Abstract
Multiple cells contribute to the function of lungs. Pulmonary neuroendocrine cells (PNECs) are important for the regulation of breathing and carcinogenesis, although they represent only a small population of the airway lining. Achaete–Scute homologue–1 (Ascl1), a proneural basic helix–loop–helix transcription factor, is critical for the development of PNECs. We postulated that Ascl1-defined cells (ASDCs) may be progenitors, and traced their fate during development and injury repair. R26R-stop-lacZ (Rosa) reporter mice were crossed with Ascl1-Cre or Ascl1-CreERTM mice, in which the Ascl1 promoter drives the expression of Cre or inducible Cre recombinase, respectively. ASDCs and their descendants will be permanently labeled. The labeled cells were characterized by immunohistochemistry, using highly specific differentiation markers. Lineage studies revealed a population that proliferates before the pseudoglandular stage, and widely contributes to different compartments. When ASDCs were labeled on Embryonic Day 9.5, they gave rise to both airway and alveolar cells, but when labeled on Embryonic Day 11.5, they only gave rise to airway cells. In postnatal naphthalene injury, ASDCs contributed to regenerating Clara cells. In conclusion, Ascl1-defined cells in the lung represent a novel multipotent lineage, indicating a close relationship of neuroendocrine cells with other cell types.
Keywords: Ascl1, lung, neuroendocrine, mouse, progenitor
Clinical Relevance
This study uncovered a new set of progenitors during lung development and injury repair. These findings may have implications for the development of several pulmonary lineages, the interaction of epithelial and mesenchymal compartments, tissue regeneration, and the pathogenesis of pulmonary diseases, including cancer.
The murine respiratory system arises from the ventral foregut endoderm at around Embryonic Day (E) 9.5. The lung-bud epithelium invades the adjacent mesenchyme and starts branching to form a tree-like structure. The epithelium begins to differentiate during the pseudoglandular stage (E9.5–E16.5) (1). Multiple cell types contribute to both the structure and function of the lung. In the alveoli, Type II pneumocytes (AT2 cells) secrete surfactant, whereas Type I cells are responsible for gas exchange. In the airways, nonciliated secretory (Clara) cells have metabolic, anti-inflammatory, and anti-neoplastic properties, whereas ciliated cells eliminate excess mucus and harmful particles. In addition, the airway lining contains rare but important pulmonary neuroendocrine cells (PNECs) that may regulate breathing and contribute to carcinogenesis (2–4). PNECs occur as either solitary cells or clusters called neuroepithelial bodies (NEBs) (5). In developing lungs, PNECs begin to differentiate before other cells, but their histogenesis has remained elusive.
Lung development is a complex process that requires mutual interactions between the mesoderm-derived mesenchyme and endoderm-derived epithelium. Both in vivo and in organ culture, a previous study demonstrated the early pseudoglandular stage of the lung as a dynamic structure, with smooth muscle and neural tissue in a prime position to influence growth and development (6). During embryonic lung development, intrinsic nerve ganglia function to innervate the airway smooth muscle (7). NEBs are extensively innervated, and may act as sensory airway receptors (8).
During injury repair, both Clara and AT2 cells are facultative progenitors that give rise to other pulmonary epithelial cells (9). On the other hand, it appears that PNECs may renew themselves but do not contribute to epithelial cells in experimental models that cause Clara cell injury (10). Although PNECs are very rare in normal adult lungs, hyperplasias emerge during many human inflammatory processes, as well as in animal models (11–15). For instance, PNECs may be involved, directly or indirectly, in the pathogenesis of cystic fibrosis (16) and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia in adults (17–19), and in neuroendocrine cell hyperplasia of infancy (20). The role of PNECs in the development, repair, regeneration, and pathophysiology of lung tissue remains poorly understood.
Transcription factors derived from both the endoderm and mesoderm play important roles in orchestrating lung development and repair (1, 21). Achaete–Scute homologue–1 (Ascl1), a proneural, basic helix–loop–helix transcription (bHLH) factor, is critical for the development of PNECs in the lung (22). Ascl1-deficient mice are defective in the differentiation of their autonomic neurons, olfactory bulb, retinal epithelium, and PNECs (22–25). Despite the total absence of PNECs, fetal lung development and differentiation are unremarkable, although Ascl1 mutant mice die shortly after birth. One of the pathways involved in determining the cell differentiation fate in airway epithelium may be the Notch/Notch-ligand “lateral inhibition” system, associated with Ascl1 and other bHLH factors (26, 27). Moreover, the expression of ASCL1 in human neuroendocrine cancer cells promotes “stemness” (28). However, limited data are available on the fate of Ascl1-defined cells (ASDCs) in the lung tissue. Understanding the lineages of ASDCs may provide strategies for the treatment of pulmonary diseases, other genetic disorders, and carcinogenesis affecting the normal function of the lung.
In this study, we aimed to identify ASDC lineages during lung development and tissue repair by crossing mice expressing Ascl-Cre or the tamoxifen (TM)–inducible Cre recombinase with R26R-stop-lacZ (Rosa) reporter mice. This approach has been successfully used to trace cells in the central nervous system (29). We show that the Ascl1 lineage in the lung includes airway lining (Clara cells, ciliated cells, and PNECs) and alveolar (AT2) epithelial cells, as well as neuronal ganglion and occasional smooth muscle cells. In conclusion, Ascl1-expressing progenitors can give rise to descendents from all three germ layers in the murine embryonic lung. Moreover, in the adult lung, Ascl1-defined progenitors can contribute to the injury repair of the airway by regenerating Clara cells.
