Abstract
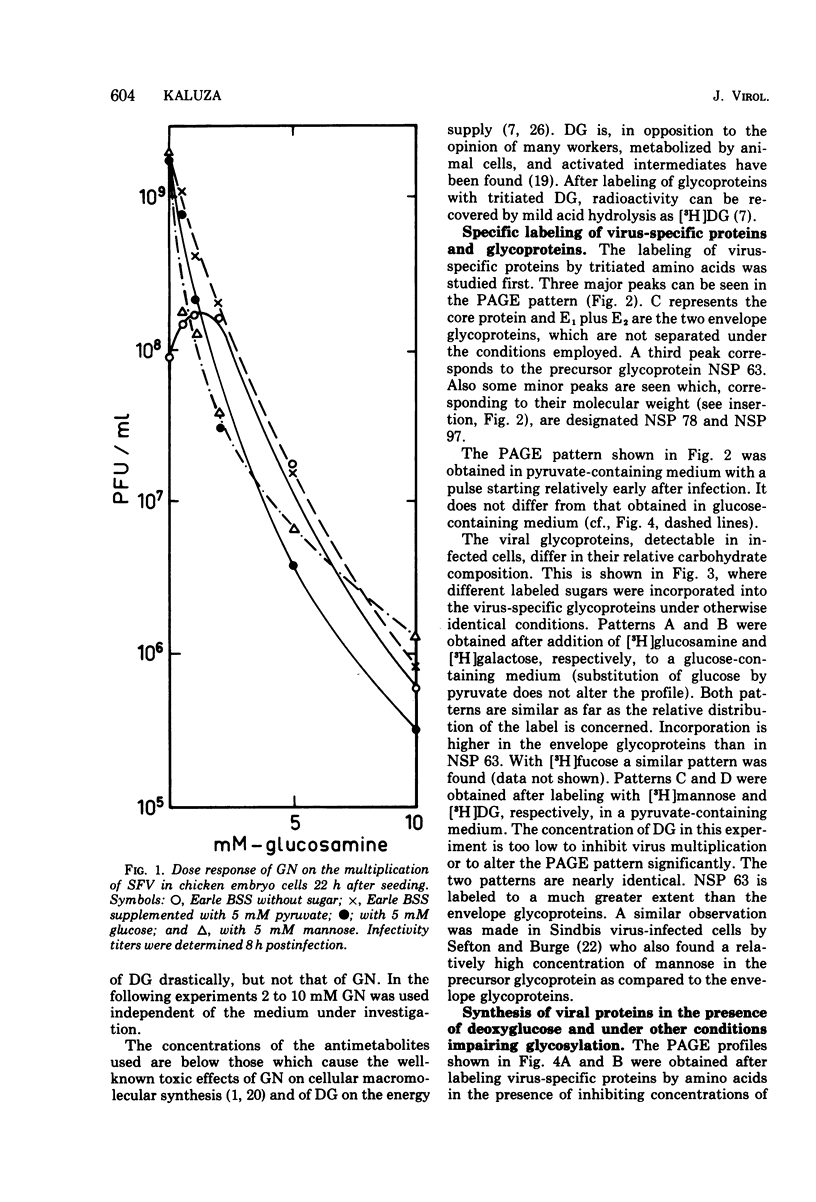
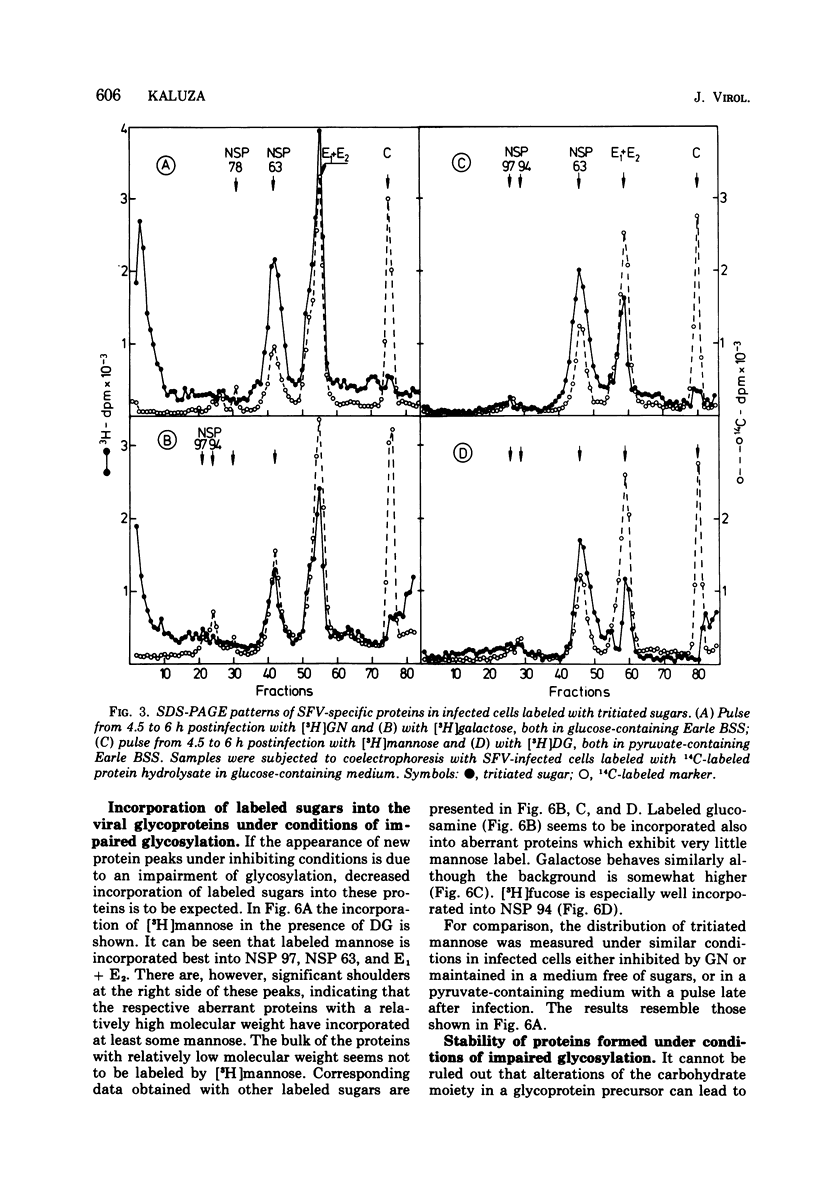
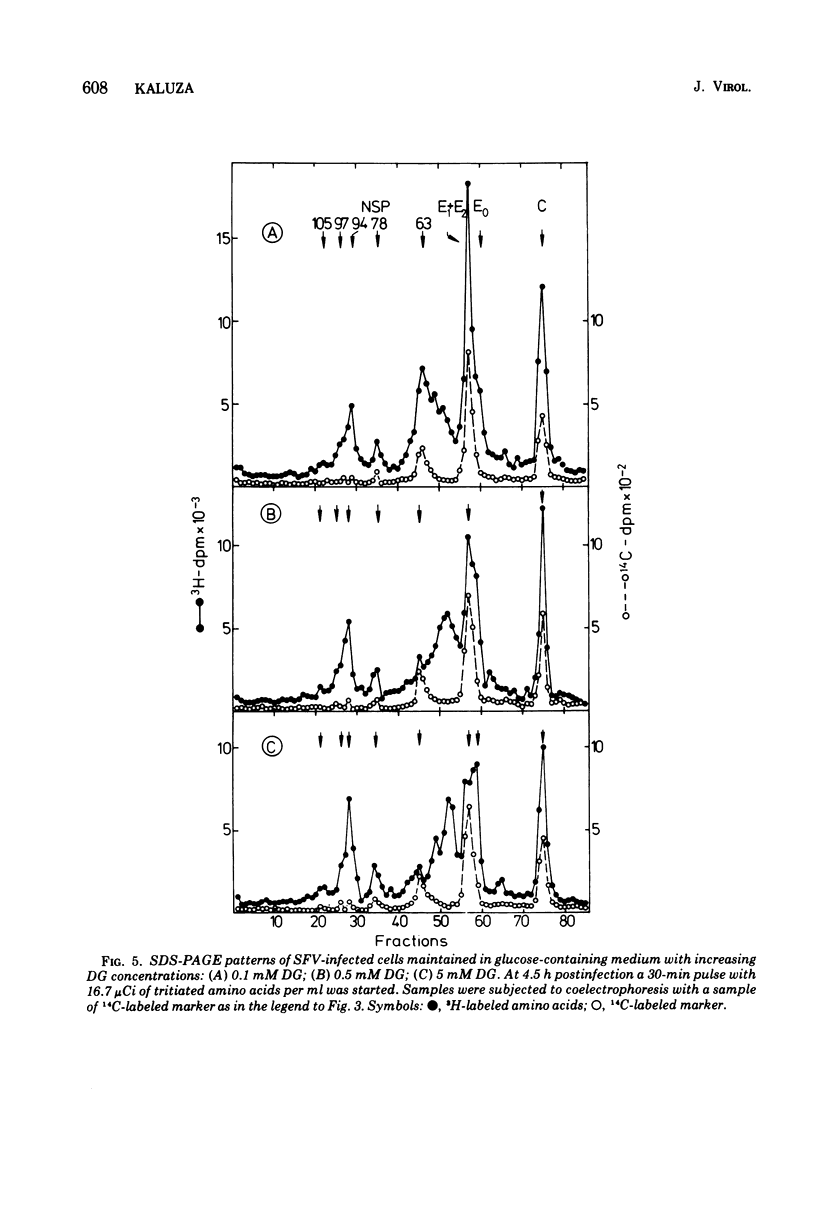
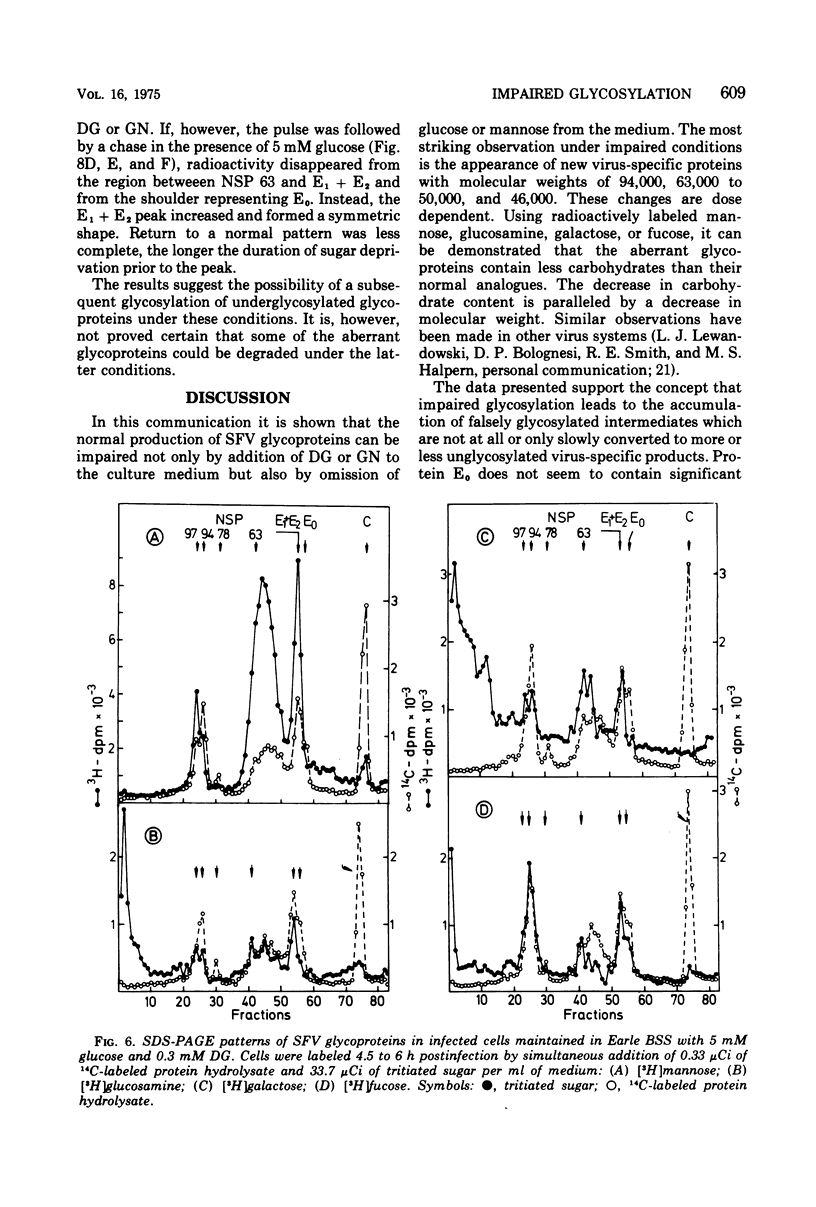
The glycoproteins of Semliki Forest virus, grown in chicken embryo cells, were labeled with radioactive sugars. The data indicate a high mannose content of the nonstructural precursor glycoprotein NSP 63. This protein can also be readily labeled with 2-deoxy-D-glucose. The envelope glycoproteins E1 and E2 are relatively rich in galactose, glucosamine, and fucose. Glycosylation can be impaired by 2-deoxy-D-glucose or D-glucosamine or by omission of sugars in the culture medium. Under these conditions characteristic changes in the electrophoretic profile of the viral polypeptides are observed: in the regions of glycoproteins NSP 97, NSP 63, and E1 and E2 new protein peaks can be detected. These polypeptides seem to be aberrant forms of the glycoproteins. When compared with the normal molecules they have lower molecular weights and contain less carbohydrates, especially mannose. Pulse-chase experiments indicate that the altered glycoproteins are degraded very slowly if at all. If, however, impairment is caused by omission of sugars in the culture medium, the radioactivity is chased after addition of glucose from the region between NSP 63 and E1 + E2 into the E1 + E2 peak. This suggests a