Abstract
Key host responses to the stress induced by environmental exposure to cigarette smoke (CS) are responsible for initiating pathogenic effects that may culminate in emphysema development. CS increases lung ceramides, sphingolipids involved in oxidative stress, structural alveolar cell apoptosis, and inhibition of apoptotic cell clearance by alveolar macrophages, leading to the development of emphysema-like pathology. RTP801, a hypoxia and oxidative stress sensor, is also increased by CS, and has been recently implicated in both apoptosis and inflammation. We investigated whether inductions of ceramide and RTP801 are mechanistically linked, and evaluated their relative importance in lung cell apoptosis and airspace enlargement in vivo. As reported, direct lung instillation of either RTP801 expression plasmid or ceramides in mice triggered alveolar cell apoptosis and oxidative stress. RTP801 overexpression up-regulated lung ceramide levels 2.6-fold. In turn, instillation of lung ceramides doubled the lung content of RTP801. Cell sorting after lung tissue dissociation into single-cell suspension showed that ceramide triggers both endothelial and epithelial cell apoptosis in vivo. Interestingly, mice lacking rtp801 were protected against ceramide-induced apoptosis of epithelial type II cells, but not type I or endothelial cells. Furthermore, rtp801-null mice were protected from ceramide-induced alveolar enlargement, and exhibited improved static lung compliance compared with wild-type mice. In conclusion, ceramide and RTP801 participate in alveolar cell apoptosis through a process of mutual up-regulation, which may result in self-amplification loops, leading to alveolar damage.
Keywords: emphysema, sphingolipids, apoptosis, cigarette smoke, stress response
Clinical Relevance
This report highlights the concept of self-amplifying injury in response to cigarette smoke exposure, the importance of alveolar type II cell apoptosis in this process, and the cell-specific apoptotic signaling of ceramide in vivo. Early interruption of such self-perpetuating stress responses may prevent emphysema development, or may allow proper repair mechanisms to prevail.
The abnormal enlargement of distal airspaces in the lungs, which occurs through the destruction of the alveolar walls, is the hallmark of emphysema, a disease included in the chronic obstructive pulmonary disease (COPD) spectrum. There is an increased need to identify molecular mechanisms that link the environmental stress induced by cigarette smoke (CS) and air pollution, which are the main causes of alveolar destruction and emphysema in COPD. COPD is a prevalent disease, which carries high morbidity and mortality rates. Although removal of the etiological agent, most commonly the exposure to CS, is an important intervention (1), the ongoing tissue destruction may progress even after CS cessation (2). There is ample evidence that mechanisms of amplification of lung injury lead to destruction of alveolar walls and persistence of inflammatory systemic and lung responses (3, 4). Ceramide and RTP801 have been identified as potential signaling relays that are engaged early by CS exposure, which may interact and therefore amplify alveolar wall injury in emphysema (5–7). Because both ceramide and RTP801 are involved in apoptosis, we sought to investigate if ceramide and RTP801 are mechanistically linked in vivo.
RTP801, also known as REDD1, is a stress response protein and signaling mediator stimulated by oxidative stress generated by CS, and triggers NF-κB–mediated inflammation in the lung as well as apoptosis of alveolar structural cells (7). The up-regulation of RTP801 was detrimental to the lung, because mice lacking RTP801 were protected against CS-induced emphysema, concomitant with an increase in trophic factors, including vascular endothelial growth factor (VEGF). The finding that lungs of patients with emphysema exhibit increased RTP801 expression (7) attracts the interest in RTP801 as a therapeutic molecular target. Previous mechanistic studies of RTP801 have been performed in the CS mouse model that recapitulates many aspects of the complex pathogenesis of this chronic lung disease. A further simplified model that replicates the paucity of VEGF signaling in the lung parenchyma of patients with emphysema and highlights the apoptotic destruction of lung alveoli is obtained by inhibition of the VEGF receptors (VEGFRs) (8). In this murine model, apoptosis-dependent emphysema develops 4 weeks after a single subcutaneous injection of the VEGFR inhibitor, SU5416 (6, 8). We previously measured robust up-regulation of ceramide production in the lungs of VEGFR-inhibited rats and mice via de novo synthesis during the first week after SU5416 injection, preceding airspace enlargement (6). The regulation of RTP801 by VEGF inhibition remains unknown.