Materials and Methods
Transgenic Mice and Tissue Collection
Ascl1-Cre mice, Ascl1-CreERTM transgenic mice, and R26-stop-lacZ or R26-stop-YFP reporter mice have been described previously (29, 30). R26-stop-lacZ and R26-stop-YFP mice are Cre recombinase reporter mice purchased from the Jackson Laboratory (Bar Harbor, ME) (31, 32). All animals were housed and handled according to a protocol approved by the Animal Care and Use Committee of the National Cancer Institute.
Mice were mated overnight, and the day of the discovery of the vaginal plug was counted as E0.5. The lung tissues from fetal (E12.5, E14.5, and E17.5) and adult (postnatal P; P ≥ 30) reporter mice were used for 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) staining and immunostaining. At least three lungs of each genotype from two litters were examined in all experiments. Transgenic adult and fetal mice were identified by PCR analysis using tail or yolk-sac DNA, and were genotyped using published primers (30, 31). The littermates without Cre or wild-type mice served as controls.
Tamoxifen Administration
The TM induction of Cre recombinase was accomplished by an intraperitoneal injection of TM (Sigma-Aldrich, St. Louis, MO) dissolved in Mazola corn oil (ACH Food Companies, Inc., Memphis, TN) to reporter mice. R26-stop-lacZ females were mated with Ascl1-CreERTM males. Pregnant females received single dose of 0.1 mg TM per gram body weight. Adult mice (4–8 weeks old) received four doses of 0.25 mg TM per gram body weight, one dose every other day.
X-Gal Staining, Immunohistochemistry, and Immunofluorescence
X-Gal staining is described in the online supplement.
Immunohistochemistry (IHC) was performed using the avidin-biotinylated peroxidase method (33), with specific antibodies (Table E1 in the online supplement). The results were analyzed with a Nikon Eclipse 400 microscope (Nikon Instruments, Inc., Lewisville, TX) and Metamorph software (Molecular Devices Corporation, Downingtown, PA). For immunofluorescence, frozen sections mounted on slides were incubated in the appropriate dilution of primary antibody in PBS, followed by the appropriate secondary antibody conjugated with Alexa Fluor 488 or 594 (Molecular Probes, Eugene, OR). Fluorescence imaging was performed on a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY). For each experiment, multiple sections from at least three different animals of each genotype were analyzed.
Naphthalene Treatment
Naphthalene (Sigma, St. Louis, MO) was dissolved to a concentration of 27.5 mg/ml in Mazola corn oil, and a single intraperitoneal injection (10 ml/kg of body weight) was administered to 2-month-old Ascl1-Cre;Rosa26R-lacZ mice or TM-induced Ascl1-CreERTM;Rosa26R-lacZ mice. Analogously, control mice received corn oil alone. After naphthalene administration, the mice were killed after 1, 2, or 5 days. The lungs were fixed in 4% paraformaldehyde for whole-mount X-Gal staining or paraffin sectioning.
Statistical Analyses
Statistical analyses were performed using SigmaStat version 3.5 software (Aspire Software International, Ashburn, VA). The Mann-Whitney rank-sum test and Student t test were used for analyses, and a P value of 0.05 or less was considered significant.
Results
Expression of Ascl1 Preceded Neuroendocrine and Epithelial Cell Differentiation in Embryonic Lungs
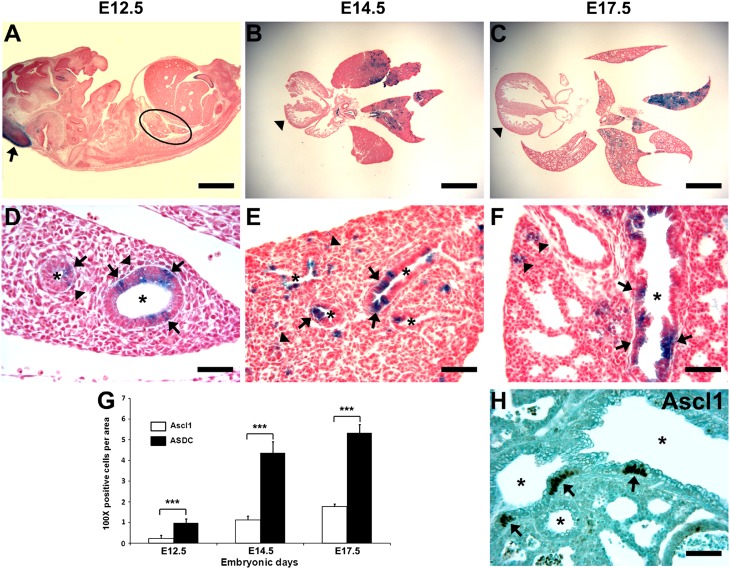
To validate that the lungs of transgenic mice used in the study were normal, we examined their histology and performed IHC, using lung sections from Ascl1-Cre;Rosa26R-lacZ mice, Ascl1-CreERTM;Rosa26R-lacZ mice, and their Cre-negative littermates at different developmental stages. Grossly and histologically, the transgenic murine lungs were unremarkable and, as expected, Ascl1-positive PNECs were the first to appear in the embryonic lung (Figures 1G, 1H and E1A in the online supplement). On E12.5, numerous, solitary Ascl1-immunoreactive cells were detected in proximal airway structures lined by layers of smooth muscle, whereas the (neuroendocrine) (NE) marker protein gene product 9.5 (PGP9.5) was expressed at low concentrations in all airway lining cells (Figures E1A and E1B). Interestingly, Ascl1 was also expressed in occasional neuronal ganglion cells intensely labeled by PGP9.5, located around the large airways (Figures E1A and E1B). By E14.5, Ascl1 and PGP9.5 were found in solitary PNECs as well as in NEBs (Figures E1D and E1E), whereas the NE marker CGRP was still negative (Figure E1F). By E17.5, many NEBs were positive for all three NE markers Ascl1, PGP9.5, and calcitonin gene-related peptide (CGRP) (Figures E1J–E1L). At later points, the NE foci were also significantly more numerous than on E12.5 (Figure 1G, P < 0.001).
