Abstract
Previous studies by our group as well as others have shown that acute adenosine exposure enhances lung vascular endothelial barrier integrity and protects against increased permeability lung edema. In contrast, there is growing evidence that sustained adenosine exposure has detrimental effects on the lungs, including lung edema. It is well established that adenosine modulates lung inflammation. However, little is known concerning the effect of sustained adenosine exposure on lung endothelial cells (ECs), which are critical to the maintenance of the alveolar–capillary barrier. We show that exogenous adenosine plus adenosine deaminase inhibitor caused sustained elevation of adenosine in lung ECs. This sustained adenosine exposure decreased EC barrier function, elevated cellular reactive oxygen species levels, and activated p38, JNK, and RhoA. Inhibition of equilibrative nucleoside transporters (ENTs) prevented sustained adenosine-induced p38 and JNK activation and EC barrier dysfunction. Inhibition of p38, JNK, or RhoA also partially attenuated sustained adenosine-induced EC barrier dysfunction. These data indicate that sustained adenosine exposure causes lung EC barrier dysfunction via ENT-dependent intracellular adenosine uptake and subsequent activation of p38, JNK, and RhoA. The antioxidant N-acetylcysteine and the NADPH inhibitor partially blunted sustained adenosine-induced JNK activation but were ineffective in attenuation of p38 activation or barrier dysfunction. p38 was activated exclusively in mitochondria, whereas JNK was activated in mitochondria and cytoplasm by sustained adenosine exposure. Our data further suggest that sustained adenosine exposure may cause mitochondrial oxidative stress, leading to activation of p38, JNK, and RhoA in mitochondria and resulting in EC barrier dysfunction.
Keywords: adenosine deaminase, equilibrative nucleoside transporters, oxidative stress, MAP kinases, RhoA
Clinical Relevance
This study documents a previously unrecognized mechanism by which sustained adenosine exposure induces lung endothelial barrier dysfunction via nucleoside transporter–mediated adenosine uptake and subsequent activation of p38, JNK, and RhoA. These results suggest that pulmonary edema caused by adenosine deaminase deficiency may be due to a direct effect of adenosine on lung endothelium. These findings offer new insights into the diverse effects of adenosine on pulmonary circulation and are relevant to increased permeability lung edema in acute lung injury and acute respiratory distress syndrome. The results also suggest a novel role for nucleoside transport inhibitors as a potential treatment for lung edema.
Increased permeability pulmonary edema is a life-threatening complication characteristic of acute lung injury (ALI) and acute respiratory distress syndrome. The purine nucleoside adenosine has been shown to protect (1–4) and cause (5) pulmonary edema and inflammation in animal models of ALI. The mechanisms governing these paradoxical effects of adenosine are poorly understood.
Adenosine is a potent signaling molecule. Under homeostatic conditions, extracellular adenosine concentrations are low (40–600 nM) (6). However, extracellular adenosine levels are increased in response to tissue injury. High plasma adenosine (10–190 μM) has been reported in patients with sepsis-induced ALI (7, 8) and tissue ischemia (9). Adenosine is also significantly elevated in the lungs of animals with ALI caused by acute hypoxia (10–13), high tidal volume ventilation (14), endotoxin (15), and bleomycin (16). Acutely elevated adenosine has been shown to have a protective, antiinflammatory effect in various animal models of ALI (1–4). However, nonsurviving patients with sepsis have much higher plasma adenosine than survivors (7), suggesting a link between high plasma adenosine and poor outcome. Adenosine is also elevated in patients with chronic lung diseases. For example, adenosine is increased in bronchoalveolar lavage fluid and in the exhaled breath condensate of patients with asthma (17, 18) and in the sputum of patients with cystic fibrosis (19). Sustained elevated adenosine also contributes to chronic lung injury (20, 21). Understanding the mechanisms underlying the tissue-protective and tissue-destructive properties of adenosine signaling is critical for advancing treatment of the various lung diseases associated with the elevation of adenosine.
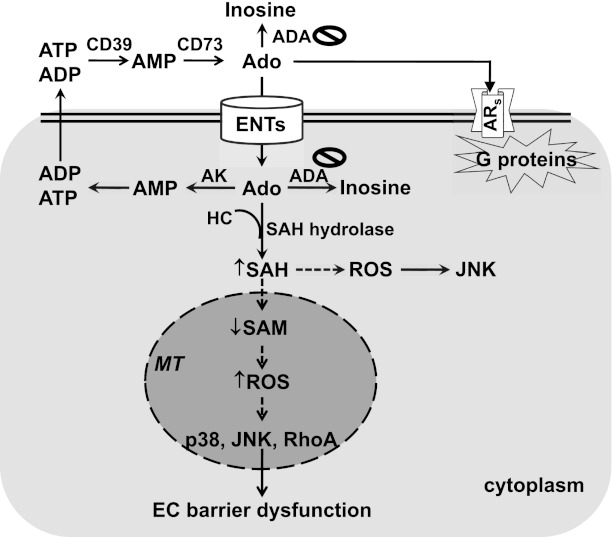
Extracellular adenosine can act via cell surface adenosine receptors (ARs) (Figure 1). Among the four types of ARs, endothelial cells predominantly express A2AR and A2BR (22–24). Extracellular adenosine can be metabolized to inosine by cell surface–bound adenosine deaminase (ADA). In addition, elevated adenosine can be taken up into cells by equilibrative nucleoside transporters (ENTs) or by concentrative nucleoside transporters. Intracellular adenosine is metabolized by intracellular ADA and/or by adenosine kinase. In addition, elevated adenosine can react with homocysteine to generate S-adenosyl-l-homocysteine (SAH) due to inhibition of the reversible enzyme SAH hydrolase. We and others have previously demonstrated that acute adenosine exposure protects against ALI and enhances endothelial barrier function via A2AR and A2BR (1–3). However, the mechanism of the deleterious effect of adenosine on the lungs and the effect of sustained adenosine exposure on lung endothelial cells are poorly understood.
Figure 1.
Proposed model. Sustained adenosine exposure causes endothelial barrier dysfunction via nucleoside transporter–mediated intracellular adenosine uptake and subsequent mitochondrial oxidative stress–induced activation of p38, JNK, and RhoA. Solid lines indicate defined pathways; dashed lines indicate speculative pathways. ADA = adenosine deaminase; Ado = adenosine; ADP = adenosine diphosphate; AK = adenosine kinase; AMP = adenosine monophosphate; ARs = adenosine receptors; ATP = adenosine-5′-triphosphate; CD39 = ectonucleotidase for ATP and ADP; CD73 = ectonucleotidase for AMP; EC = endothelial cell; ENT = equilibrative nucleoside transporter; HC = homocysteine; MT = mitochondria; ROS = reactive oxygen species; SAH = S-adenosyl-l-homocysteine; SAM = S-adenosyl-l-methionine.
