Abstract
Generally, exposure to LPS in human airways occurs in the form of aerosols and causes an acute inflammatory response or exacerbates existing chronic inflammatory conditions by enhancing airway remodeling and associated pathologies. The present study evaluated which inflammatory mediators may be responsible for the expression of Bcl-2 and mucus cell metaplasia when mice are exposed to aerosolized LPS. At 3 days after exposure, aerosolized LPS (for 20–40 min) with the estimated lung deposited dosage of 0, 0.02, 0.2, 1.4, and 20.2 μg showed a characteristic dose-dependent increase in polymorphonuclear neutrophils. Significant increases of proinflammatory mediators, including IL-1β, TNF-α, IL-6, growth-related oncogene or keratinocyte-derived cytokine, IFN-γ–induced protein-10, monocyte chemotactic protein-1, and macrophage inflammatory protein-1α, were detected at the highest doses. In addition to increased numbers of airway epithelial cells, mucus cell numbers and mucus production were increased in a dose-dependent manner. Hyperplastic epithelial cells expressed insulin-like growth factor (IGF)-1 and, similar to previous studies, increased expression of the prosurvival protein Bcl-2 and induced expression of Muc5ac. Suppression of IGF-1 expression using retroviral shRNA blocked Bcl-2 expression in human and murine airway epithelial cells and Muc5ac in primary murine airway epithelial cells. These findings show that acute inflammation induces IGF-1 to mediate Bcl-2 and Muc5ac expression in airway epithelial cells.
Keywords: LPS aerosol, mucus cell metaplasia, cell death, retroviral shRNA, cytokines
Clinical Relevance
The present study evaluated the inflammatory mediator responsible for mucus cell metaplasia and the expression of Bcl-2, a prosurvival protein that sustains hyperplastic mucus cells. A mouse model of LPS aerosol exposure was used to mimic human exposures. LPS-induced hyperplastic epithelial cells showed coinduction of insulin-like growth factor (IGF)-1, Bcl-2, and Muc5ac, the primary mucin component. More importantly, suppression of IGF-1 expression blocked Bcl-2 and MUC5AC. Thus, targeting IGF-1 could provide a rational basis for alternative intervention strategies to reduce aberrant mucus production and airway inflammation.
Acute lung inflammation can alter the pulmonary function of susceptible individuals and exacerbate the pathogenesis of chronic inflammatory lung diseases, including chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and asthma (1). Exposure to LPS or endotoxin, a constituent of outer cell membrane of gram-negative bacteria, induces airway inflammation, which is primarily characterized by increased polymorphonuclear neutrophils (PMNs) at early time points (2, 3). Because LPS is present in a variety of occupational and home environments (4) and is an active constituent of cigarette smoke (5), it is a risk factor for increasing the prevalence and severity of nonoccupational COPD (6), adult-onset of asthma (7, 8), and wheezing in children (9).
Exposure to LPS results in increased expression of several proinflammatory mediators, such as IL-1β and TNF-α, through activation of the NF-κB pathway (10, 11). In airway epithelial cells, LPS stimulation increases mucin gene expression and mucus production (12, 13). Hypersecretion of mucus overwhelms the ciliary clearance and obstructs airways, causing morbidity and mortality in chronic inflammatory respiratory lung diseases (14). In addition, acute bacterial infection contributes to the exacerbation of chronic airway diseases, specifically in patients with advanced COPD and CF, leading to increased healthcare burden and higher mortality (15).
Although 21 MUC genes have been identified (14, 16), the airway epithelial cells predominantly express the major gel-forming mucins, MUC5AC and MUC5B genes. In mice, Muc5ac is highly inducible (17), and Muc5b is primarily expressed constitutively (18). Specifically, overexpression of MUC5AC is associated with mucus cell metaplasia (MCM) and is expressed by mucus-secreting or surface epithelial goblet cells (19–22). LPS-induced Muc5ac expression involves several pathways, including the epithelial growth factor receptor (EGFR), mitogen-activated protein kinases, cyclic-AMP response element binding protein, and NF-κB pathways (21). The interplay of these pathways also regulates pathogenesis of airway remodeling by modulating proliferation and cell survival of airway epithelial cells. Bcl-2, a prosurvival protein that inhibits cell death, plays a key role in normal cellular homeostasis and regulates the integrity of the mitochondrial and endoplasmic reticulum membranes (23). Bcl-2, by interacting with sarcoendoplasmic reticulum calcium ATPase, alters apoptosis and calcium signaling in airway epithelial cells (24). Gain- and loss-of-function studies showed that Bcl-2 expression sustains hyperplastic epithelial cells, and Bcl-2 expression is elevated in airway epithelial cells of subjects with cystic fibrosis (25) and asthma (26).
Airway remodeling associated with airway inflammation is mediated at least in part by growth factors such as insulin-like growth factor (IGF)-I. IGF-1 levels are significantly elevated in endobronchial biopsies from individuals with asthma and are correlated with collagen thickening and an increased number of fibroblasts, suggesting that IGF-1 is one of the critical growth factors in the pathogenesis of airway inflammation and remodeling (27, 28). This growth factor is produced by several cell types in the lung, including bronchial epithelial cells, alveolar macrophages, and fibroblasts (29, 30). IGF-1 predominantly signals through IGF-1 receptor (IGF-1R), but signaling through transactivation of other receptor tyrosine kinases, such as EGFR and insulin receptor (IR), has also been reported (31, 32). However, little is known about the role of epithelium-derived IGF-1 in airway inflammation. IGF-1 and other inflammatory mediators regulate Bcl-2 expression in various types of cells (33, 34). The present study investigated whether LPS-induced MCM and Bcl-2 expression is mediated by IGF-1. In addition, because human lungs are exposed to LPS in the form of an aerosol, we examined the role of IGF-1 after exposure of mice to aerosolized LPS by inhalation.
