Abstract
The inflamed lung exhibits oxidative and nitrative modifications of multiple target proteins, potentially reflecting disease severity and progression. We identified sphingosine-1–phosphate receptor–3 (S1PR3), a critical signaling molecule mediating cell proliferation and vascular permeability, as a nitrated plasma protein in mice with acute lung injury (ALI). We explored S1PR3 as a potential biomarker in murine and human ALI. In vivo nitrated and total S1PR3 concentrations were determined by immunoprecipitation and microarray studies in mice, and by ELISA in human plasma. In vitro nitrated S1PR3 concentrations were evaluated in human lung vascular endothelial cells (ECs) or within microparticles shed from ECs after exposure to barrier-disrupting agonists (LPS, low-molecular-weight hyaluronan, and thrombin). The effects of S1PR3-containing microparticles on EC barrier function were assessed by transendothelial electrical resistance (TER). Nitrated S1PR3 was identified in the plasma of murine ALI and in humans with severe sepsis-induced ALI. Elevated total S1PR3 plasma concentrations (> 251 pg/ml) were linked to sepsis and ALI mortality. In vitro EC exposure to barrier-disrupting agents induced S1PR3 nitration and the shedding of S1PR3-containing microparticles, which significantly reduced TER, consistent with increased permeability. These changes were attenuated by reduced S1PR3 expression (small interfering RNAs). These results suggest that microparticles containing nitrated S1PR3 shed into the circulation during inflammatory lung states, and represent a novel ALI biomarker linked to disease severity and outcome.
Keywords: acute lung injury, sphingosine-1–phosphate receptor–3, microparticles, nitration, biomarker
Clinical Relevance
In this work, we describe sphingosine-1–phosphate receptor–3 (S1PR3), which was nitrated and released in endothelial cell microparticles, as a novel acute lung injury (ALI) biomarker, a finding confirmed in vitro as well as in vivo in murine and human ALI samples. We further demonstrate that increased S1PR3 concentrations were associated with mortality in intensive care unit patients with sepsis or ALI. This work provides strong support for the role for S1PR3 in ALI severity, and indicates S1PR3 as a novel ALI candidate biomarker and an attractive target for future therapeutic strategies.
Acute lung injury (ALI) is characterized by profound inflammation, increased vascular permeability, and alveolar flooding, a combination of events that frequently results in acute respiratory failure. Although ALI mortality rates have improved during the past four decades, these remain unacceptably high (30–40%) (1, 2). One critical barrier to improvements in ALI outcomes involves the paucity of reliable biomarkers for diagnosis, prognosis, and responses to therapy (3). Unfortunately, this search is hindered by the inherent heterogeneity of the disease, along with the lack of correlations between biochemical markers, pathophysiologic variables, and clinical outcomes (4). More recently, interest has increased in ALI biomarkers that play recognized roles in vascular homeostasis, including inflammatory factors such as IL-1β, IL-6, IL-8, and TNF-α (5), coagulation factors such as protein C and thrombomodulin (6), and endothelial cell–derived factors such as von Willebrand factor (vWF), vascular endothelial growth factor (VEGF), and angiopoietin-2 (7). These studies indicated that the marked disruption of vascular integrity and the increased vascular permeability in response to bioactive agonists, cellular components, and mechanical stresses comprise cardinal features of inflammatory lung injuries such as ALI (8, 9).
The circulating plasma proteins with post-translational modifications have been recognized as emerging biomarkers in inflammatory disorders that potentially reflect disease severity and progression (10, 11). The quantification of protein nitration or the consequent compromise in biological activities offers the potential to deliver specific and clinically relevant biomarkers for sepsis, major trauma, and ALI (12). Cerruloplasmin, transferrin, and β-chain fibrinogen are nitrated in ALI (13), and several additional proteins are implicated in murine sepsis models previously observed to undergo nitration (14).