Ceramides are signaling sphingolipids that are implicated in important cellular processes, such as apoptosis, growth arrest, or senescence, and that are also induced by various stresses, including oxidative stress associated with CS exposure. CS increases lung ceramides both in vivo and in cultured primary lung epithelial and endothelial cells and, in turn, increases in ceramide levels trigger alveolar cell apoptosis (5, 6, 9). We previously described direct intratracheal instillation of ceramides as a reductionistic model of their effects seen in the setting of CS-triggered lung injury (5, 6, 10). This approach eliminates the ceramide-independent effects caused by CS, while mimicking its induction of endogenous lung ceramides. In this model, exogenous bioactive ceramide is instilled directly into the lungs, where it up-regulates endogenous ceramides, leading to increased oxidative stress and apoptosis, resulting in airspace enlargement. These processes occur within days of ceramide instillation, and are associated with several key features seen in the chronic model of CS exposure, including macrophage influx and activation of matrix metalloproteinases (5, 6). Several outstanding questions remain, including whether the accumulation of endogenous ceramides in this model is compensated by downstream prosurvival sphingolipids, such as sphingosine-1 phosphate, and whether the noted airspace enlargement is associated with abnormalities in lung function. Furthermore, it is not known if in vivo lung epithelial and endothelial cells are equally susceptible to undergoing apoptosis in response to ceramide accumulation, and by which mechanism.
Based on recent evidence, RTP801 may represent one of the earliest stress responses to CS exposure that integrates oxidative stress with lung inflammation and apoptosis (7). Because both ceramide and RTP801 have been proposed as central amplifiers of alveolar wall destruction in emphysema, we investigated if they are mechanistically linked. We show that in vivo RTP801 and ceramide are mutually inducible, and that the up-regulation of RTP801 by ceramides is necessary for ceramide-induced apoptosis of lung epithelial cells, ultimately leading to altered lung function.
Materials and Methods
Chemicals and Reagents
All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise stated.
Animals
Animal studies were approved by the Institutional Animal Care and Use Committee at Indiana University. C57Bl/6 mice (female, 3 mo old) were used for RTP801 or ceramide C12:0 instillation and for VEGFR inhibition studies. Rtp801-null mice (C57Bl/6 × 129SvEv, females at least 3 mo old; generated by Lexicon, Inc. [The Woodlands, TX] for Quark Pharmaceutical, Inc. [Ness Ziona, Israel] as a for-fee service) or wild-type controls of similar genetic background (C57Bl/6 × 129SvEv; Taconic, Fremont, CA) were used for ceramide C16:0 instillation.
Intratracheal Instillation
Intratracheal instillation is detailed in the Materials and Methods in the online supplement. Ceramide C12:0, rtp801 expression plasmid DNA (50 μg), or their vehicles were administered with perfluorocarbon (15 μl) (5, 6), as previously described (11). Ceramide C16:0 (conjugated with polyethylene glycol 2,000) or vehicle were introduced directly into the trachea. Mice instilled with control vehicle were labeled as controls; mice that only underwent a sham delivery, without installation of either vehicle or active compound, were labeled as sham. Control and sham animals were of similar age and lot as mice that were instilled with the active compounds.
VEGFR Inhibition
VEGFR inhibition model of murine emphysema (6, 13) was obtained by injecting mice subcutaneously once with SU5416 (20 mg/kg; Calbiochem, Gibbstown, NJ), using ethanol (0.1%) as vehicle.
Inhibition of Ceramide Synthases
Inhibition of ceramide synthases in vivo was achieved by Fumonisin B1 (FB1) administration (1.1–2.2 mg/kg; 100 μl; once daily, intraperitoneal) (6).
Measurement of Lung Function
Measurement of lung function is detailed in the Materials and Methods in the online supplement.
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) fluid collection and cytospin procedures are detailed in the Materials and Methods in the online supplement.
Lung Tissue Harvesting
Lung tissue harvesting is detailed in the Materials and Methods in the online supplement. Left lung inflation was achieved under constant pressure (20 cm H2O). The right lung was snap frozen in liquid N2.
Lung tissue dissociation in single-cell suspension is detailed in the Materials and Methods in the online supplement.
Sphingolipid Measurement
Lung tissue was prepared as previously described (12) and the ceramide content was evaluated by diacylglycerol (DAG) kinase assay (13). In addition, ceramide species identification and quantification, as well as sphingosine-1 phosphate content, were determined by liquid chromatography/tandem mass spectrometry (12). Sphingolipid levels were normalized by lipid phosphorus (12).
Apoptosis
Apoptosis was measured as detailed in the Materials and Methods in the online supplement.
Detection of RTP801
Detection of RTP801 by immunohistochemistry was performed as detailed in the Materials and Methods in the online supplement using a primary antibody against RTP801 (1:200; 1 h; Proteintech, Chicago, IL). RTP801 expression was quantified on coded images using image analysis software, with integration of intensity (in pixels) and area (in pixels) of the positive staining, generating arbitrary units.
Western Blotting
Western blotting was performed as described in the Materials and Methods in the online supplement.