Materials and Methods
Animals
Male pathogen-free C57BL/6 mice (Charles River Laboratories, Wilmington, MA) aged 8 to 10 weeks were used in this study. Mice were housed in groups under specific pathogen–free conditions and provided food and water ad libitum. Mice were exposed to a 12:12 hour light:dark cycle and housed at 22.2°C with 30 to 40% humidity. Each mouse was individually weighed and randomly assigned to an experimental group. All experiments were approved by the Lovelace Respiratory Research Institute Institutional Animal Care and Use Committee. Experiments were performed at the Institute in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Exposure to Aerosolized LPS
Mice were acclimated to the nose-only exposure tubes for 2 days before LPS exposure. On the day of exposure, mice were transferred to the nose-only exposure unit by placing them individually in exposure tubes. Mice were exposed to filtered air or to LPS (Pseudomonas aeruginosa serotype 10, 900,000 EU/mg; Sigma, St. Louis, MO) that was diluted with deionized water at different doses by modifying the length of exposure from 20 to 40 minutes. The exposures yielded a target dose of 0.1, 0.01, 0.001, or 0.0001 mg of LPS by controlling exposure concentration and exposure duration for each treatment group based on an assumed deposition fraction that was dependent on the median particle size of 0.1 μm. Atmospheric oxygen content, temperature, and relative humidity were measured continuously to ensure that the environmental conditions were within acceptable limits. After exposure, animals were returned to shoe-box type cages for 3 days.
Cell Culture and Treatments
AALEB cells, a cell line derived from human airway epithelial cells by SV40 and hTERT transformation and immortalization as described previously (35), were maintained in submerged liquid culture using bronchial epithelial growth medium (Lonza, Walkersville, MD). Primary murine embryonic fibroblasts (MEFs) and mouse airway epithelial cells (MAECs) were harvested from 12-day-old embryos or from 8- to 14-week-old mice, respectively, according to standard procedures and were maintained in liquid culture as described (36, 37). The cells were treated with human or murine recombinant IL-1β for 24 hours before harvesting or fixing for further analysis.
IGF-1 Gene Silencing
Cells were transduced with retroviral expression vectors for IGF-1 shRNA or control shRNA (Origene Technologies, Inc., Rockville, MD) as per the manufacturer’s instructions. After infection, cells were treated with recombinant IL-1β, and 48 hours later cells were harvested or fixed for further analysis.
Statistical Analysis
Grouped results were expressed as means ± SEM. Data were analyzed using GraphPad Prism Software (GraphPad Software, Inc., San Diego, CA). Grouped results were analyzed using two-way ANOVA. When significant main effects were detected (P < 0.05), Fisher’s least significant difference test was used to determine differences between treatment groups.
Additional methods describing generation of LPS aerosols, particle size determination, bronchoalveolar lavage collection and cell counts, histochemical staining and analysis, luminex analysis, Muc5ac ELISA, immunofluorescent staining and image analysis, quantitative RT-PCR, and Western blot analysis are available in the online supplement.
Results
Inflammatory Response in the Bronchoalveolar Lavage Fluid
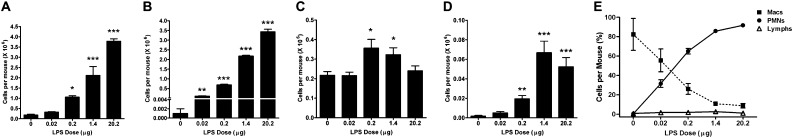
Mice were exposed to aerosolized LPS over a period of 20 to 40 minutes with the estimated lung-deposited dose of 0, 0.02, 0.2, 1.4, and 20.2 μg per mouse. At 3 days after exposure, the total number of inflammatory cells that was recovered in the bronchoalveolar lavage (BAL) fluid increased in a dose-dependent manner (Figure 1A), reaching 19-fold higher compared with air-exposed control mice at the highest dose. Cell differential analysis showed that most of the BAL cells after LPS administration were neutrophils (Figure 1B). Alveolar macrophage numbers were increased at 0.2 and 1.4 μg LPS dose but not at the highest dose tested (Figure 1C). Similar to PMNs, the number of lymphocytes also showed a dose-dependent increase (Figure 1D). Analysis of the total composition of BAL cells by calculating percentage of each BAL cell type showed a dose-dependent increase and decrease in neutrophils and macrophages, respectively (Figure 1E).
Figure 1.
Inflammatory cell numbers recovered by bronchoalveolar lavage (BAL) are increased in mice exposed to aerosolized LPS. Mice were exposed to increasing doses of aerosolized LPS or filtered air, BAL was recovered at 72 hours after exposure using 1 ml saline (0.5 ml twice), and cytospins prepared were analyzed after Wright-Giemsa staining. (A–D) Quantitative analyses of the number of total BAL cells (A), polymorphonuclear neutrophils (PMNs) (B), macrophages (C), and lymphocytes (D) in the lavage. (E) Changes in the percentages of PMNs, macrophages, and lymphocytes over the range of LPS doses are shown. Data are shown as mean ± SEM (n = 6 per group). *P < 0.05; **P < 0.01; ***P < 0.001.