Figure 1.
Distribution of Achaete–Scute homologue–1 (Ascl1) expression and of Ascl1-defined cells (ASDCs) in developing lung tissue. (A–F) Photomicrographs of ASDCs on Embryonic Day (E) 12.5, E14.5, and E17.5 during development from Ascl1-Cre;Rosa26R-lacZ mice. (A) E12.5. Whole embryo with blue in the developing midbrain (arrow). No blue cells are visible in the lung at this magnification (black ellipse). (B and C) Low-power view of lungs, with variable intensities of blue in different lobes. Heart is negative (arrowheads). (D and E) Epithelial staining along airway lining (arrows) and in parenchyma (arrowheads). (F) On E17.5, blue ASDCs are evident in the airway epithelium (arrows) and in the area of the alveolar sacs of the peripheral lung (arrowheads). X-Gal histochemical staining was performed with Nuclear Red. Asterisk indicates airway lumen. Magnification bars = 1,000 μm in A–C, and 50 μm in D–F. (G) Bar graph of Ascl1-positive pulmonary neuroendocrine cells (PNECs) and ASDCs in the developing lung (mean ± SE). The data reflect 3 mice/genotype. ***P < 0.001. (H) Ascl1 expression in lung on E17.5. Three strongly positive neuroepithelial bodies (NEBs; arrows) are evident in developing bronchioli, which are indicated by asterisks. Immunoperoxidase staining, bar = 50 μm.
Like PNECs, the non-NE epithelial cells underwent a maturation process. On E12.5, epithelial differentiation markers such as Clara cell-specific 10 kD protein (CC10), forkhead box J1 (FoxJ1), and surfactant protein C (SPC) were negative. On E14.5, immunoreactivity for the AT2 marker pro-SPC was localized in acinar tubules and buds that would later give rise to alveolar sacs (Figure E1I), whereas the early ciliated cell marker FoxJ1 was expressed more proximally (Figure E2A), as expected (34). By E17.5, the epithelial markers β-tubulin for ciliated cells and CC10 for Clara cells were detected in airways and pro-SPC was seen in alveolar sacs, consistent with the distribution in mature and normal adult lungs (Figures E1M–E1O). Throughout embryonic development as well as in adult lungs, the IHC results for transgenic mice were comparable to those from their Cre-negative littermates and wild-type mice. Therefore, transgenic strains will provide a good model to study cell lineages during lung development.
Cells Derived from the Ascl1 Lineage Become Numerous and Widely Disseminated in the Lungs during Development
To trace the lineage of ASDCs in the developing lung, we crossed Ascl1-Cre mice with Rosa26R-stop-lacZ or Rosa26R-stop-YFP reporter mice. After Cre recombination, any cells derived from the Ascl1 lineage were permanently labeled. At any given stage, β-Gal–positive or yellow fluorescent protein (YFP)-positive cells in the mice constituted an accumulative representation of ASDCs up to that stage. Ascl1-Cre;Rosa26R-stop-lacZ murine lungs were harvested at pseudoglandular (E12.5 and E14.5) and canalicular (E17.5) stages. X-Gal–stained sections revealed that ASDCs contributed to both airway and nonairway compartments during development (Figure 1). On E12.5, ASDCs mainly occupied distal airway epithelium (Figures 1A and 1D), whereas Ascl1-positive PNECs were located in the proximal airways (Figure E1A). The number of ASDCs was markedly increased during lung development (Figures 1A–1G). At all stages, a small population of ASDCs was also detected outside the airways in the mesenchymal compartment (Figures 1D–1F). The quantification of Ascl1-positive PNECs and ASDCs lining the developing airways at different stages confirmed that ASDCs were not limited to PNECs (Figure 1G), but were more abundant. The distribution of X-Gal–positive cells suggested that ASDCs gave rise to a variety of cells in all three germ cell layers.
To explore the differentiation potential of ASDCs during development, we performed IHC on X-Gal–stained sections, using the cell type–specific markers validated earlier (Figure E1). During the embryonic period on E12.5, ASDCs included Ascl1-positive solitary PNECs in the lining of proximal airways (Figure 2A), Ascl1, and neuronal marker PGP9.5–positive neuronal cells in ganglia (Figures 2A and 2B) located outside the peribronchiolar smooth muscle. Smooth muscle that is immunoreactive for α–smooth muscle actin (α-SMA) (Figure 2C) surrounds the proximal airways and developing vessels. A number of X-Gal–positive cells appear in the undifferentiated airway lining, which is positive for PGP9.5 (Figure 2B), or in the peribronchiolar (Figure 2C) and vascular smooth muscle (Figure E2C). The peripheral parenchyma is negative for the blue X-Gal–positive cells. Interestingly, the three dually positive cell types we were able to identify are derivatives from different germ layers, indicating that ASDCs may be multipotent progenitors that emerge at a very early embryonic stage.
