Abstract
Hyperthermia has been shown to confer cytoprotection and to augment apoptosis in different experimental models. We analyzed the mechanisms of both effects in the same mouse lung epithelial (MLE) cell line (MLE15). Exposing MLE15 cells to heat shock (HS; 42°C, 2 h) or febrile-range hyperthermia (39.5°C) concurrent with activation of the death receptors, TNF receptor 1 or Fas, greatly accelerated apoptosis, which was detectable within 30 minutes and was associated with accelerated activation of caspase-2, -8, and -10, and the proapoptotic protein, Bcl2-interacting domain (Bid). Caspase-3 activation and cell death were partially blocked by inhibitors targeting all three initiator caspases. Cells expressing the IκB superrepessor were more susceptible than wild-type cells to TNF-α–induced apoptosis at 37°C, but HS and febrile-range hyperthermia still increased apoptosis in these cells. Delaying HS for 3 hours after TNF-α treatment abrogated its proapoptotic effect in wild-type cells, but not in IκB superrepressor-expression cells, suggesting that TNF-α stimulates delayed resistance to the proapoptotic effects of HS through an NF-κB–dependent mechanism. Pre-exposure to 2-hour HS beginning 6 to16 hours before TNF-α treatment or Fas activation reduced apoptosis in MLE15 cells. The antiapoptotic effects of HS pretreatment were reduced in TNF-α–treated embryonic fibroblasts from heat shock factor-1 (HSF1)-deficient mice, but the proapoptotic effects of concurrent HS were preserved. Thus, depending on the temperature and timing relative to death receptor activation, hyperthermia can exert pro- and antiapoptotic effects through distinct mechanisms.
Keywords: heat shock, febrile-range hyperthermia, Fas, TNF, apoptosis
Clinical Relevance
Apoptosis is a cellular process that contributes to both health and disease, including acute lung injury, pneumonia, and asthma exacerbations. Elevated temperature is encountered during febrile illnesses, exertion, and exposure to hot temperature. Understanding how exposure to heat can modify apoptosis will allow heat and other treatments that activate similar mechanisms to be used to modify the course of disease and promote health.
Hyperthermia can either be cytoprotective or accelerate apoptosis (1–4). Exposure to nonlethal hyperthermia by itself stimulates coordinated expression of heat-inducible proteins that support cell survival in the face of cellular stress, facilitate repair of cellular damage, and confer resistance to subsequent injury (4). However, when preceded by a proinflammatory stimulus, exposure to similar or lesser hyperthermia can enhance cell death and tissue injury (3, 5–7), a phenomenon coined the “heat shock paradox.” The opposing effects of hyperthermia on cell and tissue injury and repair are well documented in the injured lung. Pre-exposure to core temperatures between 41.5°C and 43°C for 15 to 30 minutes conferred protection against lung injury induced 6 to 18 hours later in response to various triggers, including systemic LPS administration (8, 9), cecal ligation and puncture (10), lower extremity ischemia–reperfusion (11), hemorrhagic shock and resuscitation (12), and intratracheal instillation of phospholipase A2 (13). Protection in these models correlated with expression of heat shock (HS) protein (HSP) 70 in lungs and other organs (9–11, 13, 14). Mouse embryonic fibroblasts (MEFs) lacking heat shock factor-1 (HSF1), the heat-inducible transcriptional activator of the HSP genes, exhibited increased susceptibility to hyperoxia-induced cytotoxicity, which was blocked by reintroducing HSF1 expression (15). Nonthermal activation of HSF1 by infusion of sodium arsenite in rats stimulated HSP70 expression in lungs that was temporally correlated with protection against lung injury after cecal ligation and puncture (16). Collectively, these studies demonstrate that brief exposure to temperatures ≥4.5°C above basal levels conferred delayed protection against subsequent lung injury that was agonist-independent and required HSP gene expression. The protective effects attributed to the HS response include reduced inflammation, preservation of endothelial barrier function, and reduced epithelial injury (8, 11–13). HSP70 has been shown to block apoptosis by binding to Apaf-1, thereby preventing its recruitment to the apoptosome (17). More recently, Aschkenasy and colleagues (18) showed that adenoviral transfer of HSP70 via intratracheal instillation in mice subsequently subjected to cecal ligation and puncture reduced lung injury and lung cell apoptosis. These authors also showed that HSP70 transfer to mouse lung epithelial (MLE) cell line 12 (MLE12) cells reduced TNF-α–induced apoptosis. On the other hand, when HSP70 and HSP60 enter the extracellular environment, which may occur through active secretion or release from necrotic cells, these proteins exert proinflammatory activity through activation of the Toll-like receptor-4 complex (19, 20). This mechanism has been proposed to explain the HS paradox (21).
More recent studies have demonstrated that exposure to temperatures 2.5°C to 5°C above basal levels can also promote apoptosis when accompanied by a proinflammatory or apoptotic signal (5). Most of these studies attributed the proapoptotic effects of heat to interruption of NF-κB signaling (22–26) and reduced NF-κB–dependent expression of antiapoptotic proteins (27, 28). Meinander and colleagues (29) demonstrated that exposure to hyperthermia in the febrile range (∼39.5°C; febrile-range hyperthermia [FRH]) causes ubiquitinylation and degradation of the antiapoptotic protein, FLICE-like inhibitory protein (FLIP). We previously demonstrated that concurrent exposure to temperatures in the febrile or HS range augments pulmonary epithelial injury (30). Lipke and colleagues (5) confirmed these findings, and showed the epithelial injury to result from increased apoptosis, which the authors suggested was caused by inhibition of NF-κB signaling. We previously showed that exposing human neutrophils to FRH in culture accelerated activation of caspase-3 and -8 and cleavage of the proapoptotic protein, Bcl2-interacting domain (Bid), to its active form (3). The appearance of caspase-8 activation within 15 minutes of exposing neutrophils to FRH suggested that hyperthermia may directly accelerate early apoptotic signaling.
The purpose of this investigation was to explore the mechanisms underlying the opposing effects of hyperthermia on apoptosis in the same cell. Using the MLE15 cell line and MEFs from HSF1-deficient mice, and the extrinsic apoptosis inducers, TNF-α and Fas-activating antibody, we analyzed the roles of HSF1 and NF-κB in promoting and preventing apoptosis.
Materials and Methods
Cell Culture
MLE15 cells obtained from Dr. Jeffrey Whitsett (University of Cincinnati, Cincinnati, OH) were maintained in RPMI 1640 containing 0.5% insulin transferrin sodium selenite, 10 nM hydrocortisone, 0.5% transferrin, 10 nM β-estradiol, 2 mM L-glutamine, 10 mM HEPES, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 5% FBS (Life Technologies, Carlsbad, CA). MEFs from wild-type and HSF1-deficient mice obtained from Dr. Ivor Benjamin (University of Utah) were maintained in Dulbecco’s modified Eagle’s medium containing 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 5% FBS. MLE15 and MEFs were maintained at 37°C and 5% CO2.