Lung Histology and Morphometry
Standardized lung inflation, fixation, and automated morphometric analysis on coded slides were performed as previously described (14) and detailed in the Materials and Methods in the online supplement, using image analysis software (15) that calculates mean linear intercepts (MLIs) and surface-to-volume ratios as measures of airspace size.
Statistical Analysis
Statistical analysis was performed using unpaired Student t test, ANOVA, or Kruskal-Wallis one-way ANOVA on ranks. Statistical difference was accepted at P less than 0.05 (see the online supplement).
Results
RTP801 Is Up-Regulated in the Lung in Response to VEGFR Blockade
As a first step in elucidating the cross-talk between RTP801, ceramide, and apoptosis, we documented that the oxidative stress–responsive RTP801 was up-regulated in the lungs during the first 7 days after VEGFR inhibition (Figure 1A) by Western blotting of lung tissues from the previously published experiment in which VEGFR inhibitor, SU5416, and fumonisin B1 were administered to mice (6). Interestingly, treatment of mice with the ceramide synthesis inhibitor, FB1, inhibited the VEGFR inhibitor–induced RTP801 protein expression, suggesting that ceramide synthesis may be upstream of RTP801 up-regulation in this model (Figure 1A). These data, together with previous work in the CS model of emphysema, indicate a possible interrelation between RTP801 and ceramide expression in the lung. To investigate if induction of RTP801 expression can lead to increased ceramide levels, we next overexpressed RTP801 in the lung.
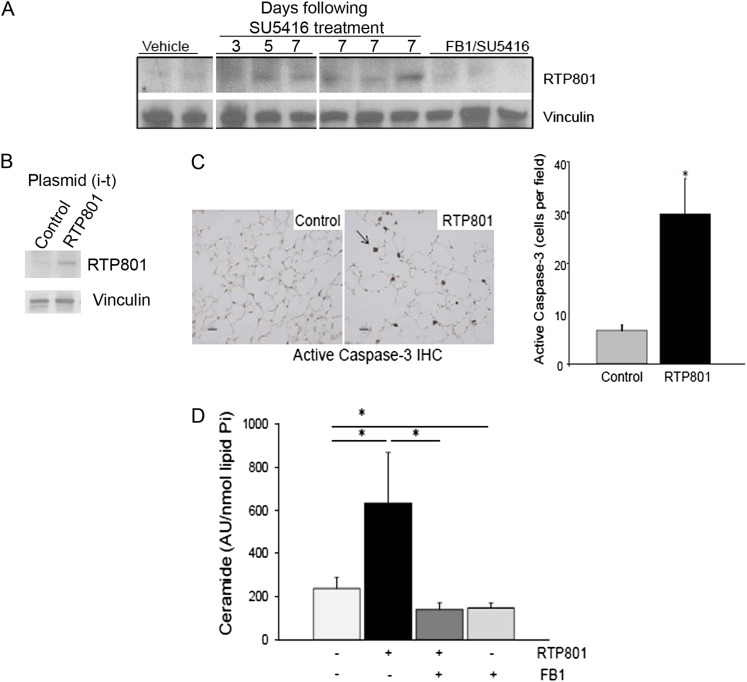
Figure 1.
Elevations of lung RTP801 are associated with increases in apoptosis and ceramide levels. (A) In a model of apoptosis-dependent emphysema by vascular growth factor receptor inhibition expression of RTP801 is increased in the lung tissue. RTP801 Western blotting in lung homogenates from individual mice treated with SU5416 (20 mg/kg, once, subcutaneously) and harvested after the indicated number of days, with vehicle control (Veh; once, subcutaneously, lungs harvested at Day 7), or with SU5416 and ceramide synthase inhibitor, Fumonisin B1 (FB1; 1.1 mg/kg, daily, intraperitoneally, lungs harvested at Day 7). Each lane represents a different mouse lung. Vinculin immunoblotting was used as a loading control. (B–E) Effects of intratracheal instillation (i-t) of RTP801-expressing plasmid (50 μg) in the lung. (B) Increased RTP801 protein measured in lung homogenates by Western blot after RTP801 instillation compared with control plasmid. (C) The left panel shows active caspase-3–positive cells detected by immunohistochemistry (IHC; brown; arrow) in fixed lung tissue from control animals after intratracheal instillation of empty plasmid (50 μg; lungs harvested after 3 d) or of RTP801-plasmid; scale bar, 10 μm. The right panel represents quantification of data from IHC. Values shown are means (+SEM); *P < 0.05 versus control; Student’s t test; n = 3 mice/group; 8–10 lung fields/mouse. (D) Total ceramide content of mouse lungs at 3 days after RTP801-plasmid instillation, with or without ceramide synthase inhibitor, FB1 (2.2 mg/kg), measured by diacylglycerol kinase assay and expressed as arbitrary units (from densitometric analysis of ceramide on thin layer chromatography plates) normalized by lipid inorganic phosphorus (lipid Pi). Values shown are means (+SEM); *P < 0.05; ANOVA; n = 3–5/group.
