating projections of medial and intralaminar thalamic nuclei to the ACC. Nuclei within these thalamic regions are implicated in the spino-ACC pathway. Stimulation of nociceptive areas in medial and intralaminar thalamic nuclei preferentially activates neurons in rostral vs. caudal ACC (28). Furthermore, neuroanatomical studies find that various medial and intralaminar thalamic nuclei project to rostral regions of ACC (area 24b) in addition to dorsal area 32 (27). The view that rostral but not more caudal areas of the ACC are involved in the affective processing of pain was proposed by Vogt et al. (34). They proposed that rostral areas of the human ACC (areas 24, 32, and 25) are involved in processing pain-related affect, whereas more caudal regions are involved in motor planning as a secondary response to nociceptor stimulation. Indeed, our data support this notion and offer causal evidence in support of the involvement of rostral vs. caudal ACC in pain-related affective processing. Although some recent human neuroimaging data suggest that mid/caudal (i.e., postgenual) portions of the ACC are preferentially activated by nociceptive stimulation (35), many studies of this nature indicate that nociceptive stimulation activates both mid/caudal and rostral (i.e., perigenual) portions of the ACC. In any case, the present data indicate that, at least in the rat, activation of caudal ACC is not necessary for pain-related aversiveness.
Based on the present results and the evidence described above, several interpretations of ACC function in pain and aversion are possible. One interpretation is that neural processing in the ACC during the acute response to hind-paw formalin injection is necessary for the affective salience of the nociceptive input. Accordingly, the affective salience of the formalin stimulus (unconditioned stimulus) would be decreased or blocked in animals lacking the rostral ACC. This disruption in unconditioned stimulus salience would disrupt neural processing in other areas involved in aversion learning circuitry, thereby impairing the association of the unconditioned stimulus (formalin) with the conditioned stimulus (environmental context) and blocking the acquisition of F-CPA. This interpretation is supported by various human and primate studies. In humans, cingulectomies or cingulumotomies decrease the perceived unpleasantness of chronic pain, whereas perception of stimulus location, intensity, and quality of the pain remain intact (9, 10). More recently, Rainville and colleagues (12) provided functional neuroimaging evidence implicating the ACC specifically in the perception of pain-related unpleasantness. Using hypnotic suggestion in a population of normal human volunteers, these investigators were able to reduce the perceived unpleasantness of a painful stimulus (47°C water) without affecting the perceived intensity of the stimulus. This reduction in perceived unpleasantness correlated with a decrease in noxious stimulus-evoked activity in the ACC but not in the somatosensory cortex, thereby demonstrating a dissociation of perceived unpleasantness from other components of nociception at both the behavioral (self-report) and anatomical levels.
An alternative explanation of the present results is that lesions of the ACC cause a disruption in neural processing relating to learning and memory. One might argue lesions of the rostral ACC reduce the animal's ability to associate the effects of the nociceptive stimulus with the distinct environmental context, rather than reflecting a reduction in the primary aversive quality of the stimulus per se. Although this interpretation is not ruled out completely by the present results, it is important to point out that lesions of the rostral ACC do not reduce the animal's ability to acquire a CPA when the unconditioned stimulus is an aversive stimulus that is non-nociceptive in nature (Fig. 4A). Additionally, other studies have found no effects of similar ACC lesions on the acquisition of morphine- and cocaine-induced conditioned place preference (36, 37). Therefore, we conclude that rostral ACC lesions do not have a general disruptive effect on learning in the place-conditioning paradigm, but rather result in a deficit relating to the acquisition or expression of pain-related CPA. This interpretation is supported by other studies indicating that the ACC contributes to pain-related learning. Several in vivo electrophysiological studies have shown that individual ACC neurons (some of them characterized as noci-responsive) can acquire responses to environmental cues that predict a painful stimulus (38–40). Moreover, lesions of area 24b in the rabbit disrupt a conditioned decrease in latency to avoid a noxious stimulus (7). Furthermore, lesions of the ACC also impair the acquisition of a classically conditioned bradycardic response to a cue predicting a noxious stimulus (41). Thus, lesions of the ACC may be specifically disrupting the aversive learning relating to nociceptive, but not non-nociceptive, stimuli.