Inhibition of ADA causes sustained elevation of circulating and tissue adenosine (25). ADA deficiency in humans is most commonly associated with combined immune deficiency and lung inflammation (26). ADA-deficient mice develop progressive respiratory complications and die at 3 weeks of age due to sustained exposure to highly elevated adenosine (27). ADA enzyme replacement is a life-saving strategy used to treat ADA-deficient patients (28, 29) and animals (30). Two weeks after withdrawal of ADA enzyme therapy, ADA-deficient mice develop increased permeability lung edema (5). Whether sustained elevated adenosine directly affects lung vascular endothelial permeability is unknown. In this study, we assessed a model of sustained adenosine exposure in cultured lung endothelial cells and demonstrated that sustained adenosine exposure caused lung endothelial barrier dysfunction via ENT-facilitated adenosine uptake and subsequent activation of p38, JNK, and RhoA.
Materials and Methods
Cells and Reagents
Bovine pulmonary artery endothelial cells (PAECs) and rat lung microvascular endothelial cells (LMVECs) were purchased from VEC Technologies (Rensselaer, NY) and were used between passages 3 through 9. Adenosine, erytho-9-(2-hydroxy-3-nonyl)adenine (EHNA), dipyridamole, nitrobenzylthioinosine, DPCPX, DPMX, MRS1754, MRS1191, N-acetylcysteine, apocynin, and SB203580 were purchased from Sigma (St. Louis, MO). Deoxycoformycin and SP600125 were from Tocris (Minneapolis, MN). Y27632 and 2,7-dichlorofluorescein diacetate were from EMD Millipore (Darmstadt, Germany). Antibodies directed against RhoA, VE-cadherin, actin, Src, and JNK were from Santa Cruz Biotechnology (Santa Cruz, CA). zVAD-fmk and β-catenin antibody were from Axxora (Farmingdale, NY) and BD Biosciences (Sparks, MD), respectively. Antibodies directed against p38, phospho-p38 (Thr180/Tyr182), HSP27, phospho-HSP27 (Ser82), phospho-JNK (T183/Y185), and phospho-Src (Y416) were from Cell Signaling (Danvers, MA). Alexa Fluor 488-conjugated phalloidin was from Molecular Probes (Grand Island, NY). pGST-C21 construct (GST-Rhotekin-RBD) was a generous gift from Dr. J. Collard (Netherlands Cancer Institute, Amsterdam, The Netherlands).
Quantification of Adenosine in ECs
As we have previously described (2), cultured ECs were rapidly collected, and the cell pellets were frozen in liquid nitrogen. Nucleosides were extracted from frozen cell pellets, and adenosine was separated and quantified using reverse-phase HPLC.
Endothelial monolayer permeability and immunofluorescence microscopy were performed as we have previously described (31).
Assessment of Reactive Oxygen Species Levels in Cultured ECs
We used dichlorofluorescein assay as described (32). Briefly, by the end of treatment, ECs were loaded with a final concentration of 10 μM 2′,7′-dichlorofluorescein diacetate for up to 2 hours at 37°C. The parallel control cells were loaded with equal volumes of DMSO to serve as a blank controlling for autofluorescence. Fluorescence was determined every 15 minutes after incubation using a Synergy 2 Fluorescence plate-reader (BioTek, Winooski, VT) set at 485 nm excitation and 528 nm emission. The data are presented as fluorescence relative to vehicle-treated ECs at 1 hour after incubation. ECs were also fixed at 1 hour after incubation with 2′,7′-dichlorofluorescein diacetate, and fluorescence was captured using fluorescence microscopy.
Membrane/Cytosol Fractionation
As we have previously described (31), EC homogenates were centrifuged at 100,000 × g for 1 hour at 4°C. The supernatant (cytosolic fraction) was collected. The pellets were resuspended in lysis buffer. The suspension was centrifuged at 15,000 × g for 10 minutes at 4°C, and the supernatant (membrane fraction) was collected.
Isolation of Mitochondria
Mitochondria were isolated as previously described by Zhuang and colleagues (33). Briefly, EC pellets were homogenized in the presence of 250 mM sucrose. Homogenates were centrifuged twice at 750 × g. The supernatants were then centrifuged at 10,000 × g. The resulting mitochondrial pellets were dissolved with SDS lysis buffer. The supernatant (nonmitochondrial cytosolic fraction) was also collected.
RhoA GTPase Activity Assay
As we have previously described (34), RhoA GTPase activity was assessed by pull-down assay using pGST-C21 beads. Gel electrophoresis and immunoblot analysis were performed as we have previously described (35).
Data Analysis
All experiments were performed at least in triplicate. Data are presented as mean ± SE. ANOVA and Tukey-Kramer post hoc test were used to analyze differences among groups. Differences among means were considered significant at P < 0.05.
Results
Exogenous Adenosine plus ADA Inhibitor Maintained Sustained Adenosine Levels in Lung ECs
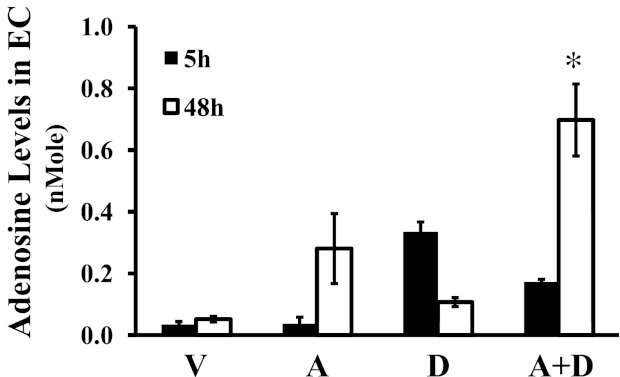
Adenosine is rapidly metabolized by ADA in cells. Not surprisingly, adenosine levels are elevated in lungs of ADA-deficient mice (5, 27, 36). To test the effect of sustained elevated adenosine on lung ECs, we established a model of sustained adenosine exposure by incubating lung ECs with adenosine plus the ADA inhibitor deoxycoformicin. Adenosine levels were significantly elevated in pulmonary artery endothelial cells (PAECs) exposed to adenosine in the presence of deoxycoformicin for 48 hours compared with PAECs exposed to vehicle, adenosine, or deoxycoformicin alone (Figure 2). This result validates our model of sustained adenosine exposure in cultured lung ECs used in this study.
Figure 2.
Effect of ADA inhibition on adenosine levels. Bovine pulmonary artery endothelial cells were incubated with vehicle (V) or 50 μM adenosine (A) in the absence or presence of 50 μM of the ADA inhibitor deoxycoformicin (D) for the indicated times. Nucleosides were extracted from lysates, and adenosine levels were quantified using HPLC (n = 3). *P < 0.05 versus ECs treated with vehicle, adenosine, or deoxycoformicin alone for 48 hours.