completion of the carbohydrate chains under these conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekesi J. G., Bekesi E., Winzler R. J. Inhibitory effect of D-glucosamine and other sugars on the biosynthesis of protein, ribonucleic acid, and deoxyribonucleic acid in normal and neoplastic tissues. J Biol Chem. 1969 Jul 25;244(14):3766–3772. [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Duda E., Schlesinger M. J. Alterations in Sindbis viral enbelope proteins by treating BHK cells with glucosamine. J Virol. 1975 Feb;15(2):416–419. doi: 10.1128/jvi.15.2.416-419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S. S., Stanley P., Taylor J. M., White D. O. Inhibition of influenza viral glycoprotein synthesis by sugars. Microbios. 1972 Jan;5(17):41–50. [PubMed] [Google Scholar]
- Garoff H., Simons K., Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974 Oct;61(2):493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J., Burke D. C. Proteins synthesized in chick cells following infection with Semliki Forest virus. J Gen Virol. 1968 Sep;3(2):175–184. doi: 10.1099/0022-1317-3-2-175. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Schmidt M. F., Scholtissek C. Effect of 2-deoxy-D-glucose on the multiplication of Semliki Forest virus and the reversal of the block by mannose. Virology. 1973 Jul;54(1):179–189. doi: 10.1016/0042-6822(73)90127-x. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Burke D. C. Studies on the structural proteins of Semliki Forest virus. J Gen Virol. 1972 Jan;14(1):87–98. doi: 10.1099/0022-1317-14-1-87. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. The effect of enzymes on structural and biological properties of Semliki forest virus. J Gen Virol. 1974 May;23(2):129–143. doi: 10.1099/0022-1317-23-2-129. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Caliguiri L. A., Choppin P. W. The proteins of the parainfluenza virus SV5. II. The carbohydrate content and glycoproteins of the virion. Virology. 1970 Oct;42(2):473–481. doi: 10.1016/0042-6822(70)90290-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laine R., Söderlund H., Renkonen O. Chemical composition of Semliki forest virus. Intervirology. 1973;1(2):110–118. doi: 10.1159/000148837. [DOI] [PubMed] [Google Scholar]
- Morser M. J., Burke D. C. Cleavage of virus-specified polypeptides in cells infected with Semliki Forest Virus. J Gen Virol. 1974 Mar;22(3):395–409. doi: 10.1099/0022-1317-22-3-395. [DOI] [PubMed] [Google Scholar]
- Morser M. J., Kennedy S. I., Burke D. C. Virus-specified polypeptides in cells infected with Semliki Forest virus. J Gen Virol. 1973 Oct;21:19–29. doi: 10.1099/0022-1317-21-1-19. [DOI] [PubMed] [Google Scholar]