RTP801 Triggers Lung Apoptosis and Increases Lung Ceramide Levels
We overexpressed RTP801 in the lungs by intratracheal instillation of RTP801 cDNA-containing plasmid using empty plasmid as negative control (16). At Day 3 after administration, when compared with control mice that received empty plasmid, the lungs of mice instilled with RTP801-expressing plasmid and exhibiting increased RTP801 protein (Figure 1B) showed increased active caspase-3 in the lung parenchyma, as measured by immunohistochemistry (Figure 1C). In these mice, there was a marked increase in lung ceramides in response to RTP801 instillation, which was prevented by pretreatment with the ceramide synthase inhibitor FB1 (Figure 1D). These results suggested that RTP801 increases were sufficient to trigger ceramide synthesis.
Direct Augmentation of Ceramides in the Lung Increases Endogenous Ceramides and Causes Apoptosis, Airspace Enlargement, and RTP801 Up-Regulation
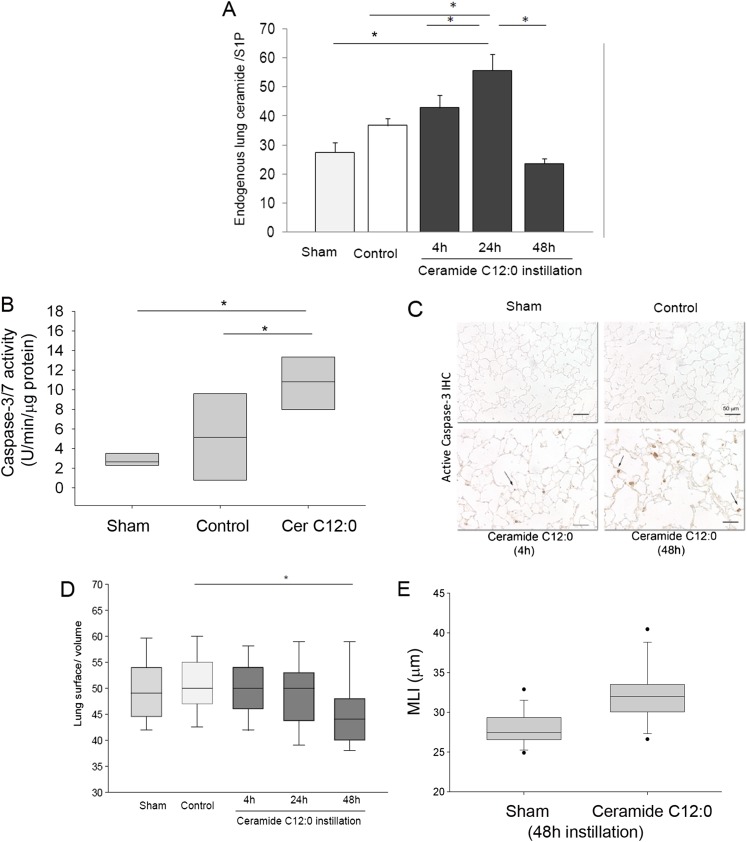
We have previously demonstrated that a single intratracheal instillation of ceramides with a 12-carbon fatty acid side chain (C12:0; 1 mg/kg), administered suspended in perfluorocarbon vehicle after solubilization in ethanol (6), causes overproduction of endogenous ceramides and lung apoptosis associated with an increase in alveolar size (5, 6). To further characterize this model, we instilled mice intratracheally with ceramide (C12:0; 1 mg/kg) or a with a control solvent (negative control) and compared them with sham-operated mice. To distinguish exogenous from endogenous ceramides, we used tandem mass spectrometry to identify and measure only endogenous sphingolipids with longer than 14-carbon fatty acid side chain. Because ceramide is the precursor of sphingosine-1 phosphate, an important prosurvival sphingolipid, we measured the ratio of endogenous ceramide to sphingosine-1 phosphate in the lungs after C12:0 ceramide instillation as a better reflection of a proapoptotic sphingolipid imbalance. The endogenous lung ceramide:S1P ratio increased at 4 hours and was highest at 24 hours after ceramide instillation, followed by a return to baseline levels at 48 hours (Figure 2A). We noted changes induced by vehicle instillation in some of the control mice that were reflected in lower median ceramide:S1P ratios. However, these changes were not significantly different from the sham group, and may have been related to perfluorocarbon itself or to the degree with which it prevented tissue hypoxia in individual mouse lungs. Ceramide induced activation of executioner caspases 3/7 as early as 4 hours that persisted for 48 hours after instillation (Figures 2B and 2C). As expected, ceramide instillation induced a decrease of alveolar surface area/lung volume and MLI, which became significant at 48 hours (Figures 2D–2E).