We sought to identify novel ALI biomarkers by investigating nitrated plasma proteins in murine ALI models. Our studies identified sphingosine-1–phosphate receptor–3 (S1PR3), a vascular barrier–regulatory member of the S1P family of receptors (S1PR1–5) and a critical signaling molecule mediating cell proliferation, adhesion, angiogenesis, and vascular permeability (15, 16), as a nitrated protein in plasma and a potential novel ALI candidate gene. We determined plasma S1PR3 concentrations in several forms of ALI, including sepsis, trauma, and ventilator-induced lung injury, to confirm our findings, and we discovered that bacterial endotoxin (LPS)–exposed mice exhibit increased concentrations of total and nitrated S1PR3 in lungs and plasma. S1PR3 concentrations in plasma from intensive care unit (ICU) patients with ALI were elevated and linked to ICU mortality. Finally, in vitro endothelial cell (EC)–based studies confirmed S1PR3 nitration and its release into the medium of cultured human pulmonary artery endothelial cells (HPAECs), which were increased by barrier-disruptive agents and mechanical stress, and which contributed to endothelial barrier disruption. Together, these experiments indicate that S1PR3 is a molecular target in ALI and a novel ALI biomarker, reflecting vascular injury and impaired vascular integrity.
Materials And Methods
Cell Culture and Reagents
HPAECs and human lung microvascular endothelial cells (HLMVECs) were obtained from Cambrex (Walkersville, MD), and cultured as previously described (17) in EBM-2 Complete Medium (Cambrex) at 37°C in a humidified atmosphere of 5% CO2 and 95% air, with Passages 6–10 used for experiments. Unless otherwise specified, reagents were obtained from Sigma (St. Louis, MO). Rabbit and murine anti-S1PR3 antibodies were purchased from Exalpha Biologicals (Watertown, MA). Murine anti-nitrotyrosine (clone 1A6) antibody was purchased from Millipore Corp. (Bedford, MA). Rabbit anti-phosphoserine and rabbit anti-phospho–threonine antibodies were purchased from Zymed (South San Francisco, CA). Murine anti–β-actin antibody and LPS were purchased from Sigma (St. Louis, MO). Secondary horseradish peroxidase (HRP)–labeled antibodies were purchased from Amersham Biosciences (Piscataway, NJ).
Animal Preparation and Experimental Intervention
Male C57BL/6J mice (aged 8–10 wk; Jackson Laboratories, Bar Harbor, ME) were anesthetized with intraperitoneal ketamine (150 mg/kg) and acetylpromazine (15 mg/kg). In LPS-induced ALI models, LPS (2.5 mg/kg) or water (control) were instilled intratracheally, as we previously described (18, 19). High-molecular-weight hyaluronan (HMWHA) (1.5 mg/kg), hepatocyte growth factor (HGF) (50 μg/kg), or saline (control) were used intravenously, 4 hours after LPS challenge (16). The animals were allowed to recover for 24 hours after challenge. Plasma and bronchoalveolar lavage fluid were subsequently collected (LPS, n = 10; PBS control, n = 8) for S1PR3 nitration analysis, protein/albumin measurement, and ELISA. The ventilator-induced lung injury (VILI) model was designed to produce ALI (tidal volume, 40 ml/kg; 4 h). Control mice received anesthesia and were intubated, but did not receive mechanical ventilation, as reported earlier (20). In specific experiments, siSTABLE specifically modified S1PR3 small interfering (si) RNAs, or siRNA controls (10 mg/kg mouse, intravenous; Dharmacon, Lafayette, CO) were administered as previously described (20). Experimental groups included (1) spontaneously breathing (SB) mice receiving control siRNA, (2) SB mice challenged with S1PR3 siRNA, (3) mice receiving high tidal ventilation (VILI) treated with control siRNA, and (4) mice receiving high tidal ventilation treated with S1PR3 siRNA (VILI-siS1PR3) (n = 4–6 for all groups). Bronchoalveolar lavage (BAL) fluid was subsequently collected for protein/albumin measurements, as described previously (20).
Immunoprecipitation and Immunoblotting
Cellular materials from treated or untreated HLMVECs were incubated with immunoprecipitation buffer, as we previous described (16). Solubilized proteins were immunoprecipitated with rabbit anti-S1PR3 antibody IgG, followed by SDS-PAGE in 4–15% polyacrylamide gels, transferred to Immobilon membranes (Millipore Corp.). After blocking nonspecific sites with 5% BSA, the blots were incubated with either murine anti-S1PR3 antibody or murine anti-nitrotyrosine antibody, followed by incubation with HRP-labeled goat anti-rabbit or goat anti-mouse IgG. The visualization of immunoreactive bands was achieved using enhanced chemiluminescence (Amersham Biosciences). Two-dimensional electrophoresis was performed as described elsewhere (21). Briefly, HLMVECs were treated with LPS (0–24 h, 1 μg/ml) and immunoprecipitated with anti-S1PR3 antibody, and eluted samples were loaded on an immobilized pH gradient (IPG) strip (Amersham Biosciences) that was rehydrated for 12 hours, followed by isoelectric focusing steps of 500 volt-hours (Vhr), 1,000 Vhr, and 8,000 Vhr, using the IPGphor IEF System (Amersham Biosciences). The second-dimension separation was run using the XCell Surelock Mini-Cell System (Invitrogen, Grand Island, NY) in 4–20% gels. Proteins were transferred onto Immobilon membranes, and developed with specific primary and secondary antibodies.