Sustained Adenosine Exposure Caused Barrier Dysfunction of Lung Macro- and Microvascular ECs
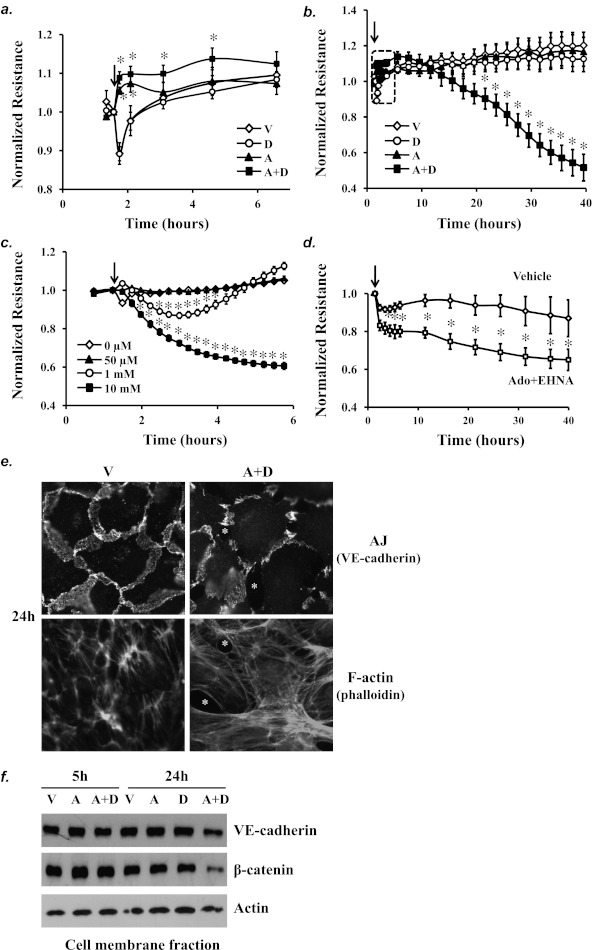
Sustained elevated adenosine causes lung edema in ADA-deficient mice (5). To evaluate the effects of sustained adenosine exposure on lung vascular endothelial permeability, pulmonary artery and lung microvascular ECs were exposed to adenosine plus ADA inhibitor for a prolonged period of time, and permeability was assessed over time by measuring transendothelial electrical resistance. Similar to adenosine alone, which acutely improves EC barrier function (Figure 3a) (2), adenosine plus deoxycoformicin (A+D) initially (within the first 5 h) enhanced EC barrier integrity, as indicated by increased electrical resistance, compared with exposure to vehicle (Figure 3a). Unlike adenosine alone, which did not affect EC barrier integrity after prolonged exposure, prolonged (A+D) exposure (20–40 h) gradually caused EC barrier dysfunction, as indicated by decreased electrical resistance, compared with exposure to vehicle or adenosine alone (Figure 3b). Deoxycoformicin alone did not affect EC barrier function (Figures 3a and 3b). These results indicate that exposure to sustained elevated adenosine causes EC barrier dysfunction. Additionally, ECs exposed to higher doses of adenosine alone exhibited progressive increased permeability (Figure 3c). These data suggest that the barrier-disruptive effect of adenosine is time and concentration dependent. Heterogeneous responses of PAECs and lung microvascular endothelial cells (LMVECs) have been recognized (37). However, the barrier disruptive effect of sustained adenosine exposure, induced by adenosine plus the ADA inhibitor EHNA, was also seen in LMVECs, where LMVECs treated with adenosine plus EHNA had increased permeability compared with LMVECs treated with vehicle (Figure 3d).
Figure 3.
Effect of sustained adenosine exposure on EC permeability and adherens junctions (AJs) and F-actin stress fiber formation. Pulmonary artery endothelial cells (PAECs) were incubated with vehicle (V) or 50 μM adenosine (A) in the absence or presence of 100 μM deoxycoformicin (D) for the indicated times (a, b) or were incubated with varying doses of adenosine or vehicle for the indicated times (c), and endothelial monolayer permeability was assessed by measuring electrical resistance across monolayer by the electrical cell impedance sensor (ECIS). The data in the dotted square of b are shown in a. (d) Rat lung microvascular endothelial cells were treated with vehicle or 100 μM adenosine (Ado) plus the ADA inhibitor erytho-9-(2-hydroxy-3-nonyl)adenine (EHNA) (10 μM) for the indicated times, and monolayer permeability was assessed by ECIS. Arrows indicate the time for addition of treatments (n = 5–8) *P < 0.05 versus vehicle-treated ECs. (e) PAECs were incubated with vehicle (V) or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) for 24 hours. AJs were assessed by immunofluorescence staining of VE-cadherin, an AJ component. F-actin stress fibers were assessed by phalloidin staining of F-actin and visualized by fluorescence microscopy. Asterisks indicate intercellular gaps. (f) PAECs were incubated with vehicle (V) or 50 μM adenosine (A) in the absence or presence of 50 μM deoxycoformicin (D) for 5 and 24 hours. Cell membrane fraction was isolated, and the protein levels of the AJ components VE-cadherin and β-catenin in cell membrane fraction were assessed by immunoblotting analysis. Actin was used to control for protein loading. Data in e and f represent three independent experiments for each.
Sustained Adenosine Exposure Decreased Adherens Junctions and Increased F-Actin Stress Fibers
Adherens junctions (AJs) and F-actin stress fibers are critical regulators of EC barrier integrity. Fluorescence microscopy demonstrated decreased AJs, increased intercellular gaps, and enhanced stress fibers in ECs treated with (A+D) for 24 hours (Figure 3e). Diminished paracellular AJs in ECs treated with (A+D) for 24 hours were also confirmed by reduced protein levels of VE-cadherin and β-catenin, components of AJs, in the membrane fraction (Figure 3f). Sustained adenosine exposure did not alter immunofluorescence staining of vinculin, a component of focal adhesion complexes (data not shown). These results suggest that sustained adenosine exposure causes lung EC barrier dysfunction by affecting AJs and F-actin stress fibers.