Figure 2.
Increases in lung ceramide content are associated with airspace enlargement, apoptosis, and RTP801 up-regulation. Effect of a single intratracheal instillation of ceramide C12:0 (5 mg/kg) in mice on (A) endogenous ceramides:sphingosine-1 phosphate (S1P) ratio in lung tissue homogenates at the indicated time in sham-operated mice (that underwent exposure of the trachea only), negative control mice (that were administered vehicle only), or mice that received exogenous C12:0. Values shown are means (+SEM); *P < 0.05*; ANOVA; n = 4–5. (B) Lung caspase-3/7 enzymatic activity was measured in whole-lung lysates of mice that were instilled with C12:0 (n = 7), in sham (n = 4), or control mice (n = 5). Box plot (box indicating the 25th and 75th percentile, with the middle line showing the median; * P < 0.05; ANOVA). (C) Lung active caspase-3 expression detected by IHC (brown, arrows) shown in representative micrographs (scale bar, 50 μm). (D and E) Lung surface:volume ratio and mean linear intercepts (MLIs) determined by standardized lung morphometry of alveolar spaces of mice after ceramide instillation time course and 48 hours, respectively. Box plot (box indicating the 25th and 75th percentile, with the middle line showing the median, and the 5th and 95th percentiles indicated by whiskers); *P < 0.05; ANOVA; n = 2 (Sham); n = 3–4/group; 8–10 lung fields/mouse.
Rtp801-Null Mice Are Protected from Ceramide-Induced Emphysema-Like Disease
To determine the link between ceramide and RTP801, we first investigated if ceramide increases are sufficient to elevate RTP801 expression in the lung. We administered, via a similar protocol of intratracheal instillation as C12:0 ceramide, a more abundant endogenous ceramide species, C16:0, using polyethylene glycol conjugation to achieve improved solubilization of this more hydrophobic molecule. Compared with control-instilled mice, the lungs of mice in which C16:0 was augmented had a significant 2-fold increase in RTP801 expression in the lung parenchyma (Figures 3A and 3B). These results suggest a mutual induction of ceramide and RTP801 in the lung.
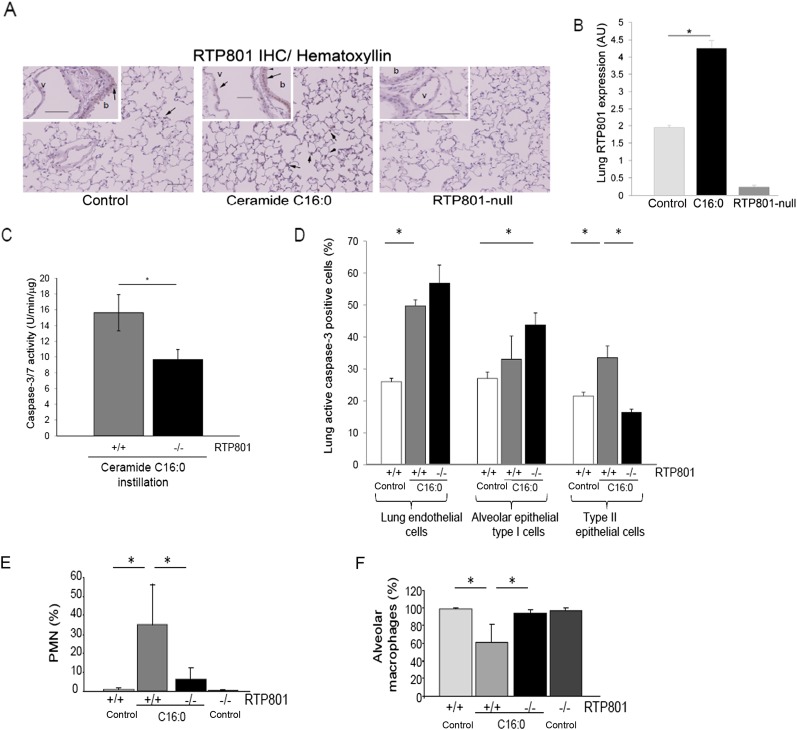
Figure 3.