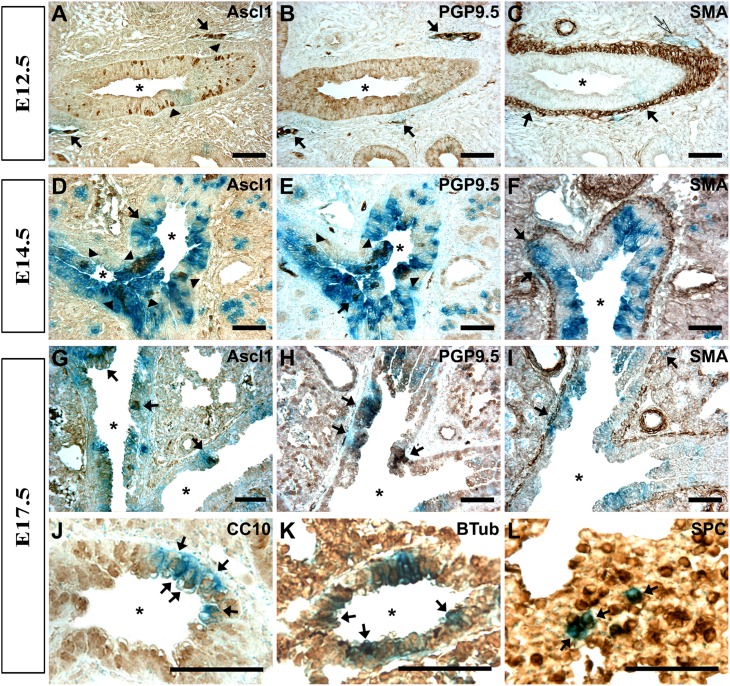
Figure 2.
Ascl1-defined progenitors contribute to both airway and nonairway compartments during lung development. Photomicrographs of colabeling in the lung by X-Gal (histochemical stain; blue) and immunohistochemistry (immunoperoxidase stain; brown). (A–C) E12.5 lung with lineage-labeled (blue) cells include Ascl1-positive brown cells (A, arrowheads) in the airways and ganglia (A, arrows), PGP9.5-positive ganglion cells (B, solid arrows; C, open arrow), and α–smooth muscle actin (α-SMA)–positive cells in the airway smooth muscle layer (C, arrows). In addition, α-SMA–positive cells are also associated with vascular smooth muscle (S2C, arrows). (D–F) In E14.5 lung, lineage-labeled cells include NEBs (arrowheads) and solitary PNECs (arrows) expressing Ascl1 (D), PGP9.5 (E), and smooth muscle cells expressing α-SMA (F). (G–L) In E17.5 lung, lineage-labeled cells include PNECs that express Ascl1 (G) or PGP9.5 (H), and α-SMA–positive smooth muscle cells (I), CC10-positive Clara cells (J), β-tubulin (BTub)–positive ciliated cells (K), and alveolar Type II (AT2) cells (L). SMA, smooth muscle antigen. Asterisks indicate airway lumen. Bars = 50 μm.
In the midpseudoglandular stage on E14.5, ASDCs contributed to both solitary PNECs and NEBs (Figures 2D and 2E), which were defined by Ascl1 and PGP9.5. In addition, ASDCs were found among FoxJ1-positive ciliated precursor cells (Figure E2B), Ascl1, and PGP9.5 (Figure 2E)–positive ganglia and α-SMA–expressing smooth muscle cells (Figure 2F). Overall on E14.5, the number of ASDCs had greatly increased, and the majority of them appeared undifferentiated or negative for the differentiation markers in both the airway lining and parenchyma.
During the canalicular period on E17.5, ASDCs still included Ascl1-positive and PGP9.5-positive NEBs, PGP9.5-positive neuronal cells/nerves (Figures 2G and 2H), and α-SMA–positive smooth muscle cells (Figure 2I). Notably, a number of ASDCs were also positive for CC10 in the airway lining Clara cells (Figure 2J), for β-tubulin in ciliated cells (Figure 2K), and for SPC in AT2 cells (Figure 2L) located in the alveolar compartment. Taken together, current data suggest that ASDCs give rise to a variety of cells in both airway and nonairway compartments during lung development.
Stage-Specific Fate of the Ascl1 Lineage in the Developing Lung
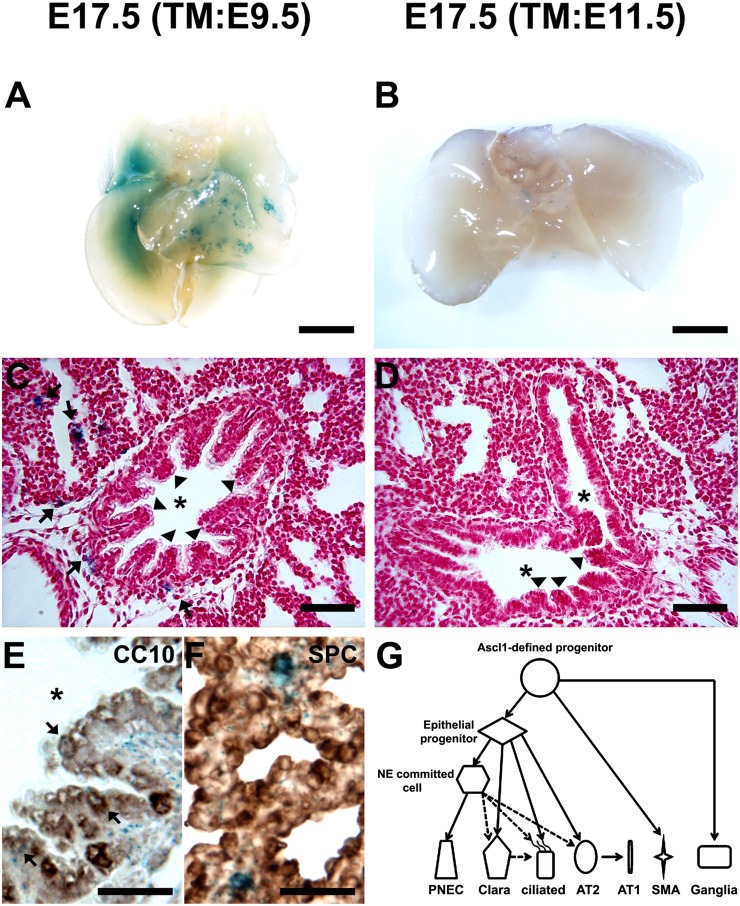
To investigate the fate of ASDCs at specific developmental stages, we used an inducible Cre recombinase transgenic strain, Ascl1-CreERTM crossed with Rosa26R reporter mice. In this model, Cre recombination will be detectable within 6 hours after TM injection, and will continue for nearly 24 hours (35). Hence, only cells expressing Ascl1 at this time period (and their descendants) will be labeled. This approach allowed us to temporally and spatially map the fate of ASDCs and their descendants as they matured. TM was injected into pregnant female mice at pseudoglandular stage E9.5 or E11.5. When the lungs were dissected during the canalicular stage on E17.5 and stained with X-Gal, more cells were labeled after the early TM injection (Figures 3A–3D). X-Gal staining results showed that the descendents of the E9.5-induced ASDCs contributed to both the airway and nonairway compartments (Figure 3C). A portion of Clara cells (Figure 3E) and AT2 cells (Figure 3F) were labeled. In contrast, the X-Gal staining of descendents from E11.5-induced ASDCs were limited only to a few cells in the airways (Figure 3D). Considering these results together with the Ascl1 lineage cells identified using Ascl1-Cre;Rosa26R-stop-lacZ embryonic lungs (Figure 2), we propose a model for Ascl1 lineage in the developing lung (Figure 3G). The Ascl1-expressing population before the pseudoglandular stage (E9.5–E16.5) can give rise to all airway cell types (PNECs, Clara, and ciliated) and alveolar AT2 cells. In addition, Ascl1-defined progenitors also give rise to neuronal cells that may develop into intrapulmonary ganglia, as well as occasional peribronchiolar smooth muscle cells.