Antibodies
Rabbit anti–poly ADP-ribose polymerase (PARP) and anti–caspase-8 antibodies were from Cell Signaling Technology (Beverly, MA), rabbit anti-Bid was from Imgenex (San Diego, CA), horseradish peroxidase–conjugated goat anti-mouse IgG, horseradish peroxidase–conjugated goat anti-rabbit IgG, and mouse anti–NF-κB p65 Cy3 conjugate were from Santa Cruz Biotechnologies (Santa Cruz, CA), rat anti–caspase-2 was from Alexis (San Diego, CA), rabbit anti–caspase-3 and mouse anti-tubulin were from Millipore (Temecula, CA), and rabbit anti–caspase-10 was from Abcam (Cambridge, MA).
Cell Heating Protocols
Cells in 35-mm plates containing 1 ml medium were warmed by transferring to incubators calibrated at 42°C or 39.5°C by electric thermometry (FLUKE Instruments, Everett, WA), as we have described previously (3). Cells were treated with recombinant mouse TNF-α (median effective dose [ED50], 0.008–0.05 ng/ml for L929 cells; R&D Systems, Minneapolis, MN) or 2.5 μg/ml agonistic Jo-2 anti–Fas antibody (BD-Pharmingen, Sparks, MD). Some cells were pretreated with an optimized concentration of 50 nM caspase-2 inhibitor I, caspase-8 inhibitor I (Calbiochem, La Jolla, CA), or the caspase-10 inhibitor, Z-Ala-Glu-Val-DL-Asp-fluoromethylketone (z-AEVD-FMK) (BioVision, Mountain View, CA) 40 minutes before TNF-α/HS treatment.
Cell Viability Assays
Survival of adherent cells was analyzed using a modified L929 bioassay protocol (31) by washing twice with 25 mM Tris, 140 mM NaCl, 3 mM KCl, 0.05% Tween-20, pH 8.0, staining with 0.04% crystal violet, washing with water, lysing in 1% SDS, and measuring absorbance at 570 nm.
Generation of IκB Superrepressor Stable Transfectants
The sequence for IκB superrepressor (IκBSR) (32) was cloned into T0 expression plasmid (Clontech, Mountain View, CA). MLE15 cells were transfected with either the IκBSR-expressing or insertless plasmid using FugeneHD (Roche Applied Science, Indianapolis, IN), selected using Zeocin (Invitrogen, Grand Island, NY), and cloned by limiting dilution.
Immunofluorescence
IκBSR-expressing monolayers on Lab-Tek slides were treated, then fixed with 4% paraformaldehyde, blocked with PBS containing 1% BSA, 1% donkey serum, 0.03% Triton X-100, anti–NF-κB p65 (Santa Cruz Biotechnologies), and donkey anti-mouse conjugated to Cy3 (Jackson Immunoresearch, West Grove, PA), counterstained with DAPI (Sigma, St. Louis, MO), and imaged by confocal microscopy using Fluo View software (Olympus America Inc., Central Valley, PA).
Fluorimetric Caspase-2, -8, and -10 Assays
Caspase-2, -10, and -8 activities in cell lysates were measured fluorimetrically using caspase-2 and -10 FAM-FLICA kits (Immunohistochemistry Technologies, Bloomington, MN), and the caspase-8 substrate, IETD-(7-amino-4-trifluoromethyl coumarin) (AFC; Calbiochem), as we have described previously (3).
Immunoblotting
Cells were lysed in Radio-Immunoprecipitation Assay buffer containing Protease Inhibitor and Phosphatase Inhibitor Cocktails (Sigma), immunoblotted, and imaged using Fuji LAS-1000 gel documentation system and ImageQuant software (Tokyo, Japan) as we have described previously (3).
Statistical Analysis
Data are presented as means (±SE). Differences between two treatments at a single time point or between two time points were analyzed by applying a Tukey honestly significant difference test to a one-way ANOVA.
Results
Exposure to Concurrent HS Augments TNF-α– and Fas-Induced Apoptosis
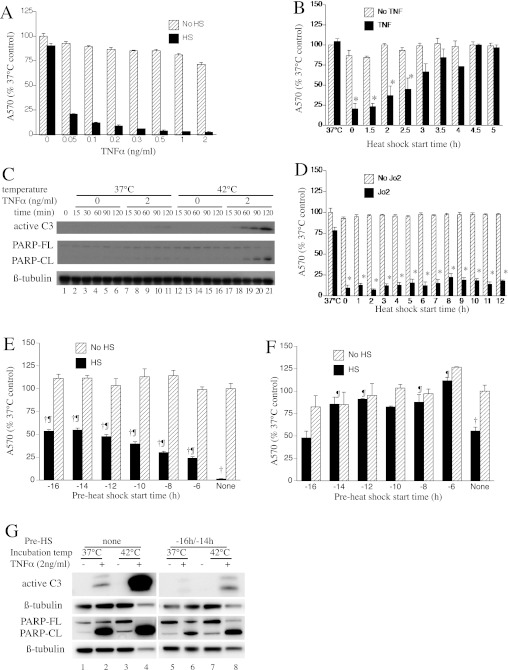
MLE15 cells were treated with 0.05 to 2 ng/ml TNF-α and incubated at 37°C for 24 hours, or were exposed to a 42°C HS for the first 2 hours, then switched back to 37°C for the remainder of the 24-hour incubation, and cell survival was assessed by crystal violet staining of the residual monolayers (Figure 1A). Exposure to HS decreased cell survival by 78 to 97% at TNF-α concentrations between 0.05 and 2 ng/ml (P < 0.05 by multivariate analysis of variance [MANOVA]), but had no effect on survival in the absence of exogenous TNF-α. To determine the optimal timing between TNF-α and HS treatments for apoptosis induction, MLE15 cells were treated with 0.3 ng/ml TNF-α and incubated for 37°C for 24 hours with or without a 2-hour HS exposure, beginning concurrently or at various times after TNF-α treatment, and cell survival was measured (Figure 1B). Reduced cell survival was only detectable in cells exposed to both TNF-α and HS, but varied with the time interval between the two exposures. Maximal cell death occurred when the cells were exposed to HS within 0.5 hour of TNF-α treatment, and gradually decreased as HS initiation was delayed. Cell death was not detectable when HS exposure was delayed for ≥3 hours after TNF-α treatment. To further evaluate the effect of HS on TNF-α–induced apoptosis, cells were treated for 15 to 120 minutes with or without 2 ng/ml TNF-α at 37°C or 42°C, followed by immunoblotting assays for active caspase-3 and PARP (Figure 1C). TNF-α stimulated capsase-3 activation and PARP cleavage at both temperatures. These molecular markers of apoptosis were increased within the initial 30 to 60 minutes in the cells incubated at 42°C. Neither caspase-3 activation nor PARP cleavage was detectable in the absence of TNF-α.