Requirement for RTP801 in ceramide-induced apoptosis of type II epithelial cells and neutrophil infiltration. (A) RTP801expression detected by IHC in the lung sections of mice after C16:0 instillation compared with control animals or rtp801-null mice. Note an increase in RTP801 (brown, arrows) in the lung parenchyma and also (see insets) in the vascular (v) endothelium as well as bronchial (b) epithelium in ceramide-instilled mice. (B) RTP801 expression in the lung parenchyma was quantified via blinded image analysis software and expressed as arbitrary units (AU). Values shown are means (+SEM); *P < 0.05; Student’s t test; n = 4 mice, except for RTP801-null, which was n = 2; 8–10 lung fields/mouse. (C–F) Measurements in response to exogenous C 16:0 instillation (5 mg/kg; 48 h) or vehicle intratracheal instillation in wild-type or rtp801-null mice. (C) Caspase-3/7 activity levels in whole-lung homogenates normalized by protein concentration. Values shown are means (+SEM); *P < 0.05; Student’s t test; n = 2–4. (D) Cell-specific apoptosis expressed as relative abundance (%) of apoptotic active caspase-3/7–positive cells among lung endothelial (CD31-positive), and alveolar epithelial type I (podoplanin positive) and type II (pro–surfactant-C positive) cells in murine lung cell suspensions, detected by flow cytometry with fluorescence-labeled specific antibodies. Values shown are means (+SEM); *P < 0.05; n = 4–5. Note that only type II epithelial cells were protected against ceramide-induced apoptosis in rtp801-null mice. (E and F) Analysis of abundance (% of total cells) of polymorphonuclear (PMN) cells (E) and alveolar macrophages (F) counted in cytospins of the bronchoalveolar lavage (BAL) fluid in mice. Values shown are means (+SEM); *P < 0.05; n = 4–5.
We next asked if the RTP801 induction was necessary for the pathogenic effects of ceramide in the lung. We instilled ceramide C16:0 intratracheally in the lungs of rtp801-null mice, which we have previously characterized in detail (7). Compared with wild-type mice, rtp801-deficient mice had significantly less caspase-3/7 activity in whole-lung tissue homogenates after ceramide augmentation (Figure 3C). Because differential susceptibility in vivo of lung alveolar cells to ceramide is not known, we evaluated caspase-3/7 activation in distinct alveolar structural cells isolated from the lung at 48 hours after ceramide instillation. We enzymatically dissociated the lung tissue in single-cell suspensions and investigated the vulnerability to apoptosis of lung endothelial and epithelial type I and type II cells in both rtp801 knockout and wild-type mice. Alveolar cell-type markers (CD31, podoplanin, and prosurfactant-C) and active caspase-3 were detected using labeling with specific antibodies followed by flow cytometry. Ceramide instillation significantly increased apoptosis in both lung endothelial and epithelial type II cells in wild-type mice (Figure 3D). Interestingly, in rtp801-null mice, of the three cell types tested, only type II pneumocytes were protected against ceramide-induced apoptosis (Figure 3D). Given that RTP801 is largely up-regulated in type II cells (but not in endothelial cells) after exposure to CS in vivo (7), these findings demonstrate concordantly that RTP801 is necessary for ceramide to activate caspase-3 in alveolar type II epithelial cells.
To determine if other features of emphysema-like disease induced by ceramide are impacted by RTP801, we measured lung inflammation by counting inflammatory cells in the BAL fluid of mice. There was a relative increase in the polymorphonuclear cells (PMNs) after ceramide instillation in wild-type mice, which was significantly reduced in the rtp801-null mice (Figure 3E). As expected, alveolar macrophages exhibited reciprocal changes (Figure 3F).
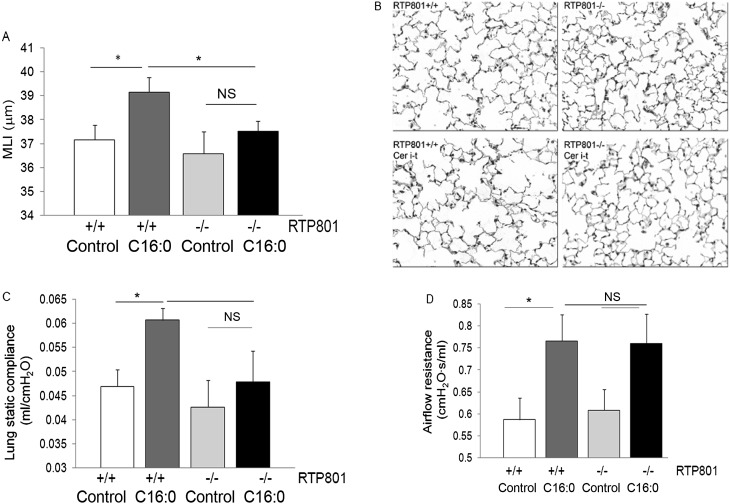
To measure the effect of ceramide 16:0 and the role of RTP801 on lung parenchyma morphology and function, after lung function testing, the left lung was inflated under constant pressure and airspace size was determined by morphometry. Compared with wild-type mice, in which ceramide augmentation increased airspace size, measured by MLI, from 37.1 to 39.1 μm (P = 0.02), the rtp801-null mice challenged with ceramide exhibited negligible changes in MLI, from 36.5 to 37.8 μm (P = 0.3; Figures 4A and 4B). Similarly, wild-type mice exhibited significant increases in lung static compliance (P = 0.01) in response to ceramide instillation, whereas rtp801-null mice did not (Figure 4C). Interestingly, ceramide instillation also elevated the airflow resistance in both wild-type and rtp801-null mice, although, in the rtp801-null mouse, the elevation induced by ceramide was not statistically significant compared with the wild-type mouse (Figure 4D).