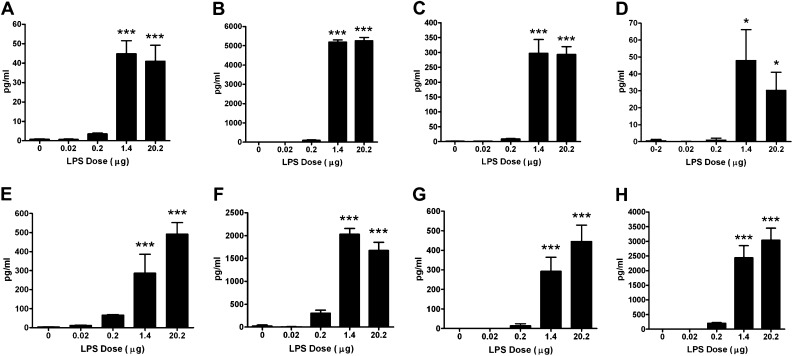
Luminex analysis of BAL supernatant showed a dose-dependent increase in proinflammatory cytokines and chemokines, including IL-1β, IL-6, TNF-α, growth-related oncogene or keratinocyte-derived cytokine (GRO/KC), IFN-γ–induced protein-10, monocyte chemotactic protein-1, and macrophage inflammatory protein-1α (Figure 2), especially at 1.4 and 20.2 μg LPS doses. A significant increase in the levels of a TH1 cytokine, IL-12, was observed in BAL samples of mice exposed to 1.4 and 20.2 μg LPS (Figure 2D). The levels of IFN-γ and IL-9 were below detection limits in all of the BAL samples tested (data not shown).
Figure 2.
Inflammatory factors are increased in the BAL supernatant of mice exposed to aerosolized LPS. Levels of IL-1β (A), IL-6 (B), TNF-α (C), IL-12 (D), growth-related oncogene or keratinocyte-derived cytokine (E), IFN-γ–induced protein (IP)-10 (F), monocyte chemotactic protein-1 (MCP-1) (G), and macrophage inflammatory protein-1α (MIP-1α) (H) in the BAL supernatant were analyzed by Luminex-based ELISA assay. Data are shown as mean ± SEM (n = 6 per group). *P < 0.05; ***P < 0.001.
Airway Epithelial Cell Hyperplasia and Mucus Cell Metaplasia
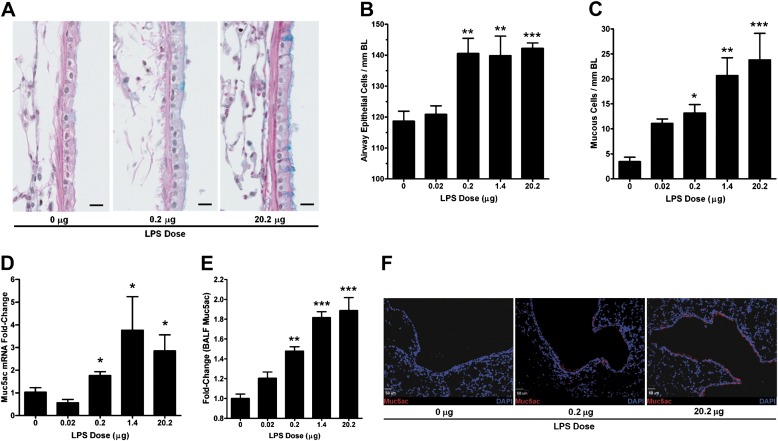
The lung sections were analyzed for changes in airway epithelium as described previously (38) by analyzing the axial airway epithelial cell and MC numbers in LPS-exposed mice (Figure 3A). Particularly, in mice exposed to 20.2 μg LPS, epithelial cells were visibly taller than in control mice, with an average height of 14 and 7 μM, respectively. The number of epithelial cells of basal lamina (BL) increased with LPS dose from 119 ± 3 to 142 ± 2 cells per mm BL in mice exposed to 0 or 20.2 μg LPS, respectively (Figure 3B). The number of MCs, as assessed by Alcian blue staining, showed a trend toward increase even at 0.02 μg LPS but significantly increased by approximately 8-fold at the highest dose (Figure 3C).
Figure 3.
Aerosolized LPS induces mucos cell metaplasia. Paraffin-embedded lung sections (5 μm) were stained with Alcian Blue (AB) and counterstained with hematoxylin and eosin (H&E), and the epithelial and mucus cells were quantified over the length of basal lamina (BL). (A) Representative photomicrographs of AB/H&E-stained axial airway epithelia from mice exposed to 0, 0.2, and 20.2 μg of aerosolized LPS (scale bar, 10 μM). (B) Number of airway epithelial cells per mm BL. (C) Number of mucus cells per mm BL over a range of LPS doses. (D) Muc5ac mRNA levels in lung tissues of mice exposed to LPS as analyzed by qRT-PCR and the fold-change (2−ddCT) calculated after normalizing to the respective CDKN1B levels. (E) Relative quantitation of the BAL Muc5ac protein levels as analyzed by ELISA. The data are shown as fold-change over control mice (0 μg of LPS). Data are shown as mean ± SEM (n = 6 per group). *P < 0.05; **P < 0.01; ***P < 0.001. (F) Fluorescent immunostaining analysis for Muc5ac expression in airway tissues from mice exposed to 0, 0.2, and 20.2 μg of aerosolized LPS. Representative micrographs show a dose-dependent increase in the number of Muc5ac-immunopositive cells (red) in mice treated with 0.2 (middle panel) and 20.2 μg (right panel) compared with 0 μg (left panel) LPS. 4',6-Diamidino-2-phenylindole was used as nuclear counter stain and is shown in blue (scale bar, 50 μM).