ELISA
Plasma from patients with severe sepsis-induced ALI (n = 23) and sepsis without ALI (n = 24) and nonsepsis ICU control subjects (n = 19) was collected according to the Chicago Consortium for Investigating ICU Genetics Protocol (and with the approval of its Institutional Review Board). Costar enzyme immunoassays/radioimmunoassays (EIA/RIA) plates (ImmunoChemistry Technologies, Bloomington, MN) were coated with capture antibodies (murine anti-S1PR3 antibody, amino acid residues 302–379) at 4°C overnight. Plates were washed and blocked with blocking buffer for 2 hours, and plasma samples from control subjects and ALI patients were added and incubated for 2 hours. After the addition of detection antibodies (rabbit anti-S1PR3, amino acid residues 140–170, polyclonal antibodies), the plates were incubated for 1 hour. Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) was next applied and incubated for 30 minutes. The concentration of S1PR3 was determined using Cytofluor 4000 (Applied Biosystems, Foster City, CA), and normalization was based on concentration–response curves, using S1PR3 recombinant protein (Novus, Littleton, CO). In addition to these studies, a limited number of plasma samples was obtained from trauma patients with ALI (n = 5) and without ALI (n = 5), and was analyzed for total and nitrated S1PR3 concentrations with immunoblotting, as already described.
Statistical Analysis
In our case studies, the data are presented as median and 5th–95th percentiles, because S1PR3 concentrations were not normally distributed in the plasma of patients. Statistical analyses were performed on the log (base 2) S1PR3 concentrations, to allow parametric tests to be performed. One-way ANOVA was used to test for significant differences between ICU patients (controls, sepsis, and sepsis-induced ALI). Otherwise, the Student t test was used, and the results are expressed as means ± SD. The significance of differences in survival rates between patients with high (above the median for the study population) or low S1PR3 concentration groups were measured by log-rank test.
Results
Plasma Concentrations of Nitrated S1PR3 Are Increased in Murine Models of ALI
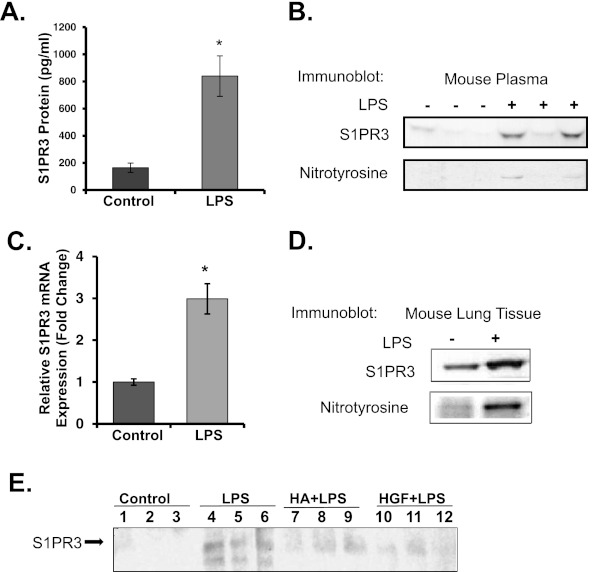
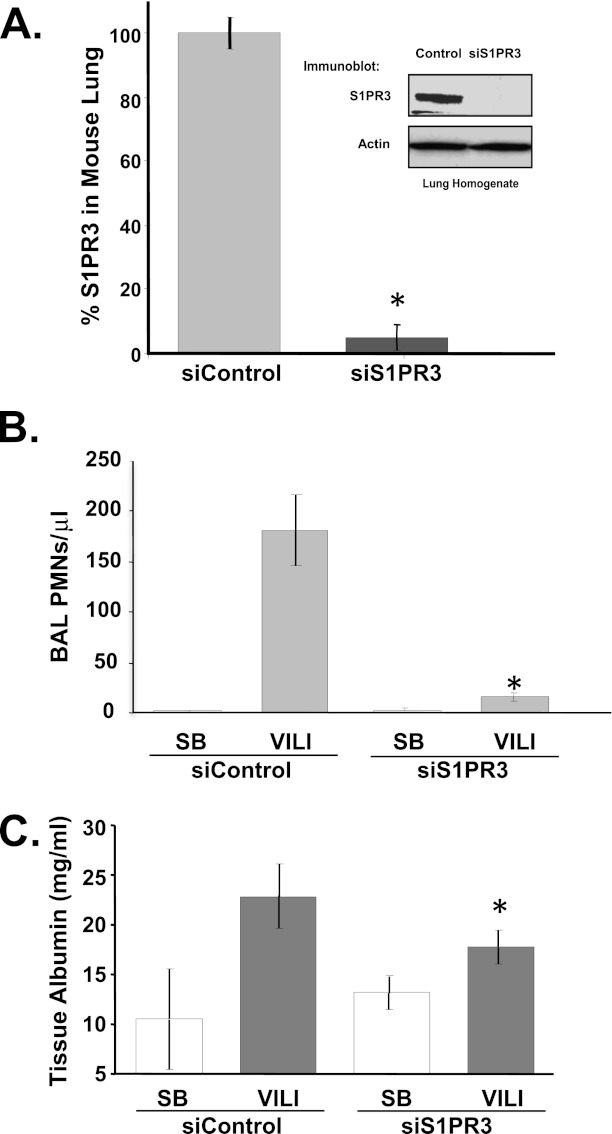