Although evidence from human and animal studies seems to support differing roles of the ACC in nociceptive processing, this distinction may be the result of differing experimental approaches. Studies of the perception of unpleasantness are only amenable to human experiments where self-report is possible, whereas the neural substrates of learning and motivated behavior are more easily studied in animals. It is possible that the perception of pain-related unpleasantness and pain-related aversion learning are not separable at the neural level. Although the ACC is necessary for perception of pain-related affect, it may also be a part of the circuitry mediating aversion learning. The ACC then not only encodes pain-related negative affect, it also is essential for the learning that underlies recognition of pain-predictive cues and avoidance. Electrophysiological studies lend support to this view of ACC processing. First, neurons in the ACC respond both to noxious stimuli and cues that predict a noxious stimulus (8, 13, 14, 38–40). Interestingly, one study examined response properties of individual ACC neurons to both a pain-predictive cue and a noxious stimulus (39). These neurons were first characterized after behavioral training as responsive to a pain-predictive visual cue (conditioned stimulus). After this initial characterization, these same neurons were tested in response to an acute noxious stimulus (electric shock). Of the initial conditioned stimulus-responsive neurons, 50% also responded to the noxious stimulus. These data support the idea that both learning (i.e., training-induced changes in neuronal firing) and unpleasantness (i.e., acute neuronal responses to a noxious stimulus after training) are encoded at the neural level within the ACC.
In conclusion, using a method that assesses pain-induced CPA, the present study provides causal evidence indicating that neurons in the rostral ACC are required for pain-related aversion learning—a process that directly reflects the affective component of pain. The present results, along with related human neuroimaging data (12), strongly support the contention that neuronal activation of the ACC is causally involved in the perception of pain-related unpleasantness. Clearly, additional studies are needed to examine how nociceptive input is encoded within the ACC, how this brain region interacts with other limbic nuclei involved in pain processing and aversion learning, and possible influences of the ACC on brainstem pain-modulating circuitry and spinal nociceptive processing (42). Because the debilitating nature of persistent pain in the clinic is related to the suffering and anxiety that the pain induces, studies of the ACC and other areas relating to pain-related affective processing may have considerable clinical significance.
Acknowledgments
We thank Dr. Sonja Potrebic for invaluable assistance regarding stereological assessment of lesion volumes. We also thank Dr. Ian Meng for discussions and Robin LeWinter for technical assistance. This work was supported by the Medical Research Council of Canada, the National Institutes of Health (Public Health Service Grants NS 21445 and NS 10816), and the Life Sciences Research Foundation.
Abbreviations
- ACC
anterior cingulate cortex
- CPA
conditioned place avoidance
- F-CPA
formalin-induced CPA
References
- 1.Fields H L. Pain. 1999;6,Suppl.:S61–S69. doi: 10.1016/S0304-3959(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 2.Melzack R, Casey K L. In: The Skin Senses. Kenshalo D, editor. Springfield, IL: Thomas; 1968. pp. 423–439. [Google Scholar]
- 3.Melzack R. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 4.Price D D. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 5.Bushnell M C, Duncan G H, Hofbauer R K, Ha B, Chen J I, Carrier B. Proc Natl Acad Sci USA. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treede R D, Kenshalo D R, Gracely R H, Jones A K. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel M, Kubota Y, Sparenborg S, Straube K, Vogt B A. Exp Brain Res. 1991;86:585–600. doi: 10.1007/BF00230532. [DOI] [PubMed] [Google Scholar]