Figure 1.
Heat shock (HS) augments extrinsic apoptosis. (A) Mouse lung epithelial (MLE) cell line 15 (MLE15) cells were incubated with indicated concentration of recombinant mouse TNF-α for 24 hours at 37°C with or without a concurrent 2-hour 42°C HS, and survival was assessed by crystal violet staining residual adherent cells and measuring absorbance at 570 nm. Cell death was different between HS and no HS cells (P < 0.05 by MANOVA). (B) MLE15 cells were incubated with or without 0.3 ng/ml TNF-α for 24 hours at 37°C with 2-hour HS beginning at the indicated time after TNF-α; 37°C indicates no HS. (C) MLE15 cells were incubated for the indicated time at 37°C or 42°C with or without 2 ng/ml TNF-α, lysed, and immunoblotted for active caspase-3 (C3), poly ADP-ribose polymerase (PARP), and β-tubulin. PARP-CL, cleaved PARP; PARP-FL, full-length PARP. (D) MLE15 cells were treated as in (B), but with 2.5 μg/ml Jo2 anti-Fas antibody. (E and F) MLE15 cells were preconditioned with 2-hour HS beginning at the indicated time before induction of apoptosis with 0.3 ng/ml TNF-α (E) or 2.5 μg/ml Jo2 antibody (F), with or without concurrent 2-hour HS, and 24-hour survival assessed by crystal violet staining. (G) MLE15 cells without (left panel) or with 2-hour HS preconditioning beginning 16 hours before a 2-hour incubation at 37°C or 42°C, with or without 2 ng/ml TNF-α, were lysed and immunoblotted for active caspase-3 and PARP. All graphs show means (±SE) of six experiments. (B and D) *P < 0.05 versus TNF-α or Jo2 without HS. (E and F) †P < 0.05 and ¶P < 0.05 versus similarly preconditioned and treated cells without concurrent HS and cells that were sham (37°C) preconditioned. A570, absorbance at 570 nm.
Although Fas and TNF receptor 1 share the same proapoptotic signaling, NF-κB signaling after Fas activation is less direct (33) and is suppressed, in part, by cross-talk with phosphoinositide-3 kinase/protein kinase B (34). To determine whether HS exerts a similar proapoptotic effect after Fas activation, we repeated the HS timing experiment in Figure 1B, except cells were treated with the agonistic anti-Fas Jo2 antibody (2.5 μg/ml) instead of TNF-α (Figure 1D). HS exposure reduced cell survival after Fas activation and, in contrast with TNF-α, HS was still effective even when delayed up to 12 hours after Fas activation, reducing cell survival by 77% compared with Fas-treated cells incubated at 37°C (P < 0.05).
To determine whether preconditioning with HS could also block TNF-α– and Fas-induced cytotoxicity in the MLE15 cells, the cells were pre-exposed to a 2-hour HS beginning 4 to 16 hours before treatment with 0.3 ng/ml TNF-α (Figure 1E) or 2.5 μg/ml Jo2 (Figure 1F) and a second concurrent 2-hour HS. Cell survival was assessed by crystal violet staining 24 hours after addition of TNF-α or Jo2. Concurrent exposure to 2-hour HS and TNF-α or Jo2 reduced cell survival by 99% and 45% compared with untreated cells (P < 0.05). Preconditioning with 2-hour HS beginning 6 to 16 hours before TNF-α reduced cell death by 23 to 54% (P < 0.05). Preconditioning with 2-hour HS beginning 6 to 14 hours before Jo2 reduced cell death by 68 to 100% (P < 0.05), whereas, preconditioning beginning 16 hours before Jo2 treatment had no effect. Pre-exposure to HS beginning 4 hours before treatment with TNF-α/HS did not confer protection (date not shown). Treatment with a higher concentration of TNF-α (8 ng/ml) without concurrent HS reduced cell survival by 80%, and pre-exposure to HS beginning 8–16 hours earlier reduced cytotoxicity by 15 to 26% (P < 0.05; see Figure E1 in the online supplement). To confirm this observation using molecular markers of apoptosis, we pre-exposed MLE15 cells to a 2-hour HS beginning 16 hours before a 2-hour incubation with 2 ng/ml TNF-α at 37° or 42°C, and immunoblotted for active caspase-3 and PARP (Figure 1G). As seen in Figure 1C, in the absence of preconditioning with HS, exposure to TNF-α and concurrent HS caused much greater caspase-3 activation and PARP cleavage than TNF-α alone (compare lanes 4 and 2). Preconditioning with HS reduced both apoptosis markers (compare lanes 6 and 8 with lanes 2 and 4).