We next determined the expression levels of Muc5ac, the major mucin component secreted by airway epithelial cells (22, 39). Muc5ac mRNA levels increased by 2- to 3-fold in LPS-challenged mice compared with control mice (Figure 3D). However, Muc5b mRNA levels were not significantly changed (see Figure E1 in the online supplement).
Muc5ac protein levels in the BAL samples increased in a dose-dependent manner with a 2-fold increase in mice exposed to 20.2 μg LPS compared with control mice (Figure 3E). Immunofluorescent staining showed that Muc5ac apoprotein expression was confined to airway epithelium (Figure 3F), and Muc5ac positivity of airway epithelial cells was significantly increased with LPS exposure.
Bcl-2 Expression in Airway Epithelial Cells
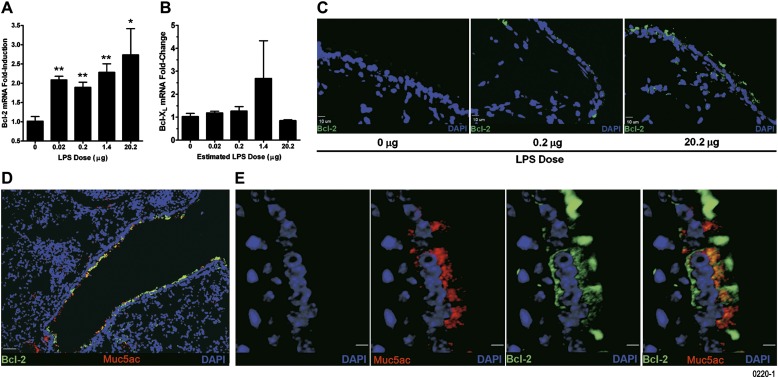
Exposure to LPS aerosols increased Bcl-2 mRNA (Figure 4A) but not Bcl-XL mRNA levels in lung tissues (Figure 4B). Bcl-2 protein levels were increased in airway epithelial cells as assessed by immunofluorescence staining (Figure 4C). Furthermore, coimmunostaining with Muc5ac- and Bcl-2–specific antibodies showed coexpression of Muc5ac apoprotein and Bcl-2 in airway epithelium of mice exposed to 20.2 μg LPS (Figure 4D). High-resolution images (Figure 4E) and capturing z-stack images of 4.2-μM lung sections with 14 optical slices of 0.3 μM each (Figure E1) in combination with the 3-D volume rendering view (Figure E2) showed that all Muc5ac-positive cells are Bcl-2 positive, whereas some Bcl-2–positive cells are negative for Muc5ac.
Figure 4.
Aerosolized LPS increases expression of Bcl-2 mRNA and protein in airway epithelial cells. Bcl-2 (A) and Bcl-XL (B) mRNA levels in the lung tissues of mice exposed to LPS as analyzed by quantitative RT-PCR. Data are shown as mean ± SEM (n = 6 per group). *P < 0.05; **P < 0.01. (C) Representative micrographs of lungs from mice exposed to 0.2 (middle panel) and 20.2 μg (right panel) compared with 0 μg (left panel) LPS showing airway epithelial cells that are Bcl-2 immunopositive (green). 4',6-Diamidino-2-phenylindole (DAPI) was used as nuclear counter stain and is shown in blue (scale bar, 10 μM). (D) A representative photomicrograph of a lung section from mice exposed to 20.2 μg of aerosolized LPS showing Bcl-2 and Muc5ac coexpression in airway epithelial cells (scale bar, 50 μM). (E) High-magnification images of Bcl-2– and Muc5ac-immunostained lung sections from mice exposed to 20.2 μg aerosolized LPS. The panels show DAPI-stained nuclei (blue), Muc5ac-positive (red), and Bcl-2–positive (green) cells. The composite image shows colocalization of Muc5ac and Bcl-2 (yellow) (scale bar, 5 μM).
IGF-1 Expression in Airway Epithelial Cells
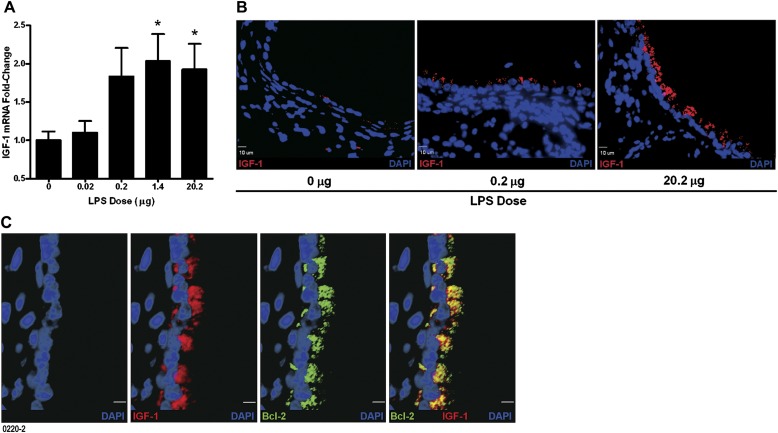
Among the growth factors, IGF-1 has been shown to modulate Bcl-2 expression (33, 34), and we have also observed an increase in IGF-1 levels in rats after LPS challenge (unpublished observation). Therefore, we investigated the levels of IGF-1 in mice exposed to aerosolized LPS. The IGF-1 mRNA levels were increased 2-fold by LPS exposure compared with control mice (Figure 5A). In the lung, IGF-1 is primarily produced by alveolar macrophages, fibroblasts, and bronchial epithelial cells (29, 30). Immunofluorescence staining showed that IGF-1 is primarily expressed in the airway epithelial cells in lungs exposed to LPS (Figure 5B). Similar to previous reports, we observed IGF-1 expression in alveolar macrophages and fibroblasts, but these findings were not analyzed in this study. In addition, coimmunostaining analysis revealed that all of the IGF-1–positive cells were Bcl-2 positive (Figure 5C;Figures E3 and E4), suggesting that IGF-1 plays a role in inducing Bcl-2 expression in airway epithelial cells.