Figure 3.
Temporal-specific fate maps of Ascl1 lineage in developing lung, and schematic summary of Ascl1 lineage. (A and B) Whole-mount X-Gal staining of Ascl1-CreERTM;Rosa26R-lacZ E17.5 lungs treated with tamoxifen (TM) at indicated embryonic stages. Bar = 1,000 μm. (C) Photomicrograph of TM injection on E9.5 labeled the cells in both airway (arrowheads) and nonairway (arrows) compartments. Bar = 50 μm. (D) The labeled cells in the nonairway compartment were dramatically decreased when TM was injected on E11.5. Bar = 50 μm. Colabeling with immunohistochemistry (IHC) showed the X-Gal–labeled (blue) cells with (E) CC10-positive Clara cells (brown; bar = 25 μm) and (F) SPC-positive AT2 cells (brown). Arrows point to the best double-positive cells. Bar = 25 μm. Asterisks indicate airway lumen. (G) Schematic of Ascl1 lineage in the developing murine lung. During embryonic lung development, Ascl1-defined progenitors gave rise to PNECs, Clara cells, ciliated cells, and AT2 cells, as well as smooth muscle cells and ganglia.
Response of Ascl1-Expressing Cells to Acute Injury in Adult Lungs
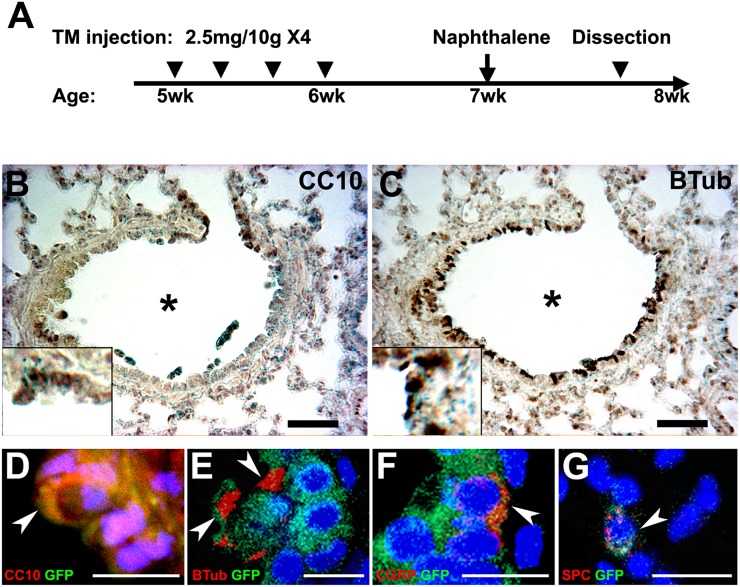
Ascl1 is critical for the development and differentiation of PNECs (26, 36). During development, we saw a marked expansion of ASDCs beyond PNECs in the airway epithelium. To study whether Ascl1-expressing cells are multipotent in the adult lung, we used the naphthalene injury repair model. In this model, naphthalene kills Clara cells, which is followed by a rapid reconstruction of the airway lining. Stage-specific inducible Ascl1-CreERTM;Rosa26R-stop-YFP mice received TM, followed by naphthalene (Figure 4A). Lungs were harvested 5 days later to monitor the repair. As shown by double-labeling in Figures 4B–4G and E3, adult Ascl1-expressing cells that were labeled before exposure gave rise not only to PNECs (Figures 4F and E3B and E3C), but also to regenerating Clara cells (Figures 4B and 4D and E3B and E3C) and ciliated cells (Figures 4C and 4E and E3B and E3C) in the airways. Notably, a subgroup of AT2 cells in the alveolar compartment was also labeled (Figure 4G), although they are generally thought not to be directly involved in acute naphthalene injury repair. As previously described, the regeneration of airway lining initially occurs in small groups or patches (44). Groups of Clara cells were located at bifurcations and distal terminal bronchioles (Figures 4B and E3B). The data suggest that adult ASDCs participate in injury repair. Moreover, even PNECs may be able to give rise to epithelial cells in response to injury.
Figure 4.