Concurrent HS Increases TNF-α–Induced Initiator Caspase Activation and Bid Cleavage
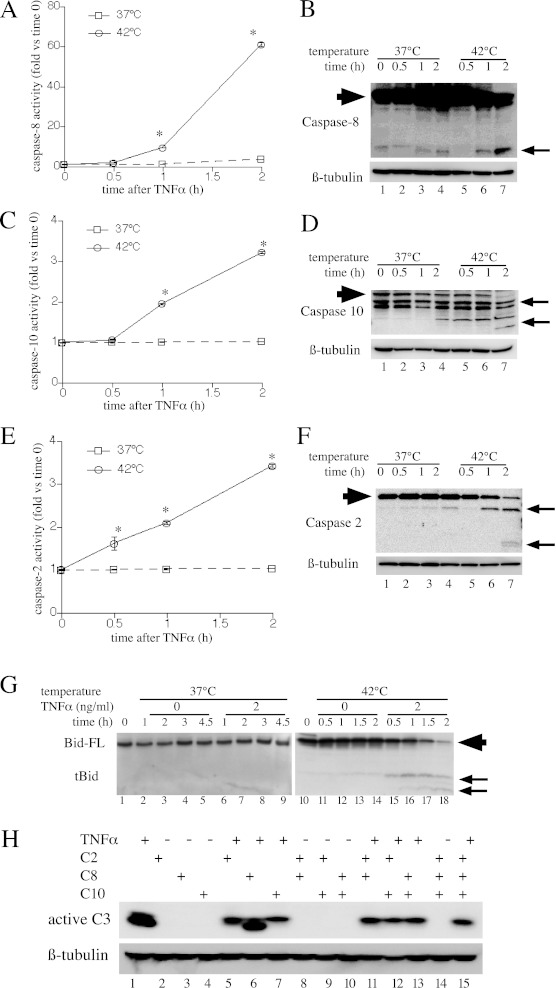
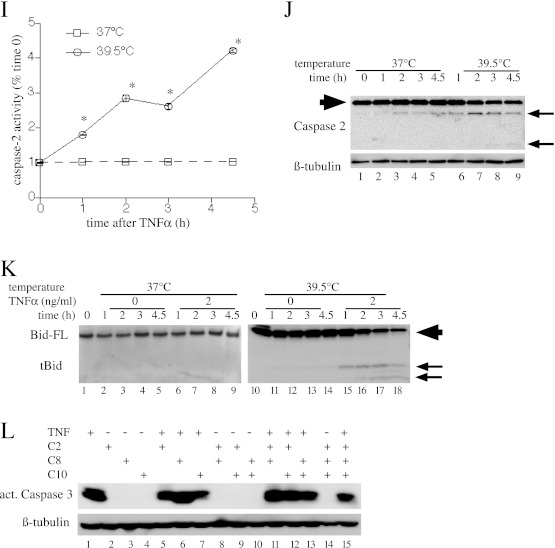
To evaluate the contribution of initiator caspase activation and cleavage of the proapoptotic Bcl-2 homology region 3-only protein, Bid, to apoptosis, we treated MLE15 cells with 2 ng/ml TNF-α and monitored caspase-2, -8, and -10 activation at 37°C and 42°C for 2 hours. Caspase activation was assessed using two complementary methods: by measuring caspase activity in cell lysates using fluorigenic substrates (Figures 2A, 2C, and 2E), and by analyzing caspase cleavage by immunoblotting (Figures 2B, 2D, and 2F). Activation of all three initiator caspases occurred within 30 to 60 minutes of TNF-α treatment in the 42°C cell cultures, but was not detectable during 2-hour incubation with TNF-α at 37°C cells. Cleavage of Bid, a substrate for caspase-2 and -8 (35) and for Jun-N-terminal kinase (36, 37), and a known mediator of TNF-α–induced apoptosis, was monitored by immunoblotting in similarly treated MLE15 cells (Figure 2G). Bid cleavage was observed within 30 minutes of treatment with TNF-α at 42°C, but was not detected up to 2 hours after TNF-α treatment in 37°C cells. Exposure to 42°C without TNF-α also stimulated Bid cleavage, although the activation was delayed and reduced compared with the TNF-α–treated cells (compare lanes 11–14 with lanes 15–18).
Figure 2.
The effects of HS on TNF-α–induced initiator caspase and Bcl2-interacting domain (Bid) activation. (A–G) MLE15 cells were incubated with 2 ng/ml TNF-α at 37°C or 42°C and sequentially lysed for fluorimetric (A, C, and E) and immunoblot (B, D, and F) analysis of caspase-8 (A and B), caspase-10 (C and D), and caspase-2 (E and F), and for immunoblot analysis of Bid (G). Caspase and Bid cleavage products are indicated by arrows (B, D, F, and G). Full-length Bid (Bid-FL) and caspases are indicated by large arrowhead. All graphs show means (±SE) of four experiments; *P < 0.05 versus 37°C. (B, D, F, and G) Each panel is representative of four similar blots. (H) MLE15 cells were pretreated with inhibitors of caspase-2, -8, and -10 (50 nM each) alone or in combination for 40 minutes at 37°C, then were incubated at 42°C with or without 2 ng/ml TNF-α and immunoblotted for activated caspase-3. A representative of three similar blots is shown. tBid, truncated Bcl2-interacting domain.
To further analyze the relative contributions of the three initiator caspases to apoptosis TNF-α and HS-treated cells, we analyzed the capacity of inhibitors of caspase-2, -8, and 10 to block caspase 3 activation in MLE15 cells after 2-hour incubation with 2 ng/ml TNF-α at 42°C (Figure 2H). The three inhibitors each partially reduced caspase-3 activation, but even combined treatment with all three inhibitors failed to abolish caspase-3 activation (lane 15).
The Proapoptotic Action of Concurrent HS in TNF-α–Treated MLE15 Cells Is NF-κB Independent
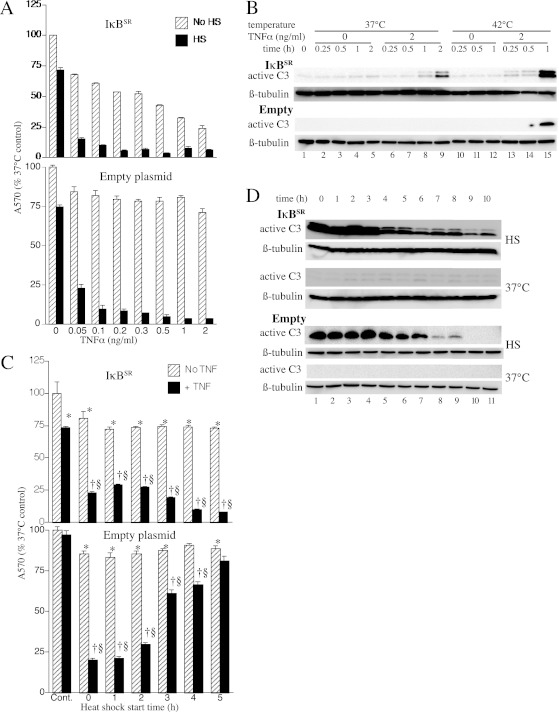
To confirm IκBSR function in the stable transfectants, we treated MLE15 cells transfected with IκBSR and control cells transfected with empty expression plasmid with TNF-α, and monitored nuclear translocation of NF-κB by immunostaining for NF-κB p65 and confocal imaging (Figure E2). Within 30 minutes of TNF-α treatment, control cells exhibited nuclear p65 staining. In contrast, nuclear p65 staining was not evident in the IκBSR-expressing cells. Having confirmed IκBSR function, we analyzed the effect of HS on TNF-α–induced apoptosis by crystal violet staining residual cells (Figures 3A and 3C) and by immunoblotting for caspase-3 (Figures 3B and 3D). In the absence of HS, the IκBSR-expressing cells exhibited increased sensitivity to TNF-α–induced apoptosis compared with wild-type and control cells (Figures 1A and 3A). TNF-α at 0.3 ng/ml reduced survival by 48% in IκBSR-expressing cells, whereas 2 ng/ml TNF-α caused only 29% reduction in survival of wild-type and control transfectants. Exposure to HS concurrent with TNF-α treatment increased apoptosis in all three cell populations (Figures 1 and 3A). However, unlike in the wild-type (Figure 1B) and control cells (Figure 3C), HS exerted cytotoxic effects in IκBSR-expressing cells even when HS was delayed for 5 hours after addition of TNF-α (Figure 3C). Immunoblotting showed similar caspase-3 activation in control and IκBSR-expressing cells when HS exposure occurred within 4 hours of TNF-α treatment, but much greater caspase-3 activation in the IκBSR-expressing than wild-type cells when HS was delayed for 7 to 12 hours (Figure 3D).