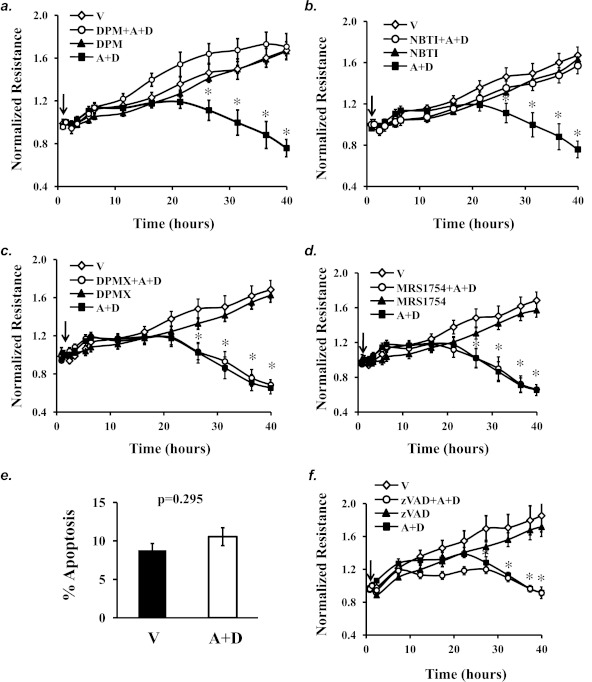
Sustained Adenosine Exposure Caused EC Barrier Dysfunction via ENTs, Not ARs or Apoptosis
We and others have previously shown that acute exposure to elevated adenosine enhanced EC barrier integrity via activation of the adenosine receptors A2AR and A2BR (1–3). However, activation of adenosine receptors was not responsible for sustained adenosine-induced lung edema (5, 38–40). To determine the role of ARs and ENTs in mediating sustained adenosine-induced EC barrier dysfunction, pharmacological inhibitors of ENTs and ARs were used. Inhibition of ENTs with dipyridamole (Figure 4a) or nitrobenzylthioinosine (NBTI) (Figure 4b) prevented (A+D)-induced EC barrier dysfunction. Additionally, neither the A2AR antagonist DPMX nor the A2BR antagonist MRS1754 prevented sustained adenosine exposure–induced EC barrier dysfunction (Figures 4c and 4d). Similarly, neither the A1R antagonist DPCPX nor the A3R antagonist MRS1191 altered sustained adenosine exposure–induced EC barrier dysfunction (data not shown). Our results indicate that sustained elevated adenosine causes lung EC barrier dysfunction via ENT-mediated intracellular events.
Figure 4.
The roles of nucleoside transporters and adenosine receptors in mediating sustained adenosine–induced barrier dysfunction. PAECs were treated with vehicle (V) or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) in the absence or presence of the ENT inhibitors dipyridamole (DPM) (10 μM) (a), nitrobenzylthioinosine (NBTI) (10 μM) (b), the A2AR inhibitor DPMX (10 μM) (c), the A2BR inhibitor MRS1754 (10 μM) (d), or the broad caspase inhibitor zVAD (100 μM) (f) for up to 40 hours. EC monolayer permeability was assessed by ECIS (a, b, c, d, f). Cells treated with vehicle or (A+D) for 40 hours in ECIS array were also fixed and subjected to apoptosis assay by DAPI staining of apoptotic nuclei (e). Arrows indicate the time for addition of treatments (n = 3–6 for each treatment for each panel). *P < 0.05 versus vehicle.
Adenosine has been shown to cause EC apoptosis (41). However, exposure of confluent endothelial monolayer to (A+D) for 40 hours did not significantly increase EC apoptosis (Figure 4e). Additionally, inhibition of apoptosis with zVAD, a broad caspase inhibitor, did not alter the disruptive effect of (A+D) on EC barrier function (Figure 4f). Our results indicate that sustained adenosine exposure–induced EC barrier dysfunction is not due to the loss of apoptotic ECs.
Sustained Adenosine Exposure Increased Cellular Oxidative Stress
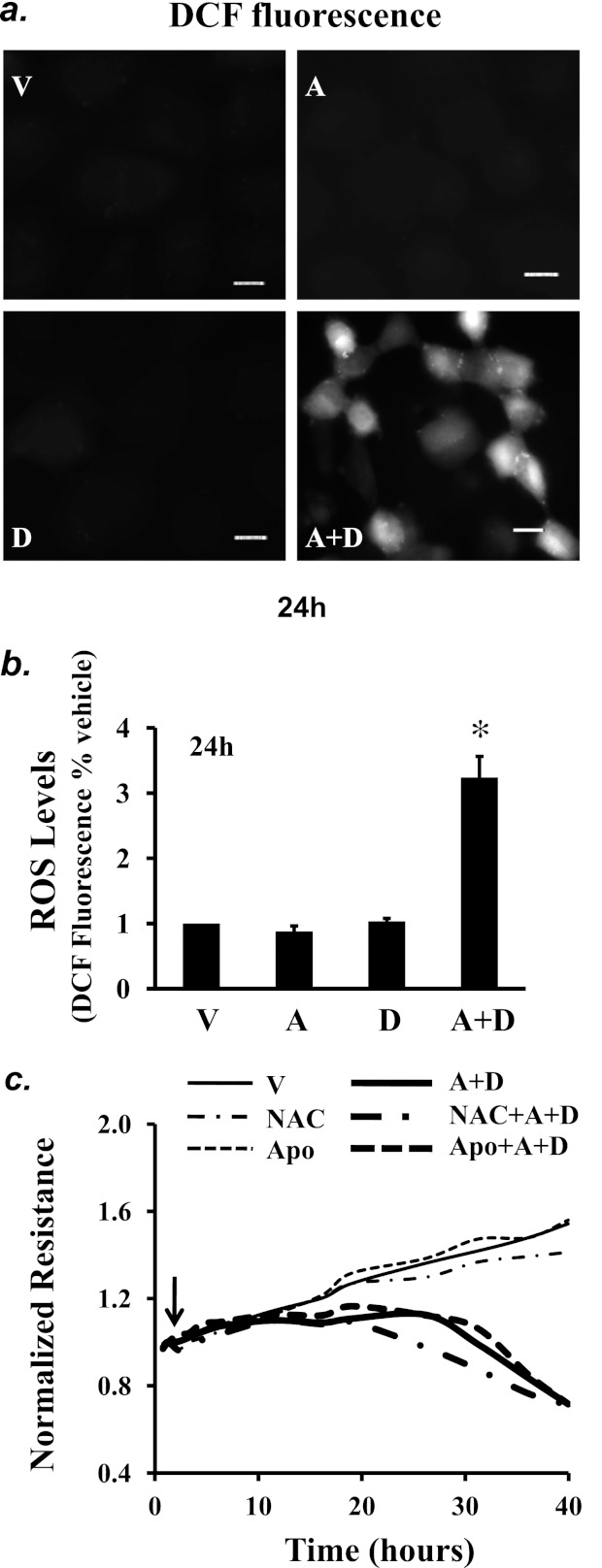
Increased oxidative stress causes EC barrier dysfunction (42). However, it is not known whether oxidative stress is implicated in sustained elevated adenosine–induced EC barrier dysfunction. We found that sustained adenosine exposure elevated reactive oxygen species (ROS) levels at 24 hours (Figures 5a and 5b). However, there was no significant increase in ROS levels in ECs exposed to (A+D) for 5 hours (data not shown). Neither antioxidant, N-acetyl-cysteine (NAC), nor the NADPH oxidase inhibitor apocynin altered sustained adenosine–induced EC barrier dysfunction (Figure 5c).
Figure 5.