Lineage-tracing of ASDCs in adult lungs during injury repair. (A) Schematic of injection schedules. Four TM injections of 2.5 mg/g body weight were administered every other day to Ascl1-CreERTM;Rosa26R-YFP mice. Seven days after the final TM injection, mice were treated with naphthalene, and were killed 5 days after naphthalene treatment. wk, weeks. (B and C) Photomicrographs of colabeling with X-Gal and immunoperoxidase staining in the lung, 5 days after naphthalene treatment, in regenerating CC10-positive Clara cells (brown) (B) and β-tubulin–positive ciliated cells (C). Bar = 50 μm. Insets show higher-magnification views. (D–G) Immunofluorescence micrographs at the same time points show colocalization (in orange/yellow) of Ascl1 lineage GFP (green) with (D) CC10 (red) in Clara cells, (E) β-tubulin (red) in ciliated cells, (F) CGRP (red) in PNECs, and (G) SPC (red) in AT2 cells. GFP, green fluorescent protein. Bars = 10 μm.
Discussion
The formation of the mammalian pulmonary system requires timely cell proliferation, migration, and differentiation of distinct cell types from multipotent progenitors. These processes are largely driven by interactions between mesenchymal and epithelial elements. Theoretically, undifferentiated epithelial cells provide the progenitor pool for lineage-committed precursors that will develop into fully specialized cells (9, 37). Our study demonstrates that progenitors defined by Ascl1 can give rise to a wide variety of pulmonary cell types. Although the proneural transcription factor Ascl1 is a critical gene for the development of PNECs in the lung (22), our in vivo genetic fate–mapping study revealed that the descendants from Ascl1-expressing cells or ASDCs in embryonic murine lungs were not restricted only to become PNECs. In contrast, the majority of ASDCs that expressed the lacZ or YFP marker after Cre recombination were epithelial. ASDCs lining the airways coexpressed the Clara, ciliated, and NE cell markers CC10, β-tubulin, and CGRP, respectively, and a substantial number of ASDCs were found in the alveolar compartment and colabeled with the AT2 marker SPC.
The peripheral lung tubules are thought to be formed by budding from the intrapulmonary conducting airways, which later leads to the formation of alveoli. Although the first Ascl1-positive cells were detected in proximal conducting airways on E12.5, the multipotent distal cells in the budding tubules were negative for Ascl1. Perl and colleagues, who used an inducible SPC promoter, discovered that by E11.5, the progenitors of the peripheral lung were highly restricted (38). Our lineage study shows that the progenitors expressing Ascl1 at the initiation of embryogenesis readily generated airway and, eventually, AT2 cells, whereas ASDCs on E9.5 gave rise to far fewer airway and alveolar cells. Importantly, the number of descendent cells from Ascl1-expressing progenitors on E11.5 was further decreased, and these progenitors were incapable of giving rise to AT2 cells. This provides new evidence supporting observations on the early restriction of murine embryonic lung progenitor cells (38). Our current data indicate that in the lineages, the expression of Ascl1 may be transient, and progenitors may already exist before the formation of lung buds. Notably, at the beginning of the pseudoglandular stage, such ASDCs may persist and retain the potential to give rise to AT2 cells. However, further studies are needed to investigate whether progenitors are homogenous, or if the subsets they give rise to will be similar.
Obviously, the cell population of major interest, although few in numbers, comprised PNECs and NEBS. They are the first cells to differentiate during lung development (5). Perl and colleagues concluded that in the very early nonoverlapping proximal (giving rise to PNECs) and distal lung, cell lineages emerge before the formation of the visible lung bud (38). In the present study, Ascl1-positive solitary PNECs were discovered on E12.5, and NEBs on E14.5. A subset of cells was also labeled with X-Gal, suggesting that they were ASDCs. Because all NE cells were not doubly labeled, we cannot rule out the possibility that different subsets of PNECs exist. However, the present study did not contain the scope to determine the histogenesis or upstream events of PNECs, but rather explored the descendants of Ascl1-defined cells.
Using the same murine strains (Ascl-Cre or Ascl1-CreERTM crossed with Rosa reporter mice), Ascl1 lineage cells have been shown to contribute to distinct cell types in the central nervous system (29). In the present study, Ascl1-positive peribronchial ganglion cells that were labeled with X-Gal were occasionally seen in developing lungs. They were positive for the neuronal marker PGP9.5. These cells may belong to the intrinsic pulmonary nervous system, or may be among the neural cells from sympathetic ganglia that migrate to the parabronchial plexus. A study of the salivary gland, another branching organ, has determined that neuronal activity preferentially affects epithelial progenitor cells (39). In the lung, intrinsic nerve ganglia that innervate airway smooth muscle are derived from neural crest cells (40–42). Our study has shown that the ganglia in the lung are Ascl1 lineage cells, which may migrate from the central nervous system at an early embryonic stage.
Both in vivo and organ culture studies have shown that in addition to neural tissue, smooth muscle plays an important role in lung growth and development (6). Our data demonstrate that Ascl1-defined progenitors give rise to both smooth muscle cells and neuronal (ganglion) cells, indicating that the expression of Ascl1 in multiple derivatives from different germ layers is characteristic of early lung development. In wild-type mice, the numbers of PNECs and NEBs increase during embryonic lung development, and reach a peak during the perinatal period (43), indicating that PNECs are important during the terminal sac stage (E17.5–P5). Ascl1−/− mice develop lungs without PNECs and NEBs. Pups all died within 24 hours after birth because of hyperventilation and severe abnormalities of the central nervous system (22). Our study shows the Ascl1 lineage cells are not limited to PNECs, but also contribute to ganglia and smooth muscle cells during lung development. These tissues are conceivably needed for the regulation of breathing. Thus, our results provide new evidence to explain why the lungs in Ascl1−/− mice could not function normally.