Figure 3.
Cytotoxic effect of HS persists when NF-κB signaling is blocked. (A) MLE15 cells stably transfected with IκB superrepressor (IκBSR) expression plasmid (upper panel) or empty plasmid (lower panel) were treated with 0.05 to 2 ng/ml TNF-α at 37°C for 24 hours with or without a concurrent 2-hour 42°C HS and survival assessed by crystal violet staining. (B) IκBSR-expressing MLE15 cells were incubated with 2 ng/ml TNF-α at 37°C or 42°C, sequentially lysed, and immunoblotted for active caspase-3. (C) MLE15 cells stably transfected with IκBSR expression plasmid (upper panel) or empty plasmid (lower panel) were incubated at 37°C for 24 hours with or without 0.3 ng/ml TNF-α and with 2-hour 42°C HS initiated at the times indicated, and survival was assessed by crystal violet staining. Control cells received no HS. (D) Wild-type (WT) and IκBSR-expressing MLE15 cells incubated with or without 0.3 ng/ml TNF-α were exposed to 2-hour 42°C HS initiated at the times indicated. Cells were lysed at the end of HS and immunoblotted for active caspase-3. Control cells (WT-37°C and IκBSR-37°C) were incubated in parallel without HS. All graphs show means (±SE) of four experiments; (A) 42°C is significantly different from no HS control by MANOVA (P < 0.0001); (C) *P < 0.05, †P < 0.05, and §P < 0.05 versus no–TNF-α control, the same HS exposure without TNF-α, and TNF-α without HS, respectively.
Cytoprotection Conferred by HS Preconditioning, but Not the Proapoptotic Effect of Concurrent HS, Is HSF1 Dependent
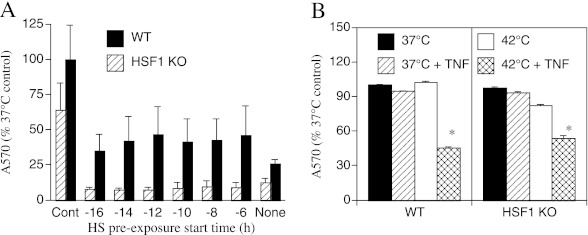
Although the cytoprotective effects of HS preconditioning have been shown to require HSF1 (15), the role of HSF1 in the proapoptotic action of HS after TNF-α treatment is not known. To analyze the requirement for HSF1 for the cytoprotective and proapoptotic effects of HS, we used MEFs obtained from wild-type and HSF1-null mice. The MEFs were pre-exposed to 2-hour HS beginning 6 to 16 hours prior to treatment with 4 ng/ml TNF-α concurrent with a second HS (Figure 4A). Concurrent exposure to TNF-α and HS reduced cell survival in wild-type and HSF1-null MEFs to 26 and 12%, respectively. Pre-exposure to HS improved survival to 45% (P = 0.04) in wild-type MEFs, but did not improve survival in the HSF1-null MEFs, thus confirming previous studies demonstrating that HSF1 is required for cytoprotection conferred by heat preconditioning (38). To analyze the HSF1 requirement for the proapoptotic effects of HS, wild-type and HSF1-null MEFs were incubated for 4 hours in the absence or presence of 4 ng/ml TNF-α with or without concurrent 2-hour HS (Figure 4B). The wild-type and HSF1-null MEFs exhibited a similar response to TNF-α and HS. Neither TNF-α nor HS alone caused detectable cytotoxicity, but concurrent exposure to TNF-α and HS reduced cell survival by 54 and 46% in the wild-type and HSF1-null MEFs, respectively.
Figure 4.
HSF1 is required for cytoprotective effect of HS pre-conditioning but not for the cytotoxic effect of concurrent HS and TNF-α treatment. (A) WT and HSF1-knockout (KO) mouse embryonic fibroblasts (MEFs) were preconditioned with 2-hour 42°C HS beginning at the indicated time, treated with 4 ng/ml TNF-α and concurrent 2-hour HS, incubated for an additional 2 hours at 37°C, and cell survival was assessed by crystal violet staining. Control-treated cells were incubated in parallel at 37°C without TNF-α or HS. (B) WT and HSF1-KO MEFs were incubated with or without 4 ng/ml TNF-α and with or without a concurrent 2-hour 42°C HS, incubated at 37°C for an additional 2 hours, and cell survival was assessed by crystal violet staining. Means (±SE) of four experiments are shown. (A) WT is significantly different from HSF1-KO (P = 0.04 by MANOVA). (B) *P < 0.05 versus all three other treatments.