Effect of sustained adenosine exposure on oxidative stress. PAECs were treated with vehicle (V) or 50 μM adenosine (A) in the absence or presence of 50 μM deoxycoformicin (D) for 24 hours. Reactive oxygen species (ROS) levels were assessed using 10 μM 2,7-dichlorofluorescein diacetate to generate dichlorofluorescein fluorescence, which was detected by fluorescence microscopy (a) and fluorescence microplate reader at Ex 485 and Em 538 (b). Scale bar = 25 μm. (b) Data presented as percentage of vehicle-treated ECs. *P < 0.05 versus vehicle-treated ECs (n = 6). (c) PAECs were treated with vehicle (V) or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) in the absence or presence of the antioxidant N-acetylcysteine (NAC) (12.5 mM) or the NAPDH inhibitor apocynin (Apo; 10 μM) for up to 40 hours, and EC monolayer permeability was assessed by ECIS. Arrow indicates the time for addition of treatments; a and c represent three independent experiments for each.
Sustained Adenosine Exposure Activated p38 and JNK via ENT-Mediated Intracellular Uptake of Adenosine
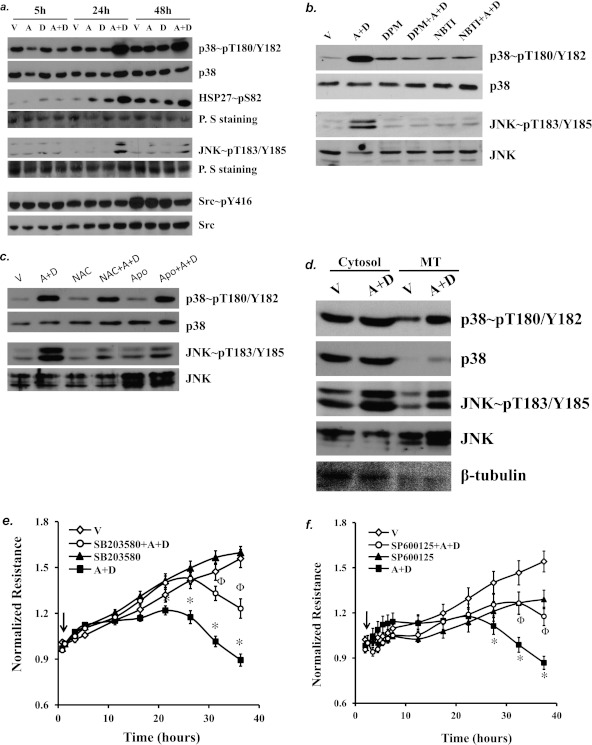
Because ROS levels were elevated by sustained adenosine exposure, we assessed activation of the multiple redox-sensitive proteins that are important in the regulation of EC barrier function. We found that sustained adenosine exposure (24 and 48 h) activated p38 and JNK but not Src (Figure 6a). Consistent with p38 activation, the p38 downstream substrate HSP27 was also activated in EC exposed to (A+D) for 24 and 48 hours (Figure 6a). The activities of p38, HSP27, and JNK were not altered by short-term (5 h) (A+D) exposure (Figure 6a). We further determined that sustained adenosine–induced activation of p38 and JNK was prevented by inhibition of ENTs with dipyridamole and NBTI (Figure 6b), indicating a dependence on ENT-mediated adenosine uptake.
Figure 6.
The role of p38 and JNK in sustained adenosine–induced EC barrier dysfunction. (a) PAECs were treated with vehicle (V) or 50 μM adenosine (A) in the absence or presence of 50 μM deoxycoformicin (D) for the indicated times (5, 24, and 48 h). Cell lysate was analyzed for activation of p38, HSP27, JNK, and Src by immunoblot analysis using antibodies directed against phospho-p38, phospho-HSP27, phospho-JNK, and phospho-Src, which correlate with activation of each protein. Ponceau S (P. S) staining was used to examine protein loading. (b) PAECs were treated with vehicle (V) or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) in the absence or presence of ENT inhibitors DPM 10 (μM) or NBTI (10 μM) for 24 hours. Activation of p38 and JNK was assessed in lysate. (c) PAECs were incubated with vehicle or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) in the absence or presence of the antioxidant NAC (12.5 mM) or the NAPDH inhibitor apocynin (Apo; 10 μM) for 24 hours, and activation of p38 and JNK was assessed. (d) PAECs were incubated with vehicle or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) for 24 hours. Cells were lysed by homogenization, and mitochondria (MT) were isolated through several centrifugations. Activation of p38 and JNK in mitochondria and nonmitochondria cytosolic fractions was assessed by immunoblot analysis. The blots were stripped and reprobed for total p38, JNK, and β-tubulin. Data are representative of three independent experiments for each panel. (e and f) PAECs were treated with vehicle or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) in the absence or presence of the p38 inhibitor SB203580 (10 μM) (e) or of the JNK inhibitor SP600125 (10 μM) (f) for up to 40 hours. Endothelial monolayer permeability was assessed by ECIS. Arrows indicate the time for addition of treatments (n = 6–10). *P < 0.05 versus vehicle. ΦP < 0.05 versus A+D.
p38 and JNK are redox-sensitive proteins. Neither the antioxidant NAC nor the NAPDH inhibitor apocynin had an effect on sustained adenosine–induced p38 activation (Figure 6c). However, sustained adenosine–induced JNK activation was partially attenuated by NAC or apocynin (Figure 6c). We further determined that p38 was activated in mitochondria, whereas JNK was activated in cytoplasm and mitochondria 24 hours after exposure to sustained adenosine (Figure 6d).
Activation of p38 and JNK Contributed to Sustained Adenosine–Induced EC Barrier Dysfunction
Using a pharmacological approach, we show that inhibition of p38 by SB203580 (Figure 6e) or inhibition of JNK by SP600125 (Figure 6f) attenuated sustained adenosine–induced endothelial barrier dysfunction. These results suggest that activation of p38 and JNK contributes to sustained adenosine–induced endothelial barrier dysfunction.
Activation of RhoA Contributed to Sustained Adenosine–Induced EC Barrier Dysfunction
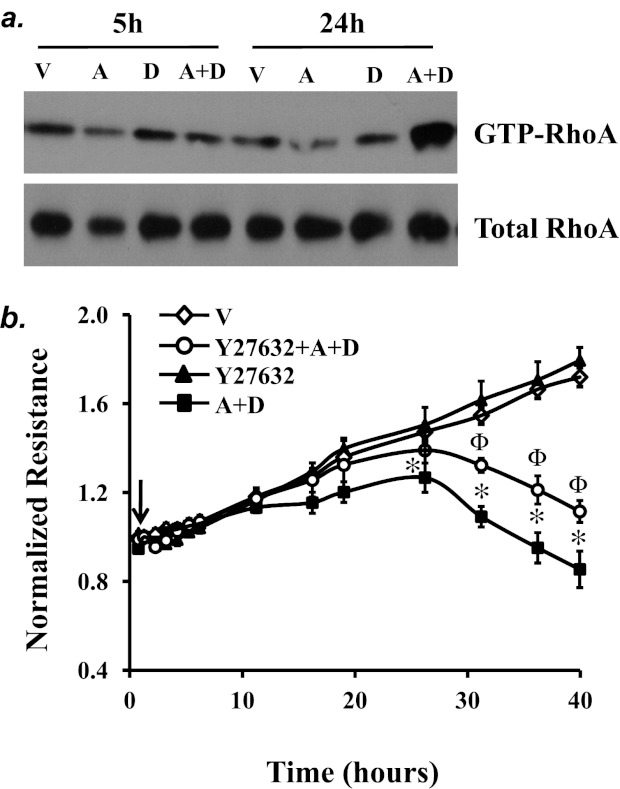
RhoA activation is important in mediating endothelial barrier dysfunction induced by various edemagenic agents via effects on AJs and stress fiber formation (43). RhoA can be activated by oxidative stress (44). Thus, we assessed activation of RhoA and found that prolonged adenosine exposure (24 h), but not short-term (5 h) exposure, activated RhoA (Figure 7a). We also noted that inhibition of RhoA signaling by the Rho kinase inhibitor Y27632 partially attenuated sustained adenosine–induced endothelial barrier dysfunction (Figure 7b), suggesting a role of RhoA in this process.
