There has been great recent interest in the bioactive lipid mediator lysophosphatidic acid (LPA) in the pathogenesis of fibrotic diseases, from both academic laboratories and pharmaceutical companies. Investigators have demonstrated critical roles for LPA signaling through one of its receptors, LPA1, in animal models of fibrosis of multiple organs, including the lung (1, 2), skin (3), and kidney (4, 5). Building on these pre-clinical studies, pharmaceutical companies will be evaluating LPA1 antagonism in upcoming clinical trials as a new therapeutic strategy for human fibrotic diseases, including idiopathic pulmonary fibrosis (IPF) and scleroderma. In this issue of the Journal, Oikonomou and colleagues (pp. 566–574) demonstrate that genetic deletion or pharmacological inhibition of autotaxin, the enzyme responsible for generating most extracellular LPA, also limits the development of lung fibrosis in the bleomycin model (6). These investigators demonstrate increased expression of autotaxin in the lungs of patients with IPF and fibrotic nonspecific interstitial pneumonia (NSIP), identifying this enzyme as an exciting new addition to the growing list of rationale drug targets for IPF and other fibrotic diseases.
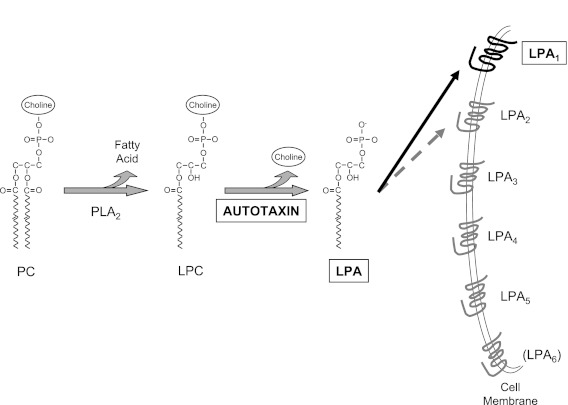
LPA is the common name for acyl-hydroxy-glycero-3-phosphates, all consisting of a glycerol phosphate backbone esterified to a single fatty acid (Figure 1), and signaling through specific G protein–coupled receptors. Five high-affinity LPA receptors have been definitively established and designated LPA1 to LPA5 (Figure 1); P2Y5 is a lower-affinity receptor that is likely to join the LPA receptor family as LPA6 (7). By signaling through these receptors, LPA mediates multiple fundamental responses to tissue injury, including responses that may be aberrant or aberrantly excessive when injury leads to fibrosis rather than to repair. LPA signaling specifically through LPA1 has pro-fibrotic effects on epithelial cells, endothelial cells, and fibroblasts: genetic deletion of this receptor reduces epithelial cell apoptosis, vascular leak, and fibroblast accumulation in the bleomycin model of lung fibrosis (1, 8). LPA1-deficient mice are consequently dramatically protected from fibrosis and mortality in this model (1). Pharmacological inhibition of LPA1 abrogates fibroblast migration in response to the bronchoalveolar lavage (BAL) of patients with IPF, demonstrating that LPA–LPA1 signaling is responsible for fibroblast recruitment in this disease (1).
Figure 1.
The autotaxin-LPA-LPA1 pathway. Generation of lysophosphatidic acid (LPA) by autotaxin, and LPA signaling through LPA1; both are required for bleomycin-induced pulmonary fibrosis in mice, and both have been implicated in the pathogenesis of idiopathic pulmonary fibrosis in humans. The majority of LPA in vivo appears to be produced by conversion of phospholipids such as phosphatidylcholine (PC) to lysophospholipids such as lysophosphatidylcholine (LPC) through the action of phospholipase A2 (PLA2) family members, followed by the conversion of these lysophospholipids to LPA through the lysophospholipase D activity of autotaxin. Once generated by autotaxin, LPA signals through specific cell surface G protein–coupled receptors: five high-affinity LPA receptors have been definitively established and designated LPA1 to LPA5; a lower-affinity receptor is likely to join the LPA receptor family as LPA6. LPA signaling specifically through LPA1 has pro-fibrotic effects on epithelial cells, endothelial cells, and fibroblasts, promoting epithelial cell apoptosis, inducing vascular leak, and directing fibroblast recruitment, proliferation, and persistence. LPA–LPA2 signaling may also have pro-fibrotic effects, inducing activation of latent TGF-β by lung epithelial cells, although LPA signaling through LPA2 on leukocytes may serve to prevent excess innate immune activation after tissue injury.
The study by Oikonomou and colleagues demonstrates that, as does LPA1 antagonism, limiting LPA synthesis by inhibiting autotaxin limits the development of pulmonary fibrosis (6). Although LPA can be produced from membrane phospholipids of cells or platelets, or from surfactant phospholipids, by at least three pathways (9), the majority of LPA in vivo appears to be produced by autotaxin, since plasma LPA concentrations in mice heterozygous for an autotaxin-null allele (autotaxin+/− mice) are approximately one half of those that are present in autotaxin+/+ mice (10, 11). The autotaxin pathway of LPA synthesis is illustrated in Figure 1: phospholipids such as phosphatidylcholine (PC) are converted to lysophospholipids such as lysophosphatidylcholine (LPC) by members of the phospholipase A2 (PLA2) family of enzymes, and these lysophospholipids are converted to LPA by the lysophospholipase D activity of autotaxin. Autotaxin was initially isolated as an “autocrine motility factor” that stimulated the migration of melanoma cells (12). Autotaxin’s identification as the lysophospholipase responsible for generating LPA from LPC came later, and its ability to generate LPA was then determined to be the mechanism by which autotaxin stimulates cell motility (13).