Figure 5.
Expression of intracellular IGF-1 is increased in airway epithelial cells by exposure to aerosolized LPS. (A) IGF-1 mRNA levels in the lung tissues of mice exposed to LPS as analyzed by quantitative RT-PCR. Data are shown as mean ± SEM (n = 6 per group). *P < 0.05. (B) The IGF-1 expression in airway epithelial cells was analyzed by fluorescent immunostaining. Representative micrographs showing increased numbers of IGF-1–immunopositive cells (red) in mice exposed to 0.2 (middle panel) and 20.2 μg (right panel) compared with 0 μg (left panel) LPS. (C) Representative high-magnification images of Bcl-2– and IGF-1–immunostained lung sections from mice exposed to 20.2 μg aerosolized LPS. The panels show DAPI-stained nuclei (blue), IGF-1–positive (red), and Bcl-2–positive (green) cells. The composite image shows colocalization of IGF-1 and Bcl-2 (yellow) (scale bar, 5 μM).
Suppression of IGF-1 Expression Blocks Bcl-2 and Muc5ac Expression
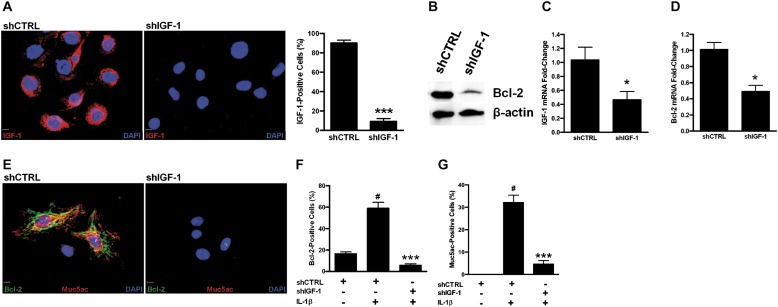
To understand the role of IGF-1, we investigated Bcl-2 and Muc5ac expression by blocking IGF-1 expression using the shRNA approach. We have investigated the effect of several cytokines and observed that IL-1β is one of the cytokines that consistently induced Bcl-2 and MUC5AC levels. In addition, the role of IL-1β in inducing MUC5AC has been reported previously (40, 41). Therefore, IL-1β was used to assess the effect of blocking IGF-1 on Bcl-2 and Muc5ac expression. Compared with cells transduced with control shRNA (shCTRL cells), AALEB cells expressing shRNA-targeting IGF-1 (shIGF-1 cells) suppressed IL-1β–mediated IGF-1 expression by 10-fold (Figure 6A). Also, the IL-1β–induced Bcl-2 levels were significantly reduced by shIGF-1– compared with shCTRL-infected cells (Figure 6B).
Figure 6.
Suppression of IGF-1 expression reduces Bcl-2 and Muc5ac levels. (A) Suppression of IGF-1 using shIGF-1 in AALEB cells, a human airway epithelial cell. A representative photomicrograph showing AALEB cells expressing shControl (shCTRL) or shIGF-1 after treatment with IL-1β (20 ng/ml) for 24 hours. DAPI (blue) was used as a nuclear counterstain (scale bar, 10 μM). Quantitative analysis of IGF-1–positive cells in shIGF-1– and shCTRL-transfected AALEB cells after 24 hours of IL-1β treatment. (B) Western blot analysis showing reduced Bcl-2 protein levels in AALEB cells infected with retroviral vector expressing shIGF-1 compared with shCTRL after 24 hours of IL-1β treatment. Expression of β-actin was used as loading control. (C) IGF-1 mRNA levels in mouse embryonic fibroblasts (MEFs) transfected with shCTRL and shIGF-1 as analyzed by quantitative RT-PCR after 24 hours IL-1β treatment. (D) Bcl-2 mRNA levels in shCTRL- and shIGF-1–transfected MEFs 24 hours after IL-1β treatment. (E) Suppression of IGF-1 using shIGF-1 in mouse airway epithelial cells (MAECs). A representative photomicrographs showing coinduction of Bcl-2 (green) and Muc5ac (red) in MAECs 24 hours after IL-1β (20 ng/ml) treatment in MAECs infected with retroviruses expressing shCTRL-1 (left panel) or shIGF-1 (right panel). DAPI (blue) was used as nuclear counterstain (scale bar, 10 μM). (F) Quantitative analysis of Bcl-2–positive cells in shIGF-1– and shCTRL-transfected cells 24 hours after IL-1β treatment. (G) Quantitative analysis of Muc5ac-positive cells in shIGF-1– and shCTRL-expressing cells 24 hours after IL-1β treatment. Data are shown as mean ± SEM (n = 5 with > 300 cells counted per treatment). *P < 0.05 and ***P < 0.001 compared with IL-1β–treated shCTRL group. #P < 0.001 compared with untreated shCTRL group.