The immunoprecipitation of plasma proteins from mice exposed to LPS followed by mass spectrometry identified a peptide S14SVSDY+45GNYDIIVR27, with a tyrosine residue in position 19 modified by nitration. In silico analysis revealed that this peptide resides within the sequence of the S1PR family of receptor proteins. We next evaluated S1PR family proteins as specific targets for nitration in well characterized murine models of ALI and in human subjects with ALI. Despite variances in concentrations of S1PR3 protein and nitration, the average S1PR3 concentrations were significantly increased in plasma from LPS-induced ALI mice, compared with control mice, by approximately 5-fold (P < 0.01) (Figures 1A, 1B, and E1). Increased S1PR3 gene and protein expression in the murine lung was assessed by Affymetrix microarrays (Affymetrix, Santa Clara, CA) (Figure 1C) or by immunoprecipitation from lung homogenates (Figure 1D), and confirmed increased concentrations of S1PR3 as well as tyrosine nitration in plasma (Figure 1B) and lung tissues (Figure 1D). Interestingly, the addition of vascular barrier–promoting agonists such as HMWHA or HGF (15, 16, 22) significantly attenuated the increase in S1PR3 recovered in plasma from LPS-challenged ALI mice (Figure 1E). In addition, the intravenous administration of siS1PR3 significantly reduced S1PR3 expression in lungs (Figure 2A), and significantly attenuated mechanical ventilation-induced leukocyte infiltration (Figure 2B) and vascular permeability (Figure 2C) in the ventilator-induced ALI murine model.
Figure 1.
LPS-induced murine acute lung injury (ALI) increases the nitration of sphingosine-1–phosphate receptor–3 (S1PR3) in lung tissues and plasma. Plasma and lung tissue were obtained from mice challenged with intratracheal LPS (2.5 mg/kg, n = 10) or H2O (control, n = 8) for 18 hours. (A) Levels of S1PR3 protein expression in plasma were detected by immunoassay (ELISA) with anti-S1PR3 antibodies. (B) Nitrated S1PR3 protein in plasma was detected by immunoprecipitation with anti-S1PR3 and probing with anti-nitrotyrosine antibodies. (C) Levels of S1PR3 gene expression in lung tissue were detected by genome-wide microarray profiling of mRNA in LPS-induced ALI mice, compared with control mice (*P < 0.01). (D) Concentrations of nitrated S1PR3 were increased in lung tissue of LPS-exposed mice compared with control mice (*P < 0.01). (E) Concentrations of S1PR3 in plasma of mice were detected by immunoblotting with anti-S1PR3 antibody, 24 hours after mice were instilled with LPS 2.5 mg/kg intratracheally, with high-molecular-weight hyaluronan (HA) 1.5 mg/kg, hepatocyte growth factor (HGF) 50 μg/kg, or saline control. These experiments were repeated three times.
Figure 2.