With the identification of important roles for LPA and LPA1 in fibrosis, autotaxin emerged as another potential therapeutic target for fibrotic diseases. However, validation of this enzyme as a target in pre-clinical models of fibrosis was hampered by lack of an effective pharmacological autotaxin inhibitor, and the unexpected lethality of global genetic deletion of autotaxin in mice. Early blood vessels formed but failed to mature properly in autotaxin−/− embryos, resulting in lethality between Embryonic Days 9.5 and 10.5, and revealing an unanticipated but critical role for this enzyme in vascular development (10, 11). Oikonomou and colleagues, working in Vassilis Aidinis’ research group, have now overcome both of these hurdles to the pre-clinical assessment of autotaxin in pulmonary fibrosis (6). These investigators generated three lines of cell-specific autotaxin-deficient mice, in which autotaxin was genetically deleted only from bronchial epithelial cells, only from alveolar epithelial cells, or only from macrophages and neutrophils. Their choice to target these cell types followed from their immunohistochemical localization of increased autotaxin in the lungs of patients with IPF to bronchiolar epithelial cells, alveolar epithelial cells, and alveolar macrophages (in addition to fibroblast-like appearing cells), and their localization of increased autotaxin in the lungs of bleomycin-challenged mice to bronchial epithelial cells and alveolar macrophages (in addition to weak expression by alveolar epithelial cells and fibroblasts). Bleomycin-induced increases in lung collagen and BAL cell counts were diminished in bronchial epithelial cell– and macrophage-specific autotaxin-deficient mice, as was the bleomycin-induced increase in BAL total protein in macrophage-specific autotaxin-deficient mice. In contrast, deletion of autotaxin specifically in alveolar epithelial cells afforded no protection from bleomycin-induced lung fibrosis. Further validating autotaxin as a therapeutic target for pulmonary fibrosis, a novel pharmacological inhibitor of this enzyme (GWJ-A-23 [14]) dramatically reduced bleomycin-induced increases in lung collagen, BAL cell counts, and BAL total protein, as well as BAL levels of both LPA and TGF-β.
Impressive reductions in bleomycin-induced fibrosis produced by pharmacological targeting of either LPA1 or autotaxin underscore the important role of the LPA pathway in fibrogenesis. Whether one of these targets will prove to be more effective than the other remains to be determined. To the extent that LPA receptors other than LPA1 mediate some of LPA’s pro-fibrotic activities, targeting autotaxin may be more effective than targeting LPA1. LPA signaling through LPA2 has been demonstrated to induce αvβ6 integrin-mediated activation of latent TGF-β by lung epithelial cells (15), and TGF-β activation by this integrin is critically required for the development of lung fibrosis (16). On the other hand, to the extent that LPA receptors other than LPA1 may mediate some of LPA’s beneficial effects, targeting autotaxin in fibrosis may have more unintended consequences. LPA signaling through LPA2 has been demonstrated to be a negative regulator of innate immune responses, such as those mediated by dendritic cells in allergic pulmonary inflammation (17), and this activity of LPA may be needed to prevent excess inflammation after tissue injury. Regardless, the elegant studies from Aidinis’ laboratory published in this issue allow autotaxin to take its place on the growing list of rationale targets for IPF. By extension, autotaxin appears to be an exciting therapeutic target for fibrotic diseases more broadly, given the important roles for LPA–LPA1 signaling that have been identified in fibrosis of multiple organs.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants R01-HL095732 and R01-HL108975, a Pulmonary Fibrosis Foundation grant, a Scleroderma Research Foundation grant, and a Nirenberg Center for Advanced Lung Disease Grant.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tager AM, Lacamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA(1) links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 2008;14:45–54 [DOI] [PubMed] [Google Scholar]
- 2.Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, Fagan P, Baccei CS, Santini AM, Hutchinson JH, et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol 2010;160:1699–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castelino FV, Seiders J, Bain G, Brooks SF, King C, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM. Genetic deletion or pharmacologic antagonism of LPA1 ameliorates dermal fibrosis in a scleroderma mouse model. Arthritis Rheum 2011;63:1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, Calise D, Chun J, Bascands JL, Saulnier-Blache JS, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol 2007;18:3110–3118 [DOI] [PubMed] [Google Scholar]
- 5.Swaney JS, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G, Santini AM, Darlington J, King CD, Baccei CS, et al. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther 2010;336:693–700 [DOI] [PubMed] [Google Scholar]
- 6.Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, Karameris A, Prestwich GD, Bouros D, Aidinis V. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol 2012;47:566–574 [DOI] [PubMed] [Google Scholar]
- 7.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 2010;50:157–186 [DOI] [PubMed] [Google Scholar]
- 8.Funke M, Xhao Z, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis following lung injury. Am J Respir Cell Mol Biol 2012;46:355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol 2004;15:477–489 [DOI] [PubMed] [Google Scholar]
- 10.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJO, Saulnier-Blache JS, Mummery CL, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 2006;26:5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 2006;281:25822–25830 [DOI] [PubMed] [Google Scholar]
- 12.Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 1992;267:2524–2529 [PubMed] [Google Scholar]
- 13.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 2002;158:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang G, Madan D, Prestwich GD. Aromatic phosphonates inhibit the lysophospholipase D activity of autotaxin. Bioorg Med Chem Lett 2011;21:5098–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am J Pathol 2009;174:1264–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328 [DOI] [PubMed] [Google Scholar]
- 17.Emo J, Meednu N, Chapman TJ, Rezaee F, Balys M, Randall T, Rangasamy T, Georas SN. LPA2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J Immunol 2012;188:3784–3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.