MEFs are easier to culture and transduce with retroviral preparations than MAECs; therefore, we first used MEFs to investigate the effect of shIGF-1 transductions on IGF-1 suppression and Bcl-2 levels. Similar to AALEBs, IL-1β–induced IGF-1 levels were significantly reduced in MEFs by retroviral expression of shIGF-1 but not by shCTRL constructs after treatment with IL-1β for 24 hours (Figure 6C). Bcl-2 mRNA levels were also significantly suppressed in shIGF-1– compared with shCTRL-infected MEFs (Figure 6D). Quantitative analysis of MAECs immunostained for Bcl-2 and Muc5ac showed a 10- and 6-fold decrease in Bcl-2 and Muc5ac positivity, respectively, in shIGF-1 compared with shCTRL-infected cells 24 hours after IL-1β treatment (Figures 6E–6G).
Discussion
The present study demonstrates that, in response to LPS-induced acute inflammation, airway epithelial cells express IGF-1, which mediates the induction of Bcl-2 and Muc5ac expression. IGF-1 expression was required for the Bcl-2 and Muc5ac expression in airway epithelial cells because suppression of IGF-1 expression reduced the IL-1β–induced Bcl-2 and Muc5ac expression. The association between LPS-induced injury and IGF-1 has been demonstrated in brain, liver, and cardiomyocytes (42–44), whereas IGF-1 and IGF-1 binding proteins, especially IGF-1 binding protein 3, modulate fibrotic lung diseases and allergic airway remodeling (30, 45). The present study demonstrates that IGF-1 is one of the main inducers of Muc5ac expression by involving Bcl-2 expression.
Exposure of mice to aerosolized LPS that mimics the clinically relevant route of human exposure causes an evenly distributed inflammation throughout the lung. Similar to what was previously observed in rats, even low levels of deposited LPS, when delivered as an aerosol, induced robust inflammation that results in much higher numbers of PMNs in the lavage fluid than when LPS is administered by intranasal or intratracheal instillation (46, 47). These findings suggest that equal distribution of LPS throughout the lung may cause more epithelial cells to secrete neutrophil chemoattractants, which results in greater inflammation. This hypothesis is supported by the increased levels of several inflammatory mediators detected as late as 72 hours after LPS challenge. In addition, the number of recruited inflammatory cells was still elevated at this time point, as shown by the number of PMNs, macrophages, and lymphocytes in the BAL.
As expected from previous studies (3, 46, 48), the response to aerosolized LPS challenge was primarily neutrophilic, although at intermediate doses increased numbers of alveolar macrophages were also observed, suggesting a recruitment and/or differentiation of monocytes. With increasing LPS doses, the number of BAL neutrophils increased dramatically, whereas the number of macrophages decreased. Approximately equal numbers of PMNs and macrophages were present in the lungs at the LPS-deposited dose of 0.02 to 0.2 μg. These findings are consistent with previous observations from rats instilled with varying doses of LPS (38).
Among the inflammatory mediators, we detected elevated levels of cytokines and chemokines, which are implicated in PMN recruitment and activation, including IL-1β, TNF-α, IL-6, GRO/KC, IP-10, monocyte chemotactic protein-1, and MIP-1α in mice receiving 1.4 and 20.2 μg of LPS. The levels of IL-12 were also elevated, indicating the activation of a TH1-type response, which results in the induction of IFN-γ levels (49). Although IFN-γ was not detected in the BAL fluid, the IFN-γ–induced chemokine IP-10 was significantly elevated by LPS exposure. The lack of detection for IFN-γ could stem from the point when BAL was harvested or could be due to IFN-γ remaining in the lung tissue rather than being secreted into the pulmonary airspaces that were sampled by the lavage.
At the lowest LPS dose (0.02 μg), only the numbers of PMNs and Bcl-2 mRNA were significantly modified. Because mRNA for this study was isolated from the whole lung, it is possible that this change reflects expression in cell types other than airway epithelial cells. However, significant changes were observed in airways, including the number of total epithelial cells and MCs only at doses greater than 0.2 μg LPS. Along with these changes, secreted mucus in the BAL fluid was significantly increased at the 0.2 μg dose and was further increased with higher LPS doses.
To study the relationship of IGF-1 and expression of Muc5ac and Bcl-2, we analyzed the airway epithelial cell responses in mice exposed to the highest LPS dose. We observed that all IGF-1–positive cells were Bcl-2 positive. The nature of epithelial cells that are Bcl-2 and IGF-1 positive but Muc5ac negative is unknown. We speculate that these cells may be in the process of differentiating into MCs and have not expressed a sufficient amount of Muc5ac to be detected but may present as MCs at later time points. It is also possible that these cells are ciliated epithelial cells that respond to LPS-induced inflammation by inducing IGF-1 and Bcl-2 expression. Additional immunostaining experiments using specific antibodies targeting cilia and Clara cells will address this question. Although Bcl-2 is a known cytosolic or organelle-membrane–associated protein, IGF-1 is a secretory protein. Whether this intracellularly induced IGF-1 is an apoprotein or is sequestered by IGF-binding proteins remains to be investigated. Nonetheless, the present data suggest that IGF-1 and Bcl-2 are regulated by a common pathway, with IGF-1 acting upstream of Bcl-2 because suppression of IGF-1 down-regulated Bcl-2 expression. In contrast to IGF-1 and Bcl-2, costaining for Bcl-2 and Muc5ac showed that not all Bcl-2–positive cells were Muc5ac positive. The presence of Bcl-2–positive and Muc5ac-negative cells may suggest that Bcl-2 is expressed earlier than Muc5ac or that Bcl-2 has a role in sustaining nonmucus cells. Our previous studies showed that Bcl-2 sustains metaplastic MCs (25) and that Bcl-2 was likely not involved in the cell cycle of airway epithelial cells because 50% of the Bcl-2–positive MCs were BrdU negative and therefore derived from nonproliferating, preexisting cells (50). The present findings suggest that Muc5ac and Bcl-2 expression are regulated by a common pathway because suppression of IGF-1 expression equally blocked their induction. Possibly, one of the redundant pathways could be mediated by EGFR because Muc5ac and Bcl-2 expression are modulated by EGFR-signaling pathways (12, 19, 40). In addition, EGFR pathways are predominantly involved in proinflammatory responses, leading to epithelial cell hyperplasia and MCM (41–43).
