Abstract
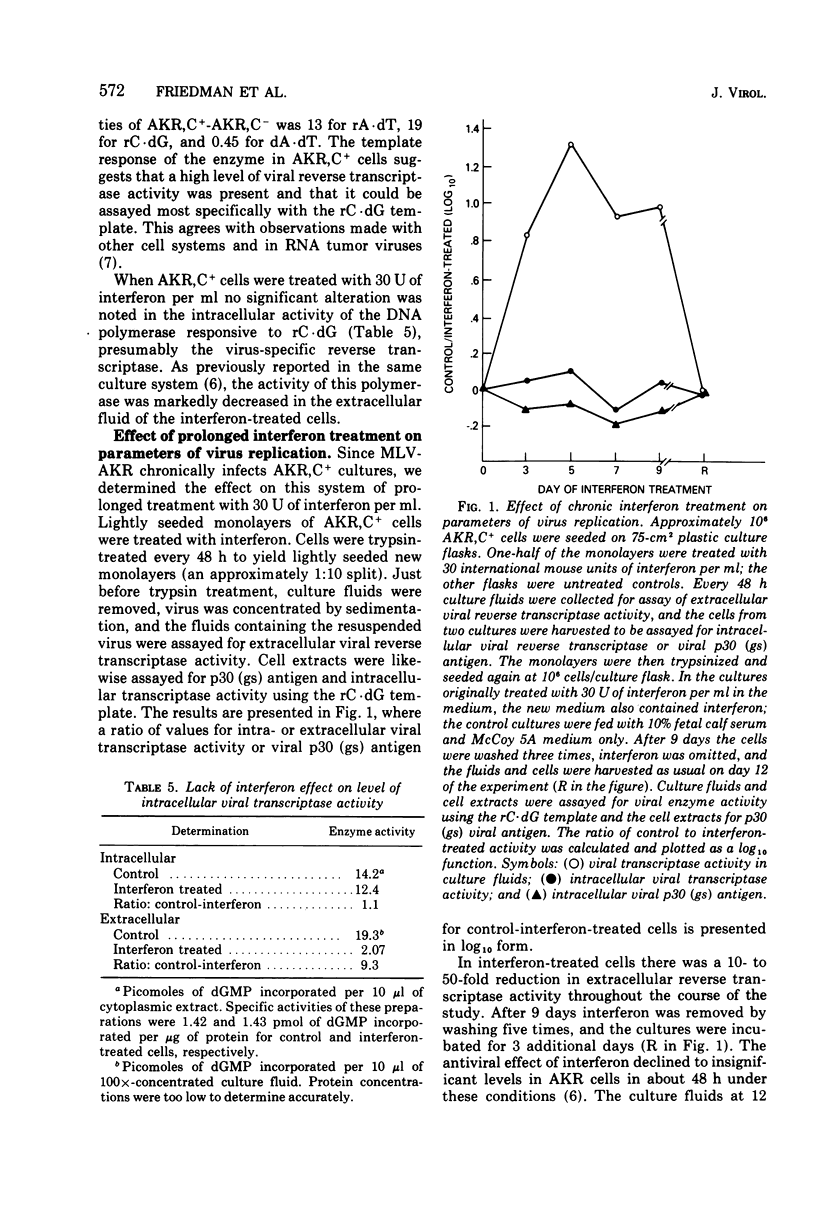
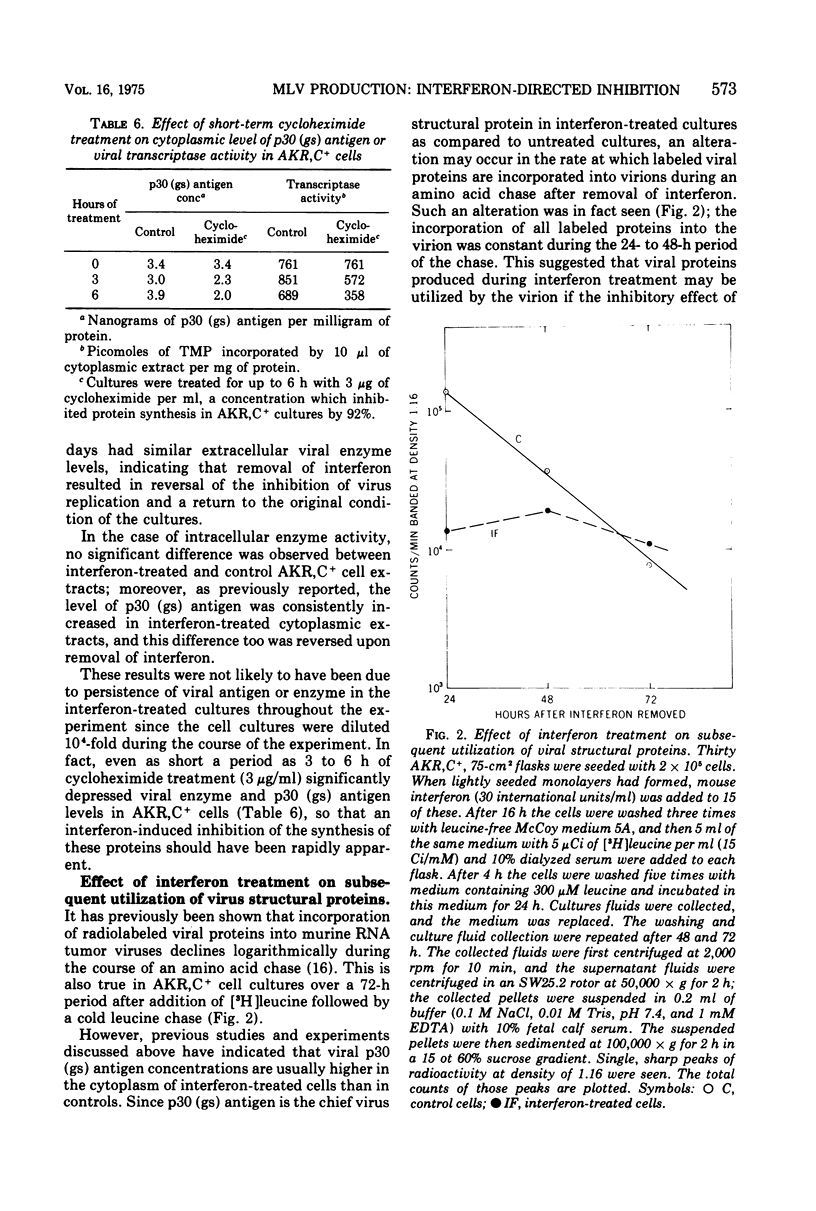
Extracellular murine leukemia virus (MLV) reverse transcriptase activity was decreased by interferon treatment in four interferon-sensitive mouse cell lines which were chronic MLV producers. In three cell lines which were relatively insensitive to interferon, extracellular enzyme activity remained unchanged by interferon treatment. The concentrations of interferon used had no effect on DNA synthesis or cell replication of AKR,C+ cells which were chronic producers of AKR-MLV. In AKR,C+ cultures interferon treatment also had no effect on the level of intracellular viral reverse transcriptase activity in spite of an inhibition of extracellular enzyme activity. Treatment of AKRC+ cultures with interferon for 9 days inhibited extracellular viral reverse transcriptase levels throughout the period of treatment; however, the intracellular enzyme activity remained unchanged, and concentrations of viral p30 (gs) antigen were increased in the interferon-treated cells. When the cells were washed to remove interferon, however, virus production rapidly rose and intracellular p30 antigen fell to the levels of untreated AKR,C+ cells. These and previously reported results suggested that in interferon-treated AKR,C+ cells virus production is inhibited at a late step in the MLV replication cycle, either directly or through the inhibition of the production of a protein required for virus assembly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Sobis H., De Somer P. Influence of interferon on virus particle formation in different oncornavirus carrier cell lines. Int J Cancer. 1973 Nov 15;12(3):646–653. doi: 10.1002/ijc.2910120313. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Esteban R. M., Metz D. H., Tovell D. R., Kerr I. M., Williamson R. Translation of RNA by L cell extracts: Effect of interferon. FEBS Lett. 1972 Aug 15;24(3):273–277. doi: 10.1016/0014-5793(72)80371-5. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Inhibition of arbovirus protein synthesis by interferon. J Virol. 1968 Oct;2(10):1081–1085. doi: 10.1128/jvi.2.10.1081-1085.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Horvath A. E., Friedman R. M. Nucleic acid and proteins isolated from a strain of murine sarcoma virus (MSV-O). Proc Soc Exp Biol Med. 1971 Jul;137(3):1075–1081. doi: 10.3181/00379727-137-35731. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy M. V., Easterbrook K. B., Lee S. H., Katz L. J., Rozee K. R. Interferon inhibition of autogenously induced virions (C particles) in synchronized L-929 cell cultures. J Natl Cancer Inst. 1974 Dec;53(6):1687–1690. [PubMed] [Google Scholar]
- Ogburn C. A., Berg K., Paucker K. Purification of mouse interferon by affinity chromatography on anti-interferon globulin-sepharose. J Immunol. 1973 Oct;111(4):1206–1218. [PubMed] [Google Scholar]
- Oxman M. N., Levin M. J. Interferon and transcription of early virus-specific RNA in cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1971 Feb;68(2):299–302. doi: 10.1073/pnas.68.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Livingston D. M. Radioimmunoassay of mammalian type C viral proteins. I. Species specific reactions of murine and feline viruses. J Immunol. 1972 Sep;109(3):570–577. [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Polypeptides of Moloney sarcoma-leukemia virions: their resolution and incorporation into extracellular virions. Virology. 1974 Oct;61(2):575–587. doi: 10.1016/0042-6822(74)90291-8. [DOI] [PubMed] [Google Scholar]