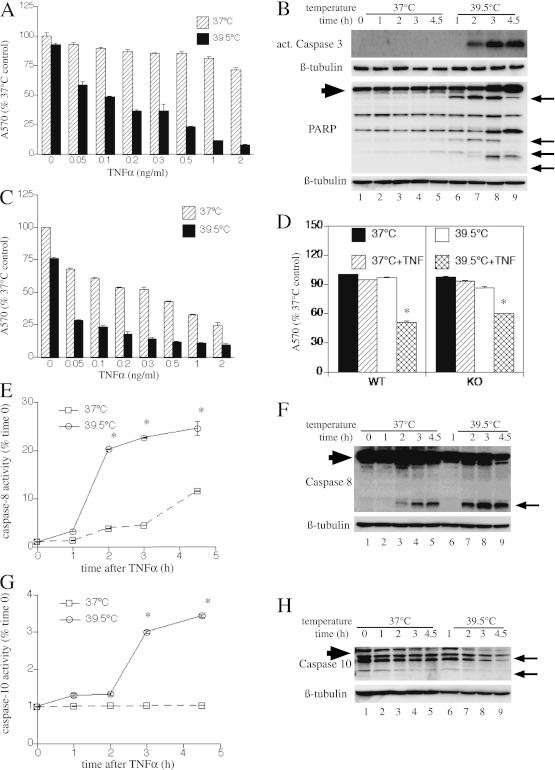
Concurrent Exposure to FRH (39.5°C) Augments Extrinsic Apoptosis through NF-κB– and HSF1-Independent Pathways
To analyze the mechanism by which concurrent exposure to more moderate and clinically relevant hyperthermia augments extrinsic apoptosis, we substituted continuous exposure to 39.5°C for the 2-hour HS. In agreement with previous findings of Lipke and colleagues (5), raising the temperature from 37°C to 39.5°C in TNF-α–treated MLE15 cells increased toxicity (Figure 5A). Similar to the effect with HS, exposure to 39.5°C for 24 hours in the absence of TNF-α did not change cell survival, but reduced survival by 42 to 92% in cells treated with 0.05–2 ng/ml TNF-α (P < 0.0001). Similarly, incubating MLE15 cells with 2.5 μg/ml Jo2 antibody for 24 hours failed to alter MLE15 survival at 37°C, but reduced survival to 18% at 39.5°C, and HS preconditioning conferred cytoprotection (P < 0.05; Figure E3). The reduced cell survival in the 39.5°C TNF-α–treated cells was accompanied by increased caspase-3 activation and PARP cleavage (Figure 5B), but onset was slower compared with cells exposed to TNF-α at 42°C (compare Figure 5B with Figure 1C). We also noted that the cells incubated at 39.5°C exhibited increases in both full-length and cleaved PARP, suggesting possible onset of secondary necrosis. To determine whether FRH may exert its proapoptotic effects by interfering with NF-κB signaling, we analyzed TNF-α–induced cytotoxicity at 37°C and 39.5°C in IκBSR-expressing MLE15 cells by crystal violet staining (Figure 5C) and activation of caspase-3 by immunoblotting (Figure E4). The IκBSR-expressing cells exhibited substantially higher TNF-α–induced cytotoxicity at 39.5°C than at 37°C, indicating that the death-promoting effects of FRH, like HS, are NF-κB independent. Likewise, we analyzed the HSF1 requirement for augmented TNF-α cell death at 39.5°C by comparing survival in wild-type and HSF1-null MEFs incubated for 4 hours with or without 4 ng/ml TNF-α at 37°C or 39.5°C (Figure 5D). Similar to HS treatment, the HSF1-null MEFs were as sensitive to TNF-α–induced toxicity at 39.5°C as wild-type MEFs.
Figure 5.
Concurrent exposure to febrile-range hyperthermia (FRH) (39.5°C) augments extrinsic apoptosis through NF-κB– and HSF1-independent pathways. (A) MLE15 cells were incubated for 24 hours at 37°C or 39.5°C with the indicated doses of TNF-α and cell survival was measured by crystal violet staining. (B) MLE15 cells were treated with 2 ng/ml TNF-α at 37°C or 39.5°C for the indicated time, and cells were lysed and immunoblotted for active caspase-3, PARP, and β-tubulin. (C) IκBSR-expressing MLE15 cells were treated as described in (A) and cell survival assessed by crystal violet staining. (D) WT and HSF1-KO MEFs were incubated for 4 hours at 37°C or 39.5°C with or without 4 ng/ml TNF-α and cell survival was assessed by crystal violet staining. (E–K) MLE15 cells were incubated with 2 ng/ml TNF-α at 37°C or 39.5°C and sequentially lysed for fluorimetric (E, G, and I) and immunoblot (F, H, and J) analysis of caspase-8 (E and F), caspase-10 (G and H), and caspase-2 (I and J), and for immunoblot analysis of Bid (K). (F, H, J, and K) Caspase and Bid cleavage products are indicated by arrows. Full-length Bid and caspases are indicated by large arrowhead. All graphs show means (±SE) of four experiments; *P < 0.05 versus 37°C. (A and C) There was a difference between 37°C and 39.5°C and between TNF-α–treated and untreated at 37°C (P < 0.0001 by MANOVA). (F, H, I, J, and K) Each panel is representative of four similar blots. (L) MLE15 cells were pretreated with inhibitors of caspase-2, -8, and -10 (50 nM) alone or in combination for 40 minutes at 37°C, then were incubated at 39.5° with or without 2 ng/ml TNF-α and immunoblotted for activated caspase-3. A representative of three similar blots is shown.
Similar to HS exposure, incubating TNF-α–treated cells at 39.5°C increased caspase-2, -8, and -10 activation and Bid cleavage (Figures 5E–5K), but more gradually than did HS exposure (compare Figures 5E–5K with Figures 2A–2G). Finally, pretreating MLE15 cells with inhibitors of caspse-2, -8, and -10, alone or in combination, demonstrated a pattern of effect similar to that in MLE15 cells exposed to TNF-α and concurrent HS (compare Figure 5L with Figure 2H). Combined treatment with all three inhibitors partially blocked caspase-3 activation in cells incubated with 2 ng/ml TNF-α for 24 hours at 39.5°C.
Discussion
This study extends previous observations of cytoprotective and cytotoxic effects of HS. Specifically, we show that HS can be both cytoprotective and cytotoxic in the same cell. Our data underscore the critical importance of timing between HS and death receptor activation, showing that exposure to more moderate and clinically relevant FRH exerts similar proapoptotic effects, and demonstrating the HSF1 and NF-κB independence and partial caspase-2, -8, and -10 dependence of the proapoptotic effects.
We used MLE15 cells, which are derived from lung tumors arising in mice transgenic for SV40 large T antigen under control of the surfactant protein (SP) C promoter, express SPA, SPB, and SPC, and have the morphologic characteristics of distal respiratory epithelial cells. Our laboratory (30) and that of Lipke and colleagues (5) have previously shown that mice coexposed to intratracheal LPS and FRH exhibit increased apoptosis and necrosis of the distal respiratory epithelium in vivo. Lipke and colleagues (5) showed that, despite expressing SV40 large T antigen, MLE15 cells undergo augmented apoptosis when coexposed to FRH and TNF-α. The methanol fixation and crystal violet staining assay used in the current study provides a high-throughput method to detect cell death, but cannot distinguish between apoptosis and primary cell detachment with or without anoikis. However, the immunoblot analysis of the adherent monolayer, demonstrating caspase-3 activation and PARP cleavage, confirmed that the observed cell death was caused by apoptosis and demonstrates a similar sensitivity to TNF-α/heat-induced apoptosis as that found by Lipke and colleagues in the same cells.