Silencing of S1PR3 protein expression protects mice from ventilator-induced lung injury (VILI). (A) S1PR3 protein concentrations in murine lung tissues were detected by immunoblotting (inset), 5 days after small interfering (si) RNA was delivered (10 mg/kg intravenous, enhanced, stable siS1PR3 or siControl). The S1PR3 proteins in murine lung tissue were significantly decreased (90%) by siS1PR3, compared with control samples (*P < 0.01 versus control). Effects of siS1PR3 on the magnitude of lung injury were assessed by numbers of polymorphonuclear leukocyte (PMN) cells (B) and albumin concentrations (C) in bronchoalveolar lavage (BAL) after ventilator-induced lung injury (VILI) with a tidal volume of 40 ml/kg for 4 hours in spontaneously breathing (SB) mice (*P < 0.05, versus VILI from siControl), compared with the siControl group. Data are representative of independent experiments from four to six mice.
Plasma S1PR3 Concentrations Are Increased in Human ALI and Are Associated with Mortality Risk
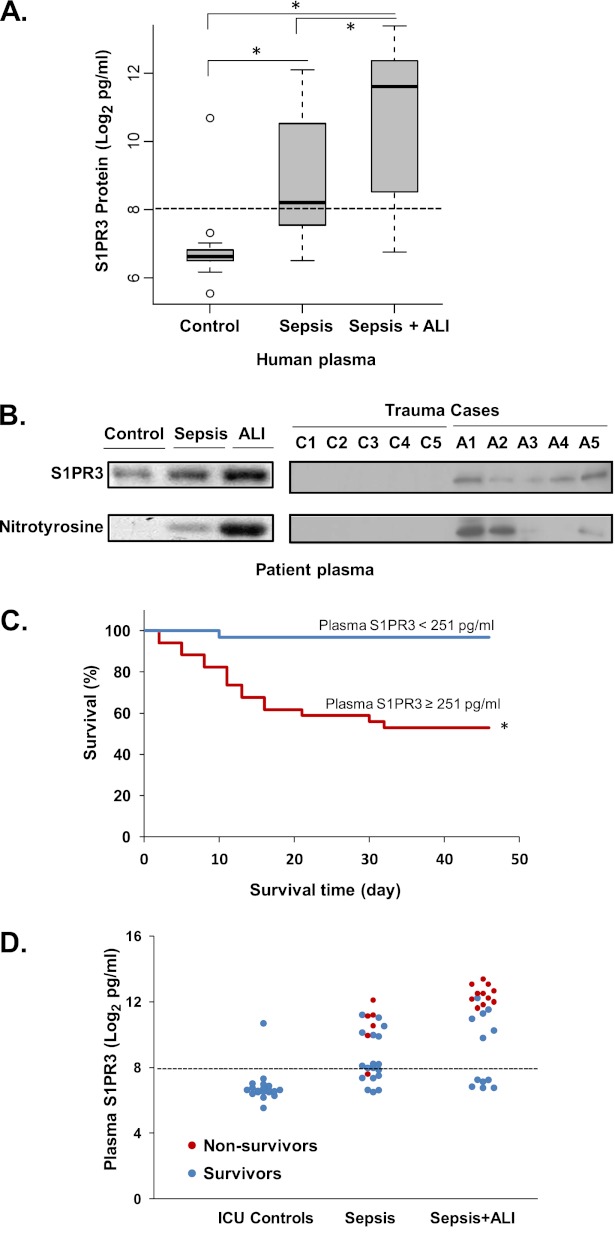
Plasma S1PR3 protein concentrations were investigated in 66 human ICU subjects with the clinical characteristics listed in Table 1. Plasma S1PR3 concentrations were significantly different in three groups: severe sepsis, severe sepsis-induced ALI, and ICU control subjects (P < 0.01), according to ANOVA. Plasma S1PR3 protein concentrations were significantly increased in 23 severe sepsis-induced cases of ALI (median, 3,136 pg/ml; range, 520–5,305 pg/ml), compared with 19 ICU control subjects (median, 98 pg/ml; range, 90–113 pg/ml) (P < 0.01). Plasma S1PR3 protein concentrations in 24 patients with sepsis without ALI were also significantly increased (median, 296 pg/ml; range, 190–1,468 pg/ml) (Figure 3A). The median value for ELISA-detected plasma S1PR3 protein concentrations in all ICU subjects was 251 pg/ml. The sensitivity and specificity for high plasma S1PR3 concentrations (> 251 pg/ml) to predict severe sepsis-induced ALI were 74% and 65%, respectively. We further detected that both total and nitrated S1PR3 were significantly increased in sepsis-induced ALI by immunoprecipitation. Given the diversity of ALI-inducing stimuli, we also assessed plasma S1PR3 concentrations in a limited number of trauma-induced ALI cases (the clinical characteristics were described previously) (23). Total and nitrated S1PR3 concentrations in plasma from a small sample of trauma-induced ALI cases (n = 5) were also significantly increased, compared with trauma ICU controls without ALI (n = 5) (Figure 3B). Importantly, plasma S1PR3 concentrations greater than 251 pg/ml were significantly associated with increased mortality in both sepsis and ALI ICU cases (log-rank test, P < 0.01) (Figure 3C). Patients with higher plasma S1PR3 concentrations (> 251 pg/ml) were more likely to be non-ICU survivors (Figure 3D). The sensitivity and specificity for increased S1PR3 in plasma (> 251 pg/ml) to predict mortality in ICU patients were 94% and 67%, respectively. Comparisons of the receiver operating characteristics curve determined that the predictive value of S1PR3 (> 1465 pg/ml) was better than Acute Physiology and Chronic Health Evaluation II (APACHE II) (area under the curve, 0.94 versus 0.80; P < 0.01) (Figure E4).