Figure 7.
The role of RhoA in sustained adenosine-induced EC barrier dysfunction. (a) PAECs were treated with vehicle (V) or 50 μM adenosine (A) in the absence or presence of 50 μM deoxycoformicin (D) for the indicated times. Active RhoA was assessed by pull-down assay using RBD-GST beads, which bind to GTP-RhoA. Total RhoA in the lysates was evaluated. Data represent three independent experiments. (b) PAECs were treated with vehicle (V) or 50 μM adenosine (A) plus 50 μM deoxycoformicin (D) in the absence or presence of the Rho kinase inhibitor Y27632 (10 μM) for the indicated times, and endothelial permeability was assessed by ECIS. Arrow indicates the time for addition of treatments (n = 4). *P < 0.05 versus vehicle. ΦP < 0.05 versus A+D.
Discussion
We and others have previously demonstrated that acute adenosine exposure enhances lung endothelial barrier integrity and protects against increased permeability lung edema in animal models of ALI via the adenosine receptors A2AR and A2BR (1–3). Conversely, it is well documented that sustained adenosine exposure has detrimental effects on the lungs, including lung edema (5). Many studies have demonstrated the important roles of inflammatory cells in mediating sustained elevated adenosine–induced lung inflammation and fibrosis (16, 20, 21, 27). However, the mechanisms underlying the development of lung edema caused by sustained adenosine exposure are unknown. Disruption of lung vascular endothelial barrier integrity causes increased permeability lung edema. In contrast to the effect of acute adenosine exposure, we demonstrated that sustained adenosine exposure caused endothelial barrier dysfunction of lung macro- and microvascular endothelial cells; this effect was associated with disruption of AJs and increased F-actin stress fiber formation. The endothelial barrier damaging effect of sustained adenosine was mediated by nucleoside transporters but not by adenosine receptors. We further demonstrated that sustained adenosine exposure caused endothelial barrier dysfunction via activation of p38, JNK, and RhoA.
ADA deficiency in humans is associated with severe combined immune deficiency and lung inflammation (26). Adenosine and deoxyadenosine are known substrates for ADA. Studies from ADA-deficient mice demonstrate that chronic elevation of deoxyadenosine in the thymus and spleen is responsible for severe combined immune deficiency (45), whereas sustained increased adenosine in the lung causes a defective pulmonary phenotype (27). It has been shown that A3R-deficient mice exhibit enhanced bleomycin-induced lung inflammation (38). A1R/ADA and A2AR/ADA double-deficient mice have enhanced lung inflammation compared with ADA-deficient mice (39, 40). These findings suggest that signaling through A1R, A2AR, and A3R have antiinflammatory effects on lungs. Although A2BR mediates proinflammatory effects of adenosine (46, 47), A2BR/ADA double-deficient mice exhibited exacerbated pulmonary vascular permeability compared with ADA-deficient mice (5), suggesting that A2BR is not responsible for sustained adenosine–induced lung edema. Similar to other G-protein–coupled receptors, prolonged exposure to adenosine leads to desensitization and internalization of adenosine receptors (48). Therefore, we speculate that sustained elevated adenosine is taken up into cells by nucleoside transporters. ECs predominantly express ENT1 and ENT2, with ENT1 expressed at twice the level of ENT2 (49). ENT1 has a 2.8-fold higher affinity for adenosine than ENT2 (50). Sodium-dependent concentrative nucleoside transporters have very low affinity for adenosine and limited expression in ECs (51). Based on expression level and adenosine affinity, ENT1 probably plays a major role in adenosine transport in ECs. The ENT1–specific inhibitors dipyridamole and NBTI (52, 53), but not antagonists against any adenosine receptors, prevented sustained adenosine exposure–induced endothelial barrier dysfunction. Our results suggest an important role of ENT1–mediated adenosine uptake and intracellular metabolism in mediating endothelial barrier dysfunction.
We have previously shown that adenosine increases SAH via inhibition of SAH hydrolase (41). Increased cytosolic SAH decreases mitochondrial S-adenosylmethionine (SAM) in rat hepatocytes (54). As a precursor to glutathione, SAM in mitochondria is essential for the maintenance of mitochondrial function by limiting oxidative stress. Thus, it is possible that sustained adenosine exposure increases mitochondrial oxidative stress via decreased mitochondrial SAM (Figure 1). Further studies are necessary to test this hypothesis.
ROS are important for the maintenance of EC function (55). However, increased oxidant stress increases EC permeability (42). p38 and JNK are redox-sensitive proteins (56). We show that sustained adenosine exposure greatly elevated cellular ROS levels and activated p38 and JNK. We also show that inhibition of p38 and JNK blunted sustained adenosine–induced EC barrier dysfunction. However, neither the antioxidant N-acetyl-cysteine (NAC) nor the NADPH oxidase inhibitor apocynin blunted sustained adenosine–induced EC barrier dysfunction. p38 was activated exclusively in mitochondria, whereas JNK was activated in cytoplasm and mitochondria by sustained adenosine exposure. NAC and apocynin partially attenuated JNK activation, but had no effect on p38 activation, upon sustained adenosine exposure. Our data suggest that sustained adenosine exposure may cause mitochondrial oxidative stress, leading to activation of p38 and JNK in mitochondria and subsequent EC barrier dysfunction (Figure 1). We speculate that NAC and apocynin were ineffective in inhibiting mitochondrial oxidative stress, thereby failing to inhibit p38 and JNK activation in mitochondria and barrier dysfunction. Further studies are necessary to assess mitochondrial oxidative stress and to determine if mitochondria-specific antioxidants could attenuate or prevent sustained adenosine–induced p38 and JNK activation and barrier dysfunction. Our data also suggest that JNK was activated in cytoplasm via oxidative stress, but this activation of JNK does not contribute to sustained adenosine–induced barrier dysfunction (Figure 1). Nevertheless, our data do not rule out ROS-independent mechanisms by which sustained adenosine activates p38 and JNK in mitochondria, leading to barrier dysfunction.