Preconditioning with HS mitigated the apoptosis induced by subsequent treatment with TNF-α or Fas activation. The cytoprotective effect of HS required at least 4 hours of recovery at 37°C before death receptor activation with TNF-α or anti-Fas antibody, and was not evident in MEFs lacking an intact HSF1 gene. These results are consistent with the current paradigm of HS-induced cytoprotection exerted through HSF1-dependent expression of HSP genes (39). Although originally discovered in the context of thermal injury, many subsequent studies have reported that HS confers protection against diverse cellular injuries, including TNF-α–induced cell death (40). Protection of WEHI-164 cells against TNF-α–induced cell death by HS preconditioning required active gene transcription and protein synthesis (40), was associated with increased HSP70 expression, and could be reproduced by overexpressing HSP70 (41). Kim and colleagues (42) showed that carbon monoxide preconditioning also increases HSP70 expression and confers protection against TNF-α–induced apoptosis, and both responses were abolished in cells transfected with HSP70-targeting small interfering RNA or cells from HSF1-null mice. Collectively, these results demonstrate that HSF1-mediated HSP expression is critical for the cytoprotection conferred by HS preconditioning. The loss of HS-conferred cytoprotection for TNF-α–induced apoptosis in HSF1-deficient MEFs found in the current study is consistent with this paradigm. In contrast, the proapoptotic effects of concurrent HS and TNF-α exposure were comparable in HSF1-deficient and HSF1-sufficient MEFs, demonstrating a critical difference in mechanism of the cytoprotective and proapoptotic effects of HS.
Previous studies of the synergy between hyperthermia and TNF-α for apoptosis induction have attributed the proapoptotic effects of hyperthermia to interruption of NF-κB signaling and reduced expression of NF-κB–dependent antiapoptotic gene expression (22–28). Lipke and colleagues (5) made similar conclusions about the apoptotic effects of 39.5°C exposure in TNF-α–treated MLE15 based on reduced TNF-α–stimulated NF-κB reporter plasmid activity at 39.5°C versus 37°C, and enhanced TNF-α–induced cell death in the presence of the NF-κB inhibitor, BMS 345.541. In the current study, we detected enhanced apoptosis as early as 30 minutes after coexposure to TNF-α and hyperthermia, which is much more rapid than the reported loss of NF-κB signaling, which required several hours and correlated with HSP gene expression (22, 23, 25, 26). To confirm an NF-κB–independent mechanism for the proapoptotic activity of hyperthermia, we created MLE15 clones stably transfected with IκBSR, which is incapable of being targeted for proteolysis and prevents nuclear translocation of NF-κB (32). We confirmed IκBSR function by showing that TNF-α–induced NF-κB nuclear translocation did not occur in the IκBSR-expressing cells. Although the IκBSR-expressing cells showed increased sensitivity to TNF-α–induced cell death at 37°C (compare Figures 1A and 3A), coexposure to HS (Figures 3A and 3B) or 39.5°C (Figure 5C) still greatly augmented cell death and caspase-3 activation, which was evident within 15 minutes of TNF-α treatment. Thus, even when NF-κB signaling is disrupted, exposure to hyperthermia still accelerates and enhances the early phase of apoptosis.
HS was only cytotoxic if applied within 3 hours of TNF-α in wild-type MLE15 cells, but protection against delayed HS was lost in the IκBSR-expressing cells (compare Figures 1B and 3C). These results are consistent with the expected time course of TNF-α–induced NF-κB activation and antiapoptotic gene expression (43–45). Wild-type cells treated with anti-Fas antibody exhibited prolonged susceptibility to the apoptotic effects of HS that resembled the pattern seen in the TNF-α–treated IκBSR-expressing cells. Although Fas activation has been shown to activate NF-κB in some cells, the process appears to be indirect (33) and less robust (34) compared with TNF-α treatment. In fact, in some cells, Fas activation has been shown to repress NF-κB activation by activating proteolysis of NF-κB signaling elements (46).
The results of the current study suggest that hyperthermia engages at least two mechanisms that regulate apoptosis: one that acts very early and is NF-κB independent, and a second that is delayed and is blocked by NF-κB–dependent gene expression. A more detailed characterization of the early mechanism is required, but we have already shown that all initiator caspases and Bid undergo accelerated and augmented activation. It is possible that apoptosis detected as early as 15 minutes after TNF-α exposure is most likely the result of modifications of the Fas and TNF receptor 1 signaling complex, such as inactivation of associated FLIP (29), increased specific activity of one or more initiator caspases, or accelerated assembly of the death-inducing signaling complex.
In summary, we have demonstrated the HS paradox—the capacity of the same HS exposure to confer cytoprotection or augment apoptosis—in the same cell, and have defined the temporal relationship with extrinsic apoptosis inducers that determines its ultimate effect. We have confirmed the HSF1 dependence of cytoprotection, while demonstrating that the proapoptotic effect is HSF1 independent. Furthermore, we have shown that both augmentation of apoptosis by exposures to HS and febrile-range temperatures is rapid and, at least in its early stages, is independent of NF-κB signaling. More complete understanding of how changes in temperature within the clinically relevant range modify cell survival and death is necessary for better understanding of disease pathophysiology, improvement of body temperature management during illness, and identification of new therapeutic targets.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants GM069431 (I.S.S.) and GM066855, HL69057 and HL085256 (J.D.H.), and by Veterans Affairs Merit Review grants to J.D.H. and I.S.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0105OC on September 6, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hasday JD, Shah N, Mackowiak PA, Tulapurkar M, Nagarsekar A, Singh I. Fever, hyperthermia, and the lung: it's all about context and timing. Trans Am Clin Climatol Assoc 2011;122:34–47 [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HP, Morse D, Choi AM. Heat-shock proteins: new keys to the development of cytoprotective therapies. Expert Opin Ther Targets 2006;10:759–769 [DOI] [PubMed] [Google Scholar]
- 3.Nagarsekar A, Greenberg RS, Shah NG, Singh IS, Hasday JD. Febrile-range hyperthermia accelerates caspase-dependent apoptosis in human neutrophils. J Immunol 2008;181:2636–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell 2010;40:253–266 [DOI] [PubMed] [Google Scholar]