Figure 4.
Requirement for RTP801 in ceramide-induced changes of lung alveolar morphology and function. (A–D) Measurements in response to exogenous ceramide 16:0 (5 mg/kg; 48 h) or vehicle (control) intratracheal instillation (i-t) in wild-type or rtp801-null mice. MLI (A) expressed as mean (+SEM); n = 5; 8–10 lung fields/mouse; *P < 0.05 or NS (not statistically significant); two-way ANOVA; measured by automated morphometry of lung parenchyma on paraffin-embedded lung sections stained with hematoxylin-eosin (B). (C) Lung compliance measured with Flexivent (Scireq, Montreal, PQ, Canada) in anesthetized mice. Values shown are means (+SEM); n = 4 (wild-type) or 5 (rtp801-null); *P < 0.05; two-way ANOVA. (D) Airflow resistance measured with Flexivent in anesthetized mice. * P < 0.05; two-way ANOVA; n = 5.
Discussion
This report brings to light an important novel interaction of two mediators of alveolar injury, ceramide and RTP801, which have been shown to lead to airspace destruction in emphysema. Using in vivo gain-of-function approaches, we have shown that ceramide augmentation induces apoptosis of both lung endothelial cells and type II epithelial cells, and is associated with infiltration of PMNs and enlargement of airspaces accompanied by an increase in static compliance, suggesting an emphysema phenotype. These phenotypes were inhibited in mice lacking rtp801, which showed lack of apoptosis of epithelial type II cells in response to ceramide. These results causally implicate RTP801 as an upstream sensor of lung cellular stresses in lung epithelial cells. Although alveolar destruction also involves capillary endothelial cells, our data position type II cells as key in the amplification of the alveolar destruction mediated by the interaction of ceramide and RTP801. Interestingly, type I cells were not only unprotected from apoptosis in the RTP801 null mouse, but they exhibited heightened caspase-3 activation in response to ceramides. This may suggest a possible cross-talk between stress-protected alveolar type II cells, which are considered precursors of type I cells, and the type I cells, which are bona fide structural components of the alveolocapillary membrane. It is possible that rescued type II cells may replace dying type I cells at a rate sufficient to preserve airspace size, and/or that RTP801 has opposing roles in the two cell types, being proapoptotic in type II cells, but antiapoptotic in type I cells. In addition, our results do not rule out that other, yet-untested cell types in the lung undergo RTP801-dependent apoptosis in response to ceramide.
A common denominator linking ceramide and RTP801 up-regulation could be oxidative stress, a known inducer of both molecules, which is also generated downstream of both ceramide and RTP801 up-regulation (5–7, 16). Oxidative stress caused directly by CS or endogenous sources, such as inflammatory and parenchymal cells, plays a central unifying role in all stages of emphysema. Reduction in antioxidant defenses in nuclear receptor factor (erythroid-derived 2)-like 2 (nrf2) knockout mice leads to increased susceptibility to emphysema, which is characterized by alveolar cell death (17). RTP801, a negative regulator of mammalian target of rapamycin signaling, is therefore activated as part of a prototypic response to adverse cell stress responses. However, its activation promotes cellular injuries triggered by CS, including further oxidative stress, cell death, and alveolar inflammation. Excessive reactive oxygen species generation could also have been responsible for RTP801 increases in response to VEGFR inhibition (15). In addition, we have shown that ceramide-induced airspace enlargement is dependent on oxidative stress (5), which may have also up-regulated RTP801 in response to intratracheal ceramide administration.
Ceramide is a prototypic lipid signaling molecule, which we have shown to amplify lung injury due to CS (5, 6, 9, 10, 18). Our prior studies have also highlighted that ceramide not only amplifies oxidative stress, but that its synthesis is triggered by reactive oxygen species (5). We present novel evidence that RTP801 is not only sufficient, but also necessary, to mediate increases in lung ceramide via the de novo pathway of ceramide synthesis. These data position RTP801 in type II cells upstream of ceramide synthesis in vivo in settings that reproduce features of CS-induced alveolar injury. Whether ceramide mediates all or part of RTP801-dependent pathogenesis due to CS will await further experimentation in mice deficient in ceramide synthetic enzymes.