We and others have previously shown that p38 mediates EC barrier dysfunction and lung edema (34, 57). The p38 downstream targetHSP27 modulates actin filament dynamics via its phosphorylation, thus affecting endothelial permeability. Increased HSP27 phosphorylation has been associated with LPS-induced lung edema (58). Activation of p38 and HSP27 has also been implicated in HMGB1-induced vascular barrier disruption in cultured lung ECs (59). Our data show that sustained adenosine exposure activated p38/ HSP27 and JNK via nucleoside transporters and that inhibition of p38 and JNK attenuated sustained adenosine–induced EC barrier dysfunction. Thus, activation of p38/HSP27 and JNK contributes to sustained adenosine exposure–induced endothelial barrier dysfunction.
RhoA activation significantly contributes to endothelial barrier dysfunction caused by various edemagenic agents (43). RhoA is activated by guanine nucleotide exchange factors and inhibited by GTPase-activating proteins and guanine nucleotide dissociation inhibitors (43). RhoA is also activated through direct oxidation of two cysteine residues located in the redox-sensitive motif GXXXCGK(S/T)C by ROS (44). ROS-mediated RhoA activation has been seen in vascular smooth muscle (60). In this study, sustained adenosine exposure increased oxidative stress and RhoA activity. Inhibition of RhoA kinase partially blunted sustained adenosine–induced EC barrier dysfunction. Thus, our data suggest that RhoA activation, likely via oxidation, at least in part contributes to sustained adenosine–induced endothelial barrier dysfunction. Further studies are required to address whether sustained adenosine–induced RhoA activation is dependent on mitochondrial oxidative stress and which site(s) of RhoA is oxidized.
In summary, this study demonstrates that exogenous adenosine plus ADA inhibitor causes sustained elevation of adenosine in cultured lung ECs. Sustained exposure of pulmonary macro- and microvascular ECs to elevated adenosine increases monolayer permeability through nucleoside transporter–facilitated intracellular adenosine uptake and subsequent activation of p38, JNK, and RhoA (Figure 1). These findings offer new insights into the diverse effects of adenosine on pulmonary circulation.
Supplementary Material
Acknowledgments
The authors thank Dr. Sharon Rounds for encouragement and helpful discussions and revisions of this manuscript.
Footnotes
This work was supported by a VA Merit Review (Sharon Rounds), by National Institutes of Health grant HL64936 (Sharon Rounds), and by an ATS/ PHA Research Award (Q.L.).
Some of the results were presented at the American Thoracic Society International Conference, Denver, Colorado, May 13–18, 2011 and have been published in abstract form (Am J Respir Crit Care Med 2011;183:A1978).
Originally Published in Press as DOI: 10.1165/rcmb.2012-0012OC on June 28, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2b adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 2008;118:3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu Q, Harrington EO, Newton J, Casserly B, Radin G, Warburton R, Zhou Y, Blackburn MR, Rounds S. Adenosine protected against pulmonary edema through transporter- and receptor a2-mediated endothelial barrier enhancement. Am J Physiol Lung Cell Mol Physiol 2010;298:L755–L767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross SD, Tribble CG, Linden J, Gangemi JJ, Lanpher BC, Wang AY, Kron IL. Selective adenosine-a2a activation reduces lung reperfusion injury following transplantation. J Heart Lung Transplant 1999;18:994–1002 [DOI] [PubMed] [Google Scholar]
- 4.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor a2a stimulation in lipopolysaccharide-induced lung injury. J Immunol 2007;179:1254–1263 [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Mohsenin A, Morschl E, Young HW, Molina JG, Ma W, Sun CX, Martinez-Valdez H, Blackburn MR. Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the a2b adenosine receptor. J Immunol 2009;182:8037–8046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arch JR, Newsholme EA. The control of the metabolism and the hormonal role of adenosine. Essays Biochem 1978;14:82–123 [PubMed] [Google Scholar]
- 7.Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med 2000;28:3198–3202 [DOI] [PubMed] [Google Scholar]
- 8.Stringer KA, Serkova NJ, Karnovsky A, Guire K, Paine R, III, Standiford TJ. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma (1)h-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am J Physiol Lung Cell Mol Physiol 2011;300:L4–L11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperlágh B, Dóda M, Baranyi M, Haskó G. Ischemic-like condition releases norepinephrine and purines from different sources in superfused rat spleen strips. J Neuroimmunol 2000;111:45–54 [DOI] [PubMed] [Google Scholar]
- 10.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, et al. Hif-1-dependent repression of equilibrative nucleoside transporter (ent) in hypoxia. J Exp Med 2005;202:1493–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. Hif-dependent induction of adenosine a2b receptor in hypoxia. FASEB J 2006;20:2242–2250 [DOI] [PubMed] [Google Scholar]
- 12.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. Hif-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 2008;111:5571–5580 [DOI] [PubMed] [Google Scholar]
- 13.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (cd73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 2002;110:993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases cd39 and cd73 in innate protection during acute lung injury. J Immunol 2007;178:8127–8137 [DOI] [PubMed] [Google Scholar]
- 15.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the a2b adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 2010;184:5271–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volmer JB, Thompson LF, Blackburn MR. Ecto-5′-nucleotidase (cd73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J Immunol 2006;176:4449–4458 [DOI] [PubMed] [Google Scholar]
- 17.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 1993;148:91–97 [DOI] [PubMed] [Google Scholar]
- 18.Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar Vilagos G, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J 2002;20:1393–1398 [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Wang W, Parker W, Clancy JP. Adenosine regulation of cystic fibrosis transmembrane conductance regulator through prostenoids in airway epithelia. Am J Respir Cell Mol Biol 2006;34:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates il-13-induced inflammation and remodeling in the lung and interacts in an il-13-adenosine amplification pathway. J Clin Invest 2003;112:332–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, Boyden L, Lifton RP, Sun CX, Young HW, et al. Adenosine metabolism and murine strain-specific il-4-induced inflammation, emphysema, and fibrosis. J Clin Invest 2006;116:1274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of a2b receptors in angiogenic factor regulation. Circ Res 2002;90:531–538 [DOI] [PubMed] [Google Scholar]
- 23.Umapathy NS, Fan Z, Zemskov EA, Alieva IB, Black SM, Verin AD. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vascul Pharmacol 2010;52:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt AW, Steinert JR, Wheeler-Jones CP, Morgan AJ, Sugden D, Pearson JD, Sobrevia L, Mann GE. Early activation of the p42/p44mapk pathway mediates adenosine-induced nitric oxide production in human endothelial cells: a novel calcium-insensitive mechanism. FASEB J 2002;16:1584–1594 [DOI] [PubMed] [Google Scholar]