- Ranki M., Käriäinen L., Renkonen O. Semliki forest virus glycoproteins and canavanine. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):760–768. doi: 10.1111/j.1699-0463.1972.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Schmidt M. F., Schwarz R. T., Scholtissek C. Nucleoside-diphosphate derivatives of 2-deoxy-D-glucose in animal cells. Eur J Biochem. 1974 Nov 1;49(1):237–247. doi: 10.1111/j.1432-1033.1974.tb03828.x. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Detection of an unstable RNA in chick fibroblasts after reduction of the UTP pool by glucosamine. Eur J Biochem. 1971 Dec;24(2):358–365. doi: 10.1111/j.1432-1033.1971.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Klenk H. D. Inhibition of glycosylation of the influenza virus hemagglutinin. J Virol. 1974 Nov;14(5):1023–1034. doi: 10.1128/jvi.14.5.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Burge B. W. Biosynthesis of the Sindbis virus carbohydrates. J Virol. 1973 Dec;12(6):1366–1374. doi: 10.1128/jvi.12.6.1366-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- WOODWARD G. E., HUDSON M. T. The effect of 2-desoxy-D-glucose on glycolysis and respiration of tumor and normal tissues. Cancer Res. 1954 Sep;14(8):599–605. [PubMed] [Google Scholar]
- ZIMMERMANN T., SCHAEFER W. Effect of p-fluorophenyl-alanine of fowl plague virus multiplication. Virology. 1960 Aug;11:676–698. doi: 10.1016/0042-6822(60)90114-8. [DOI] [PubMed] [Google Scholar]