In conclusion, induced IGF-1 expression was required for the Bcl-2 and Muc5ac expression; suppression of IGF-1 expression reduced the inflammation associated increase in Bcl-2 and Muc5ac expression. Our findings may have relevance to patients with cystic fibrosis who develop excessive inflammation, Bcl-2 expression (25), and MCM due to bacterial infections (51). Thus, targeting IGF-1 expression in airway epithelial cells may provide a useful alternative for developing novel intervention strategies to reduce aberrant epithelial cell hyperplasia, mucus production, and overall airway inflammation.
Supplementary Material
Acknowledgments
The authors thank Ifeoma Romaine for help in selected experiments.
Footnotes
This work was supported by National Institute of Health grants HL68111 and ES015482.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0079OC on August 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 2008;8:183–192 [DOI] [PubMed] [Google Scholar]
- 2.Tesfaigzi Y, Fischer MJ, Martin AJ, Seagrave J. Bcl-2 in LPS- and allergen-induced hyperplastic mucous cells in airway epithelia of Brown Norway rats. Am J Physiol Lung Cell Mol Physiol 2000;279:L1210–L1217 [DOI] [PubMed] [Google Scholar]
- 3.Harris JF, Aden J, Lyons CR, Tesfaigzi Y. Resolution of LPS-induced airway inflammation and goblet cell hyperplasia is independent of IL-18. Respir Res 2007;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rylander E, Eriksson M, Pershagen G, Nordvall L, Ehrnst A, Ziegler T. Wheezing bronchitis in children: incidence, viral infections, and other risk factors in a defined population. Pediatr Allergy Immunol 1996;7:6–11 [DOI] [PubMed] [Google Scholar]
- 5.Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. Bacterial endotoxin is an active component of cigarette smoke. Chest 1999;115:829–835 [DOI] [PubMed] [Google Scholar]
- 6.Schwartz DA, Donham KJ, Olenchock SA, Popendorf WJ, Van Fossen DS, Burmeister LF, Merchant JA. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. Am J Respir Crit Care Med 1995;151:47–53 [DOI] [PubMed] [Google Scholar]
- 7.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med 1996;154:1641–1646 [DOI] [PubMed] [Google Scholar]
- 8.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med 2005;172:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med 2001;163:322–328 [DOI] [PubMed] [Google Scholar]
- 10.Blackwell TS, Lancaster LH, Blackwell TR, Venkatakrishnan A, Christman JW. Differential NF-kappaB activation after intratracheal endotoxin. Am J Physiol 1999;277:L823–L830 [DOI] [PubMed] [Google Scholar]
- 11.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol 2007;178:6504–6513 [DOI] [PubMed] [Google Scholar]
- 12.Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, Nadel JA, Prince A, Basbaum CB. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA 1997;94:967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohri K, Ueki IF, Shim JJ, Burgel PR, Oh YM, Tam DC, Dao-Pick T, Nadel JA. Pseudomonas aeruginosa induces Muc5ac production via epidermal growth factor receptor. Eur Respir J 2002;20:1263–1270 [DOI] [PubMed] [Google Scholar]
- 14.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol 2006;34:661–665 [DOI] [PubMed] [Google Scholar]
- 15.Vestbo J. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J 2004;24:206–210 [DOI] [PubMed] [Google Scholar]
- 16.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol 2008;70:405–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol 2000;22:253–260 [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13–2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 2008;586:1977–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose MC, Piazza FM, Chen YA, Alimam MZ, Bautista MV, Letwin N, Rajput B. Model systems for investigating mucin gene expression in airway diseases. J Aerosol Med 2000;13:245–261 [DOI] [PubMed] [Google Scholar]
- 20.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523 [DOI] [PubMed] [Google Scholar]
- 21.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest 2009;135:505–512 [DOI] [PubMed] [Google Scholar]
- 22.Young HW, Williams OW, Chandra D, Bellinghausen LK, Perez G, Suarez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol 2007;37:273–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youle RJ, Strasser A. The Bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008;9:47–59 [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Ahmad A, Dremina ES, Sharov VS, Guo X, Jones TN, Loader JE, Tatreau JR, Perraud AL, Schoneich C, et al. Bcl-2 suppresses sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression in cystic fibrosis airways: role in oxidant-mediated cell death. Am J Respir Crit Care Med 2009;179:816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris JF, Fischer MJ, Hotchkiss JR, Monia BP, Randell SH, Harkema JR, Tesfaigzi Y. Bcl-2 sustains increased mucous and epithelial cell numbers in metaplastic airway epithelium. Am J Respir Crit Care Med 2005;171:764–772 [DOI] [PubMed] [Google Scholar]
- 26.Vignola AM, Chiappara G, Siena L, Bruno A, Gagliardo R, Merendino AM, Polla BS, Arrigo AP, Bonsignore G, Bousquet J, et al. Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. J Allergy Clin Immunol 2001;108:738–746 [DOI] [PubMed] [Google Scholar]