- 25.Ely SW, Matherne GP, Coleman SD, Berne RM. Inhibition of adenosine metabolism increases myocardial interstitial adenosine concentrations and coronary flow. J Mol Cell Cardiol 1992;24:1321–1332 [DOI] [PubMed] [Google Scholar]
- 26.Hershfield M. Adenosine deaminase deficiency. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. Genereviews. Seattle, WA: University of Washington; 1993 [accessed 19 July, 2012]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1483/
- 27.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med 2000;192:159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bordignon C, Mavilio F, Ferrari G, Servida P, Ugazio AG, Notarangelo LD, Gilboa E, Rossini S, O'Reilly RJ, Smith CA, et al. Transfer of the ada gene into bone marrow cells and peripheral blood lymphocytes for the treatment of patients affected by ada-deficient scid. Hum Gene Ther 1993;4:513–520 [DOI] [PubMed] [Google Scholar]
- 29.Hershfield MS, Chaffee S, Sorensen RU. Enzyme replacement therapy with polyethylene glycol-adenosine deaminase in adenosine deaminase deficiency: overview and case reports of three patients, including two now receiving gene therapy. Pediatr Res 1993;33(Suppl):S42–S47[Discussion, S47–S48.] [DOI] [PubMed] [Google Scholar]
- 30.Blackburn MR, Aldrich M, Volmer JB, Chen W, Zhong H, Kelly S, Hershfield MS, Datta SK, Kellems RE. The use of enzyme therapy to regulate the metabolic and phenotypic consequences of adenosine deaminase deficiency in mice: differential impact on pulmonary and immunologic abnormalities. J Biol Chem 2000;275:32114–32121 [DOI] [PubMed] [Google Scholar]
- 31.Lu Q, Harrington EO, Hai CM, Newton J, Garber M, Hirase T, Rounds S. Isoprenylcysteine carboxyl methyltransferase modulates endothelial monolayer permeability: involvement of rhoa carboxyl methylation. Circ Res 2004;94:306–315 [DOI] [PubMed] [Google Scholar]
- 32.Podowski M, Calvi CL, Cheadle C, Tuder RM, Biswals S, Neptune ER. Complex integration of matrix, oxidative stress, and apoptosis in genetic emphysema. Am J Pathol 2009;175:84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang S, Demirs JT, Kochevar IE. P38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem 2000;275:25939–25948 [DOI] [PubMed] [Google Scholar]
- 34.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves smad2-dependent p38 activation and subsequent rhoa activation. J Appl Physiol 2006;101:375–384 [DOI] [PubMed] [Google Scholar]
- 35.Bellas RE, Harrington EO, Sheahan KL, Newton J, Marcus C, Rounds S. Fak blunts adenosine-homocysteine-induced endothelial cell apoptosis: requirement for pi 3-kinase. Am J Physiol 2002;282:L1135–L1142 [DOI] [PubMed] [Google Scholar]
- 36.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol 2005;175:1937–1946 [DOI] [PubMed] [Google Scholar]
- 37.Stevens T. Microheterogeneity of lung endothelium. In: Aird WC, editor. Endothelial biomedicine. New York: Cambridge University Press; 2007. pp. 1161–1170.
- 38.Morschl E, Molina JG, Volmer JB, Mohsenin A, Pero RS, Hong JS, Kheradmand F, Lee JJ, Blackburn MR. A3 adenosine receptor signaling influences pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol 2008;39:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohsenin A, Mi T, Xia Y, Kellems RE, Chen JF, Blackburn MR. Genetic removal of the a2a adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. Am J Physiol Lung Cell Mol Physiol 2007;293:L753–L761 [DOI] [PubMed] [Google Scholar]
- 40.Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the a1 adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest 2005;115:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rounds S, Yee WL, Dawicki DD, Harrington E, Parks N, Cutaia MV. Mechanism of extracellular atp- and adenosine-induced apoptosis of cultured pulmonary artery endothelial cells. Am J Physiol 1998;275:L379–L388 [DOI] [PubMed] [Google Scholar]
- 42.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 2001;280:C719–C741 [DOI] [PubMed] [Google Scholar]
- 43.Tzima E. Role of small gtpases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 2006;98:176–185 [DOI] [PubMed] [Google Scholar]
- 44.Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of rhoa by reactive oxygen species requires a redox-sensitive motif. PLoS ONE 2009;4:e8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackburn MR, Datta SK, Kellems RE. Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency. J Biol Chem 1998;273:5093–5100 [DOI] [PubMed] [Google Scholar]
- 46.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of a2b adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest 2006;116:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaynagetdinov R, Ryzhov S, Goldstein AE, Yin H, Novitskiy SV, Goleniewska K, Polosukhin VV, Newcomb DC, Mitchell D, Morschl E, et al. Attenuation of chronic pulmonary inflammation in a2b adenosine receptor knockout mice. Am J Respir Cell Mol Biol 2010;42:564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International union of pharmacology: Xxv. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001;53:527–552 [PMC free article] [PubMed] [Google Scholar]
- 49.Archer RG, Pitelka V, Hammond JR. Nucleoside transporter subtype expression and function in rat skeletal muscle microvascular endothelial cells. Br J Pharmacol 2004;143:202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward JL, Sherali A, Mo ZP, Tse CM. Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ent1 and ent2, stably expressed in nucleoside transporter-deficient pk15 cells: Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J Biol Chem 2000;275:8375–8381 [DOI] [PubMed] [Google Scholar]
- 51.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol 2007;27:1004–1013 [DOI] [PubMed] [Google Scholar]
- 52.Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today 1999;5:216–224 [DOI] [PubMed] [Google Scholar]
- 53.Visser F, Vickers MF, Ng AM, Baldwin SA, Young JD, Cass CE. Mutation of residue 33 of human equilibrative nucleoside transporters 1 and 2 alters sensitivity to inhibition of transport by dilazep and dipyridamole. J Biol Chem 2002;277:395–401 [DOI] [PubMed] [Google Scholar]
- 54.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type ii cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 1998;101:761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abid MR, Spokes KC, Shih SC, Aird WC. Nadph oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem 2007;282:35373–35385 [DOI] [PubMed] [Google Scholar]
- 56.Matsuzawa A, Ichijo H. Redox control of cell fate by map kinase: physiological roles of ask1-map kinase pathway in stress signaling. Biochim Biophys Acta 2008;1780:1325–1336 [DOI] [PubMed] [Google Scholar]
- 57.Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 map kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal 2003;5:723–730 [DOI] [PubMed] [Google Scholar]
- 58.Hirano S, Rees RS, Yancy SL, Welsh MJ, Remick DG, Yamada T, Hata J, Gilmont RR. Endothelial barrier dysfunction caused by lps correlates with phosphorylation of hsp27 in vivo. Cell Biol Toxicol 2004;20:1–14 [DOI] [PubMed] [Google Scholar]
- 59.Wolfson RK, Chiang ET, Garcia JG. Hmgb1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res 2011;81:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin L, Ying Z, Webb RC. Activation of rho/rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 2004;287:H1495–H1500 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.