The significance of increased airflow resistance in response to ceramide elevation in wild-type mice is yet to be clarified. This airway response was associated with increased RTP801 expression in the larger airways as well as airway inflammation, as suggested by elevated levels of inflammatory cells (PMNs) in the BAL fluid of wild-type animals, which was largely RTP801 dependent. The mechanism by which RTP801 triggers large airway inflammation remains unknown.
With the initial discovery of the role of RTP801 and mammalian target of rapamycin signaling in CS-induced emphysema, we proposed that RTP801 functions as a sensor mechanism, integrating environmental stress responses with lung injury. The fact that VEGFR inhibition also triggers RTP801 expression further expands this concept, in that alterations in alveolar maintenance, such as those caused by interruption of VEGF survival signals, may also be interpreted as part of a lung cellular stress response. CS has been shown to decrease VEGF signaling in the alveolar lung tissue, and VEGFR blockade is sufficient to up-regulate ceramides, induce oxidative stress, and trigger alveolar cell apoptosis, events that precede the onset of airspace enlargement (6, 8, 15, 19). Furthermore, as increases in lung ceramide activate RTP801 expression; endogenous lung stress responses may perpetuate this pathogenic loop. It will be important to elucidate whether lung injury is more severe when these responses become repetitive, possibly exhausting repair mechanisms.
In conclusion, this report further highlights the concept of self-amplifying injury in response to CS exposure, the importance of alveolar type II cell apoptosis in this process, and the cell-specific apoptotic signaling of ceramide in vivo. Early interruption of such self-perpetuating stress responses may prevent emphysema development, or may allow proper repair mechanisms to prevail.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants R01 HL077328 (I.P.), R01 ES01628 (I.P.), and R21 DA029249 (I.P.), and in part by a grant from Quark Pharmaceuticals Inc.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0254OC on September 28, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Godtfredsen NS, Lam TH, Hansel TT, Leon ME, Gray N, Dresler C, Burns DM, Prescott E, Vestbo J. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J 2008;32:844–853 [DOI] [PubMed] [Google Scholar]
- 2.Miller M, Cho JY, Pham A, Friedman PJ, Ramsdell J, Broide DH. Persistent airway inflammation and emphysema progression on CT scan in ex-smokers observed for 4 years. Chest 2011;139:1380–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med 2001;164:469–473 [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 2003;28:551–554 [DOI] [PubMed] [Google Scholar]
- 5.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, et al. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 2008;295:L44–L53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, Gandjeva A, Zhen L, Chukwueke U, Mao T, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke–induced pulmonary injury and emphysema. Nat Med 2010;16:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medler TR, Petrusca DN, Lee PJ, Hubbard WC, Berdyshev EV, Skirball J, Kamocki K, Schuchman E, Tuder RM, Petrache I. Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am J Respir Cell Mol Biol 2008;38:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrusca DN, Gu Y, Adamowicz JJ, Rush NI, Hubbard WC, Smith PA, Berdyshev EV, Birukov KG, Lee CH, Tuder RM, et al. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem 2010;285:40322–40332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss DJ, Strandjord TP, Liggitt D, Clark JG. Perflubron enhances adenovirus-mediated gene expression in lungs of transgenic mice with chronic alveolar filling. Hum Gene Ther 1999;10:2287–2293 [DOI] [PubMed] [Google Scholar]
- 12.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, Schweitzer KS, Skobeleva A, Rajashekhar G, Hubbard WC, et al. Stimulation of sphingosine 1 phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med 2010;181:344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry DK, Bielawska A, Hannun YA. Quantitative determination of ceramide using diglyceride kinase. Methods Enzymol 2000;312:22–31 [DOI] [PubMed] [Google Scholar]
- 14.Clauss M, Voswinckel R, Rajashekhar G, Sigua NL, Fehrenbach H, Rush N, Schweitzer K, Yildirim AÖ, Kamocki K, Fisher AJ, et al. Lung endothelial monocyte-activating protein 2 is a mediator of cigarette smoke–induced emphysema in mice. J Clin Invest 2011;121:2470–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97 [DOI] [PubMed] [Google Scholar]
- 16.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, et al. Identification of a novel hypoxia-inducible factor 1–responsive gene, RTP801, involved in apoptosis. Mol Cell Biol 2002;22:2283–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of NRF2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J Clin Invest 2004;114:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweitzer KS, Hatoum H, Brown MB, Gupta M, Justice MJ, Beteck B, Van Demark M, Gu Y, Presson RG, Jr, Hubbard WC, et al. Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: role of oxidative stress and ceramides. Am J Physiol Lung Cell Mol Physiol 2011;301:L836–L846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuder RM, Wood K, Taraseviciene L, Flores SC, Voekel NF. Cigarette smoke extract decreases the expression of vascular endothelial growth factor by cultured cells and triggers apoptosis of pulmonary endothelial cells. Chest 2000;117:241S–242S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.