- 27.Hoshino M, Nakamura Y, Sim JJ, Yamashiro Y, Uchida K, Hosaka K, Isogai S. Inhaled corticosteroid reduced lamina reticularis of the basement membrane by modulation of insulin-like growth factor (IGF)-I expression in bronchial asthma. Clin Exp Allergy 1998;28:568–577 [DOI] [PubMed] [Google Scholar]
- 28.Yamashita N, Tashimo H, Ishida H, Matsuo Y, Arai H, Nagase H, Adachi T, Ohta K. Role of insulin-like growth factor I in allergen-induced airway inflammation and remodeling. Cell Immunol 2005;235:85–91 [DOI] [PubMed] [Google Scholar]
- 29.Cambrey AD, Kwon OJ, Gray AJ, Harrison NK, Yacoub M, Barnes PJ, Laurent GJ, Chung KF. Insulin-like growth factor I is a major fibroblast mitogen produced by primary cultures of human airway epithelial cells. Clin Sci (Lond) 1995;89:611–617 [DOI] [PubMed] [Google Scholar]
- 30.Uh ST, Inoue Y, King TE, Jr, Chan ED, Newman LS, Riches DW. Morphometric analysis of insulin-like growth factor-I localization in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;158:1626–1635 [DOI] [PubMed] [Google Scholar]
- 31.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in Cos-7 cells. J Biol Chem 2000;275:22583–22589 [DOI] [PubMed] [Google Scholar]
- 32.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res 2010;16:2505–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minshall C, Arkins S, Straza J, Conners J, Dantzer R, Freund GG, Kelley KW. IL-4 and insulin-like growth factor-I inhibit the decline in Bcl-2 and promote the survival of IL-3-deprived myeloid progenitors. J Immunol 1997;159:1225–1232 [PubMed] [Google Scholar]
- 34.Pugazhenthi S, Miller E, Sable C, Young P, Heidenreich KA, Boxer LM, Reusch JE. Insulin-like growth factor-I induces Bcl-2 promoter through the transcription factor camp-response element-binding protein. J Biol Chem 1999;274:27529–27535 [DOI] [PubMed] [Google Scholar]
- 35.Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, Fleming MD, Olsen JC, Miller SW, Weinberg RA, et al. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene 2002;21:4577–4586 [DOI] [PubMed] [Google Scholar]
- 36.Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 1998;279:1954–1958 [DOI] [PubMed] [Google Scholar]
- 37.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321 [DOI] [PubMed] [Google Scholar]
- 38.Foster JE, Gott K, Schuyler MR, Kozak W, Tesfaigzi Y. LPS-induced neutrophilic inflammation and Bcl-2 expression in metaplastic mucous cells. Am J Physiol Lung Cell Mol Physiol 2003;285:L405–L414 [DOI] [PubMed] [Google Scholar]
- 39.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, Yoon JH. Interleukin-1 beta and tumor necrosis factor-alpha induce Muc5ac overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem 2003;278:23243–23250 [DOI] [PubMed] [Google Scholar]
- 41.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway Muc5ac expression by IL-1beta and IL-17a: the NF-kappaB paradigm. J Immunol 2009;183:6236–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long E, Huynh HT, Zhao X. Involvement of insulin-like growth factor-1 and its binding proteins in proliferation and differentiation of murine bone marrow-derived macrophage precursors. Endocrine 1998;9:185–192 [DOI] [PubMed] [Google Scholar]
- 43.Wang XZ, Chen ZX, Zhang LJ, Chen YX, Li D, Chen FL, Huang YH. Expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor and its intervention by interleukin-10 in experimental hepatic fibrosis. World J Gastroenterol 2003;9:1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao P, Turdi S, Dong F, Xiao X, Su G, Zhu X, Scott GI, Ren J. Cardiac-specific overexpression of insulin-like growth factor I (IGF-1) rescues lipopolysaccharide-induced cardiac dysfunction and activation of stress signaling in murine cardiomyocytes. Shock 2009;32:100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veraldi KL, Gibson BT, Yasuoka H, Myerburg MM, Kelly EA, Balzar S, Jarjour NN, Pilewski JM, Wenzel SE, Feghali-Bostwick CA. Role of insulin-like growth factor binding protein-3 in allergic airway remodeling. Am J Respir Crit Care Med 2009;180:611–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith KR, Leonard D, McDonald JD, Tesfaigzi Y. Inflammation, mucous cell metaplasia, and Bcl-2 expression in response to inhaled lipopolysaccharide aerosol and effect of rolipram. Toxicol Appl Pharmacol 2011;253:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tesfaigzi Y, Rudolph K, Fischer MJ, Conn CA. Bcl-2 mediates sex-specific differences in recovery of mice from LPS-induced signs of sickness independent of IL-6. J Appl Physiol 2001;91:2182–2189 [DOI] [PubMed] [Google Scholar]
- 48.Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA. Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 2002;283:L952–L962 [DOI] [PubMed] [Google Scholar]
- 49.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 1998;16:495–521 [DOI] [PubMed] [Google Scholar]
- 50.Tesfaigzi Y, Harris JF, Hotchkiss JA, Harkema JR. DNA synthesis and Bcl-2 expression during development of mucous cell metaplasia in airway epithelium of rats exposed to LPS. Am J Physiol Lung Cell Mol Physiol 2004;286:L268–L274 [DOI] [PubMed] [Google Scholar]
- 51.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest 2008;133:489–495 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.