- 5.Lipke AB, Matute-Bello G, Herrero R, Kurahashi K, Wong VA, Mongovin SM, Martin TR. Febrile-range hyperthermia augments lipopolysaccharide-induced lung injury by a mechanism of enhanced alveolar epithelial apoptosis. J Immunol 2010;184:3801–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abello PA, Buchman TG. Heat shock–induced cell death in murine microvascular endothelial cells depends on priming with tumor necrosis factor-alpha or interferon-gamma. Shock 1994;2:320–323 [DOI] [PubMed] [Google Scholar]
- 7.Buchman TG, Abello PA, Smith EH, Bulkley GB. Induction of heat shock response leads to apoptosis in endothelial cells previously exposed to endotoxin. Am J Physiol 1993;265:H165–H170 [DOI] [PubMed] [Google Scholar]
- 8.Chu EK, Ribeiro SP, Slutsky AS. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med 1997;25:1727–1732 [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol 1993;265:R1447–R1457 [DOI] [PubMed] [Google Scholar]
- 10.Villar J, Ribeiro SP, Mullen JBM, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med 1994;22:914–921 [PubMed] [Google Scholar]
- 11.Javadpour M, Kelly CJ, Chen G, Stokes K, Leahy A, Bouchier-Hayes DJ. Thermotolerance induces heat shock protein 72 expression and protects against ischaemia–reperfusion–induced lung injury. Br J Surg 1998;85:943–946 [DOI] [PubMed] [Google Scholar]
- 12.Pittet JF, Lu LN, Geiser T, Lee H, Matthay MA, Welch WJ. Stress preconditioning attenuates oxidative injury to the alveolar epithelium of the lung following haemorrhage in rats. J Physiol 2002;538:583–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar J, Edelson JD, Post M, Mullen JB, Slutsky AS. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am Rev Respir Dis 1993;147:177–181 [DOI] [PubMed] [Google Scholar]
- 14.Hiratsuka M, Yano M, Mora BN, Nagahiro I, Cooper JD, Patterson GA. Heat shock pretreatment protects pulmonary isografts from subsequent ischemia–reperfusion injury. J Heart Lung Transplant 1998;17:1238–1246 [PubMed] [Google Scholar]
- 15.Malhotra V, Kooy NW, Denenberg AG, Dunsmore KE, Wong HR. Ablation of the heat shock factor-1 increases susceptibility to hyperoxia-mediated cellular injury. Exp Lung Res 2002;28:609–622 [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit Care Med 1994;22:922–929 (see comments) [DOI] [PubMed] [Google Scholar]
- 17.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2000;2:469–475 [DOI] [PubMed] [Google Scholar]
- 18.Aschkenasy G, Bromberg Z, Raj N, Deutschman CS, Weiss YG. Enhanced Hsp70 expression protects against acute lung injury by modulating apoptotic pathways. PLoS ONE 2011;6:e26956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 2000;6:435–442 [DOI] [PubMed] [Google Scholar]
- 20.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol 2000;164:558–561 [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW. Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm Allergy Drug Targets 2007;6:91–100 [DOI] [PubMed] [Google Scholar]
- 22.Curry HA, Clemens RA, Shah S, Bradbury CM, Botero A, Goswami P, Gius D. Heat shock inhibits radiation-induced activation of NF-kappaB via inhibition of I-kappaB kinase. J Biol Chem 1999;274:23061–23067 [DOI] [PubMed] [Google Scholar]
- 23.DeMeester SL, Buchman TG, Qiu Y, Jacob AK, Dunnigan K, Hotchkiss RS, Karl I, Cobb JP. Heat shock induces IkappaB-alpha and prevents stress-induced endothelial cell apoptosis. Arch Surg 1997;132:1283–1287, discussion 1287–1288 [DOI] [PubMed] [Google Scholar]
- 24.Kim WJ, Back SH, Kim V, Ryu I, Jang SK. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol Cell Biol 2005;25:2450–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong HR, Ryan M, Wispe J. Stress response decreases NF-κB nuclear translocation and increases I-κBα expression in A549 cells. J Clin Invest 1997;99:2423–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol 2000;164:5416–5423 [DOI] [PubMed] [Google Scholar]
- 27.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol 2002;3:221–227 [DOI] [PubMed] [Google Scholar]
- 28.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB–regulated X-chromosome–linked iap gene expression protects endothelial cells from tumor necrosis factor alpha–induced apoptosis. J Exp Med 1998;188:211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meinander A, Soderstrom TS, Kaunisto A, Poukkula M, Sistonen L, Eriksson JE. Fever-like hyperthermia controls T lymphocyte persistence by inducing degradation of cellular FLIPshort. J Immunol 2007;178:3944–3953 [DOI] [PubMed] [Google Scholar]
- 30.Rice P, Martin E, He J-R, Frank M, DeTolla L, Hester L, O’Neill T, Manka C, Singh I, Hasday J. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol 2005;174:3676–3685 [DOI] [PubMed] [Google Scholar]
- 31.Flick DA, Gifford GE. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods 1984;68:167–175 [DOI] [PubMed] [Google Scholar]
- 32.Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol 1995;15:2809–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SM, Kim EJ, Suk K, Lee WH. Stimulation of Fas (CD95) induces production of pro-inflammatory mediators through ERK/JNK-dependent activation of NF-kappaB in THP-1 cells. Cell Immunol 2011;271:157–162 [DOI] [PubMed] [Google Scholar]
- 34.Iyer AK, Azad N, Talbot S, Stehlik C, Lu B, Wang L, Rojanasakul Y. Antioxidant c-FLIP inhibits Fas ligand–induced NF-kappaB activation in a phosphatidylinositol 3-kinase/Akt–dependent manner. J Immunol 2011;187:3256–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsson M, Vakifahmetoglu H, Abruzzo PM, Hogstrand K, Grandien A, Zhivotovsky B. DISC-mediated activation of caspase-2 in DNA damage–induced apoptosis. Oncogene 2009;28:1949–1959 [DOI] [PubMed] [Google Scholar]
- 36.Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor–induced apoptosis. Mol Cell Biol 2002;22:3415–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell 2003;115:61–70 [DOI] [PubMed] [Google Scholar]
- 38.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem 1998;273:7523–7528 [DOI] [PubMed] [Google Scholar]
- 39.Cotto JJ, Morimoto RI. Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors. Biochem Soc Symp 1999;64:105–118 [PubMed] [Google Scholar]
- 40.Jaattela M, Saksela K, Saksela E. Heat shock protects WEHI-164 target cells from the cytolysis by tumor necrosis factors alpha and beta. Eur J Immunol 1989;19:1413–1417 [DOI] [PubMed] [Google Scholar]
- 41.Jaattela M, Wissing D, Bauer PA, Li GC. Major heat shock protein Hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J 1992;11:3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J Immunol 2005;175:2622–2629 [DOI] [PubMed] [Google Scholar]
- 43.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 2001;21:5299–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee R, Collins T. Nuclear factor-kappaB and cell survival: IAPs call for support. Circ Res 2001;88:262–264 [DOI] [PubMed] [Google Scholar]
- 45.Chu B, McKinsey T, Liu L, Gentry J, Malim M, Ballard D. Suppression of tumor necrosis factor–induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kB control. Proc Natl Acad Sci USA 1997;94:10057–10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravi R, Bedi A, Fuchs EJ. CD95 (Fas)-induced caspase-mediated proteolysis of NF-kappaB. Cancer Res 1998;58:882–886 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.