To elucidate the progenitor properties of ASDCs in adult lung epithelium, we used the well-characterized naphthalene model. A single injection of naphthalene depletes pulmonary Clara cells, followed by rapid repair and transient PNEC hyperplasia. In most cases, the epithelium has fully recovered from the injury in 1–3 weeks (44). The bronchiolar airway cells resistant to naphthalene treatment include ciliated cells, PNECs, rare “variant” Clara (vC) cells, and bronchioalveolar stem cells (BASCs). Previous studies suggest that ciliated cells neither proliferate nor transdifferentiate, and PNECs only proliferate, whereas vC cells and BASCs lead to epithelial regeneration during injury repair (45–48). Our results show that both embryonic and adult ASDCs have the ability to give rise to regenerated Clara cells after naphthalene injury. Like the Clara-cell repair that starts from “regenerative zones” (44), CC10-positive ASDCs were also found in groups. However, more studies are needed to identify the subgroups of Ascl1-defined progenitors contributing to repair. Interestingly, during injury repair, the adult Ascl1 lineage included AT2 cells in the alveolar compartment. Two intriguing possibilities are evident: (1) Ascl1-defined AT2 cells come from BASCs that are part of ASDCs. Because the BASCs express both Clara and AT2 cell markers and can generate Clara cells after naphthalene injury, they may also give rise to AT2 cells during injury repair (49). (2) A subgroup of AT2 cells transiently expresses Ascl1 in response to naphthalene, which could alter alveolar homeostasis. Further study is needed to determine whether BASCs are Ascl1 lineage progenitors, or if they transiently express Ascl1 after naphthalene treatment.
Although the murine models in the present study were previously used to trace ASDCs in the central nervous system, interpretative caution should be exercised, because several limitations may apply (29, 30). This was potentially the first time, to the best of our knowledge, that these two transgenic murine strains were used in lung research. That is why we carefully compared the development and repair processes in transgenic mice and their littermates with wild-type control mice, and detected no substantial discrepancies. To eliminate the potential interference from detection systems, we used both X-Gal–based and YFP-based methods. Nevertheless, as illustrated by Miyoshi and colleagues in brain tissue (50), the conditional or TM-inducible Cre may have failed to label some cells in lung tissue. Because the strains rely on bacterial artificial chromosome techniques, transgene expression may not be 100% reliable because of the possible position effects or lack of transcription regulatory regions. Moreover, all the cells derived from Ascl1 are not expected to be marked, because the induction of Cre is unlikely to be 100% efficient in deleting the sequence of stop codon from the reporter. Ultimately, other strains and techniques will be required to address such concerns more definitely. Although more ASDCs may exist than the ones that we detected, important questions that remain to be answered include: What are the critical factors involved in the multidirectional differentiation of Ascl1-defined progenitors? Can Ascl1-expressing progenitor cells give rise to other defined epithelial progenitors (e.g., BASCs or vC cells)?
Taken together, this study uncovered a new set of progenitors during lung development and injury repair. Our findings may have implications for the development of several pulmonary lineages, the interaction of epithelial and mesenchymal compartments, tissue regeneration, and the pathogenesis of pulmonary diseases, including cancer.
Supplementary Material
Acknowledgments
The authors thank Dr. Jane Johnson for the Ascl-transgenic murine strains and Dr. Yvona Ward for help with confocal microscopy. The authors also thank Dr. Susan Pilch at the National Institutes of Health library for comments on the manuscript.
Footnotes
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (R.I.L.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0027OC on August 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010;18:8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Lommel A, Bolle T, Fannes W, Lauweryns JM. The pulmonary neuroendocrine system: the past decade. Arch Histol Cytol 1999;62:1–16 [DOI] [PubMed] [Google Scholar]
- 3.Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease: recent advances. Pediatr Dev Pathol 2007;10:419–435 [DOI] [PubMed] [Google Scholar]
- 4.Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754–764 [DOI] [PubMed] [Google Scholar]
- 5.Linnoila RI. Functional facets of the pulmonary neuroendocrine system. Lab Invest 2006;86:425–444 [DOI] [PubMed] [Google Scholar]
- 6.Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn 2001;221:48–60 [DOI] [PubMed] [Google Scholar]
- 7.Sparrow MP, Lamb JP. Ontogeny of airway smooth muscle: structure, innervation, myogenesis and function in the fetal lung. Respir Physiol Neurobiol 2003;137:361–372 [DOI] [PubMed] [Google Scholar]
- 8.Canning BJ, Widdicombe JG. Innervation of the airways: introduction. Respir Physiol 2001;125:1–2 [DOI] [PubMed] [Google Scholar]
- 9.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 2006;133:2455–2465 [DOI] [PubMed] [Google Scholar]
- 10.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional Clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L1256–1263 [DOI] [PubMed] [Google Scholar]
- 11.Linnoila RI, Nettesheim P, DiAugustine RP. Lung endocrine-like cells in hamsters treated with diethylnitrosamine: alterations in vivo and in cell culture. Proc Natl Acad Sci U S A 1981;78:5170–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linnoila IR. Pulmonary endocrine cells in vivo and in vitro. : Kaliner MA, Barnes PJ, Kunkel GHH, Baraniuk JN, Neuropeptides in respiratory medicine. New York: Marcel Dekker, Inc.; 1994. pp. 197–224 [Google Scholar]
- 13.Elizegi E, Pino I, Vicent S, Blanco D, Saffiotti U, Montuenga LM. Hyperplasia of alveolar neuroendocrine cells in rat lung carcinogenesis by silica with selective expression of proadrenomedullin-derived peptides and amidating enzymes. Lab Invest 2001;81:1627–1638 [DOI] [PubMed] [Google Scholar]