- 8.Hutchison W D, Davis K D, Lozano A M, Tasker R R, Dostrovsky J O. Nat Neurosci. 1999;2:403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- 9.Foltz E L, White L E., Jr J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 10.Hurt R W, Ballantine H T., Jr Clin Neurosurg. 1974;21:334–351. doi: 10.1093/neurosurgery/21.cn_suppl_1.334. [DOI] [PubMed] [Google Scholar]
- 11.Casey K L. Proc Natl Acad Sci USA. 1999;96:7668–7674. doi: 10.1073/pnas.96.14.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainville P, Duncan G H, Price D D, Carrier B, Bushnell M C. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 13.Sikes R W, Vogt B A. J Neurophysiol. 1992;68:1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- 14.Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, Nomura H, Hibiya J, Fujita S, Sumino R. Brain Res. 1996;735:83–92. doi: 10.1016/0006-8993(96)00561-6. [DOI] [PubMed] [Google Scholar]
- 15.Liu R J, Qiang M, Qiao J T. Neuroscience. 1998;85:1073–1087. doi: 10.1016/s0306-4522(97)00662-3. [DOI] [PubMed] [Google Scholar]
- 16.Allen G V, Hopkins D A. J Comp Neurol. 1989;286:311–336. doi: 10.1002/cne.902860303. [DOI] [PubMed] [Google Scholar]
- 17.Cassell M D, Wright D J. Brain Res Bull. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- 18.Reep R L, Corwin J V. Brain Res. 1999;841:43–52. doi: 10.1016/s0006-8993(99)01779-5. [DOI] [PubMed] [Google Scholar]
- 19.Christie M J, Summers R J, Stephenson J A, Cook C J, Beart P M. Neuroscience. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- 20.Royce G J. Exp Brain Res. 1983;50:157–165. doi: 10.1007/BF00239179. [DOI] [PubMed] [Google Scholar]
- 21.Wyss J M, Sripanidkulchai K. Brain Res. 1984;293:1–15. doi: 10.1016/0006-8993(84)91448-3. [DOI] [PubMed] [Google Scholar]
- 22.Manning B H, Fields H L, Matthies B K. Soc Neurosci Abstr. 2000;26:431. [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow N R, Gilbert D, Koob G F. In: Neuromethods: Psychopharmacology. Boulton A A, Baker G B, Greenshaw A J, editors. Clifton, NJ: Humana; 2000. pp. 399–446. [Google Scholar]
- 25.Manning B H. J Neurosci. 1998;18:9453–9470. doi: 10.1523/JNEUROSCI.18-22-09453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard C V, Reed M G. Unbiased Stereology: Three Dimensional Measurement in Microscopy. New York: Springer; 1998. [Google Scholar]
- 27.Berendse H W, Groenewegen H J. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- 28.Hsu M M, Shyu B C. NeuroReport. 1997;8:2701–2707. doi: 10.1097/00001756-199708180-00013. [DOI] [PubMed] [Google Scholar]
- 29.Vogt B A, Peters A. J Comp Neurol. 1981;195:603–625. doi: 10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- 30.Weissenborn R, Robbins T W, Everitt B J. Psychopharmacology. 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- 31.Shippenberg T S, Bals-Kubik R, Herz A. J Pharmacol Exp Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- 32.Puig S, Sorkin L S. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 33.Dubuisson D, Dennis S G. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 34.Vogt B A, Derbyshire S, Jones A K. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 35.Davis K D, Taylor S J, Crawley A P, Wood M L, Mikulis D J. J Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- 36.Tzschentke T M, Schmidt W J. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- 37.Tzschentke T M, Schmidt W J. Behav Brain Res. 1998;97:115–127. doi: 10.1016/s0166-4328(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 38.Nishijo H, Yamamoto Y, Ono T, Uwano T, Yamashita J, Yamashima T. Neurosci Lett. 1997;227:79–82. doi: 10.1016/s0304-3940(97)00310-8. [DOI] [PubMed] [Google Scholar]
- 39.Koyama T, Tanaka Y Z, Mikami A. NeuroReport. 1998;9:2663–2667. doi: 10.1097/00001756-199808030-00044. [DOI] [PubMed] [Google Scholar]
- 40.Koyama T, Kato K, Mikami A. Neurosci Lett. 2000;283:17–20. doi: 10.1016/s0304-3940(00)00894-6. [DOI] [PubMed] [Google Scholar]
- 41.Buchanan S L, Powell D A. Neurosci Lett. 1982;29:261–268. doi: 10.1016/0304-3940(82)90327-5. [DOI] [PubMed] [Google Scholar]
- 42.Calejesan A A, Kim S J, Zhuo M. Eur J Pain. 2000;4:83–96. doi: 10.1053/eujp.1999.0158. [DOI] [PubMed] [Google Scholar]