- 14.Sunday ME, Haley KJ, Sikorski K, Graham SA, Emanuel RL, Zhang F, Mu Q, Shahsafaei A, Hatzis D. Calcitonin driven v-Ha-ras induces multilineage pulmonary epithelial hyperplasias and neoplasms. Oncogene 1999;18:4336–4347 [DOI] [PubMed] [Google Scholar]
- 15.Sunday ME, Yoder BA, Cuttitta F, Haley KJ, Emanuel RL. Bombesin-like peptide mediates lung injury in a baboon model of bronchopulmonary dysplasia. J Clin Invest 1998;102:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan J, Bear C, Farragher S, Cutz E, Yeger H. Cystic fibrosis transmembrane conductance regulator modulates neurosecretory function in pulmonary neuroendocrine cell-related tumor cell line models. Am J Respir Cell Mol Biol 2002;27:553–560 [DOI] [PubMed] [Google Scholar]
- 17.Aguayo SM, Miller YE, Waldron JA, Jr, Bogin RM, Sunday ME, Staton GW, Jr, Beam WR, King TE., Jr Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med 1992;327:1285–1288 [DOI] [PubMed] [Google Scholar]
- 18.Armas OA, White DA, Erlandson RA, Rosai J. Diffuse idiopathic pulmonary neuroendocrine cell proliferation presenting as interstitial lung disease. Am J Surg Pathol 1995;19:963–970 [DOI] [PubMed] [Google Scholar]
- 19.Lim C, Stanford D, Young I, McCaughan B, Cooper W. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a report of two cases. Pathol Int 2010;60:538–541 [DOI] [PubMed] [Google Scholar]
- 20.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol 2005;40:157–165 [DOI] [PubMed] [Google Scholar]
- 21.Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev 2007;87:219–244 [DOI] [PubMed] [Google Scholar]
- 22.Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, Baylin SB, Ball DW. An Achaete-Scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997;386:852–855 [DOI] [PubMed] [Google Scholar]
- 23.Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian Achaete-Scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 1993;75:463–476 [DOI] [PubMed] [Google Scholar]
- 24.Sommer L, Shah N, Rao M, Anderson DJ. The cellular function of MASH1 in autonomic neurogenesis. Neuron 1995;15:1245–1258 [DOI] [PubMed] [Google Scholar]
- 25.Tomita K, Nakanishi S, Guillemot F, Kageyama R. MASH1 promotes neuronal differentiation in the retina. Genes Cells 1996;1:765–774 [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix–loop–helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 2000;127:3913–3921 [DOI] [PubMed] [Google Scholar]
- 27.Ball DW. Achaete-Scute homolog–1 and Notch in lung neuroendocrine development and cancer. Cancer Lett 2004;204:159–169 [DOI] [PubMed] [Google Scholar]
- 28.Jiang T, Collins BJ, Jin N, Watkins DN, Brock MV, Matsui W, Nelkin BD, Ball DW. Achaete-Scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res 2009;69:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EJ, Battiste J, Nakagawa Y, Johnson JE. Ascl1 (MASH1) lineage cells contribute to discrete cell populations in CNS architecture. Mol Cell Neurosci 2008;38:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development 2007;134:285–293 [DOI] [PubMed] [Google Scholar]
- 31.Soriano P. Generalized lacZ expression with the Rosa26 Cre reporter strain. Nat Genet 1999;21:70–71 [DOI] [PubMed] [Google Scholar]
- 32.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the Rosa26 locus. BMC Dev Biol 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linnoila RI, Jensen SM, Steinberg SM, Mulshine JL, Eggleston JC, Gazdar AF. Peripheral airway cell marker expression in non–small cell lung carcinoma: association with distinct clinicopathologic features. Am J Clin Pathol 1992;97:233–243 [DOI] [PubMed] [Google Scholar]
- 34.Tichelaar JW, Wert SE, Costa RH, Kimura S, Whitsett JA. HNF-3/forkhead homologue–4 (HFH-4) is expressed in ciliated epithelial cells in the developing mouse lung. J Histochem Cytochem 1999;47:823–832 [DOI] [PubMed] [Google Scholar]
- 35.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 2002;244:305–318 [DOI] [PubMed] [Google Scholar]
- 36.Miki M, Ball DW, Linnoila RI. Insights into the Achaete-Scute homolog–1 gene (hASH1) in normal and neoplastic human lung. Lung Cancer 2012;75:58–65 [DOI] [PubMed] [Google Scholar]
- 37.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006;133:1611–1624 [DOI] [PubMed] [Google Scholar]
- 38.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A 2002;99:10482–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010;329:1645–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freem LJ, Escot S, Tannahill D, Druckenbrod NR, Thapar N, Burns AJ. The intrinsic innervation of the lung is derived from neural crest cells as shown by optical projection tomography in Wnt1-Cre;YFP reporter mice. J Anat 2010;217:651–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langsdorf A, Radzikinas K, Kroten A, Jain S, Ai X. Neural crest cell origin and signals for intrinsic neurogenesis in the mammalian respiratory tract. Am J Respir Cell Mol Biol 2011;44:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothman TP, Le Douarin NM, Fontaine-Perus JC, Gershon MD. Developmental potential of neural crest–derived cells migrating from segments of developing quail bowel back-grafted into younger chick host embryos. Development 1990;109:411–423 [DOI] [PubMed] [Google Scholar]
- 43.Sunday ME, Cutz E. The role of neuroendocrine cells in fetal and post-natal lung. : Mendelson CR, editor Endocrinology of the lung. Totowa, NJ: Humana Press; 2000. pp. 209–336 [Google Scholar]
- 44.Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995;269:L791–799 [DOI] [PubMed] [Google Scholar]
- 45.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835 [DOI] [PubMed] [Google Scholar]
- 46.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 2008;5:328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A 2007;104:410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Mitsialis SA, Kourembanas S, Kim CF. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2012;302:L829–L837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci 2010;30:1582–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.