TABLE 1.
CLINICAL CHARACTERISTICS OF THE STUDY POPULATION
| ICU Control Subjects | Patients with Sepsis | Patients with ALI | |
| N | 19 | 24 | 23 |
| Sex, M/F | 9/10 | 12/12 | 12/11 |
| Race, African American/European American | 11/8 | 12/11 | 8/12 |
| Age, yr (mean ± SD) | 59 ± 17 | 61 ± 16 | 52 ± 19 |
| APACHE II (mean ± SD) | 19 ± 6.6 | 28 ± 6.5* | 26 ± 8.9* |
| Mortality, % | 0 | 25 | 47 |
| TOC, d | 3 (2–5) | 5 (3–9) | 4 (3–7) |
| Mechanical ventilation, % | 47 | 58 | 100 |
| Cancer, % | 32 | 42 | 30 |
| CLD, % | 5 | 8 | 9 |
| ESRF, % | 21 | 29 | 26 |
| COPD, % | 16 | 17 | 4 |
| Diabetes, % | 53 | 29 | 26 |
| CHF, % | 37 | 29 | 13 |
| AIDS, % | 5 | 4 | 9 |
| Site of infection | |||
| Lung, % | NA | 63 | 61 |
| Abdomen, % | NA | 12 | 17 |
| UTI, % | NA | 8 | 9 |
| Blood, % | NA | 4 | 9 |
| Other, % | NA | 13 | 6 |
Definition of abbreviations: ALI, acute lung injury; APACHE II, Acute Physiology and Chronic Health Evaluation; TOC, time of collection; CHF, congestive heart failure; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; ESRF, endstage renal failure; F, female; IVU, intensive care unit; M, male; NA, not applicable; UTI, urinary tract infection.
P < 0.05 for significant difference, versus ICU control subjects.
Figure 3.
Increased nitrated S1PR3 concentrations in plasma from sepsis-induced and trauma-induced ALI cases confer increased risk of sepsis/ALI mortality. (A) S1PR3 protein concentrations in human plasma from patients with sepsis (n = 24), sepsis-induced ALI cases (n = 23), and intensive care unit (ICU) control subjects (n = 19) were determined using murine and rabbit anti-human S1PR3 with ELISA. Plasma S1PR3 concentrations are indicated as log2 pg/ml, and the median concentration is 28 (251 pg/ml), indicated as a dotted line (*P < 0.05). (B) Total S1PR3 and tyrosine-nitrated S1PR3 concentrations in plasma from patients with sepsis (n = 3), sepsis-induced ALI cases (n = 3), control subjects (n = 3), patients with trauma-induced ALI (n = 5), and trauma cases without ALI (n = 5) were detected by immunoprecipitation with anti-S1PR3 and blotting with anti-nitrotyrosine antibodies. (C) Kaplan-Meier plot shows the survival rate of ICU patients. The survival rate of ICU patients (including ICU controls, sepsis only, and sepsis-induced ALI) with high S1PR3 concentrations in plasma (≥ 251 pg/ml) was compared with that of patients with low S1PR3 concentrations (< 251 pg/ml). *P < 0.01, according to log-rank test. (D) Comparison of plasma S1PR3 protein concentrations in survival cases (blue dots) and nonsurvival cases (red dots) in ICU control, sepsis, and sepsis-induced ALI groups.
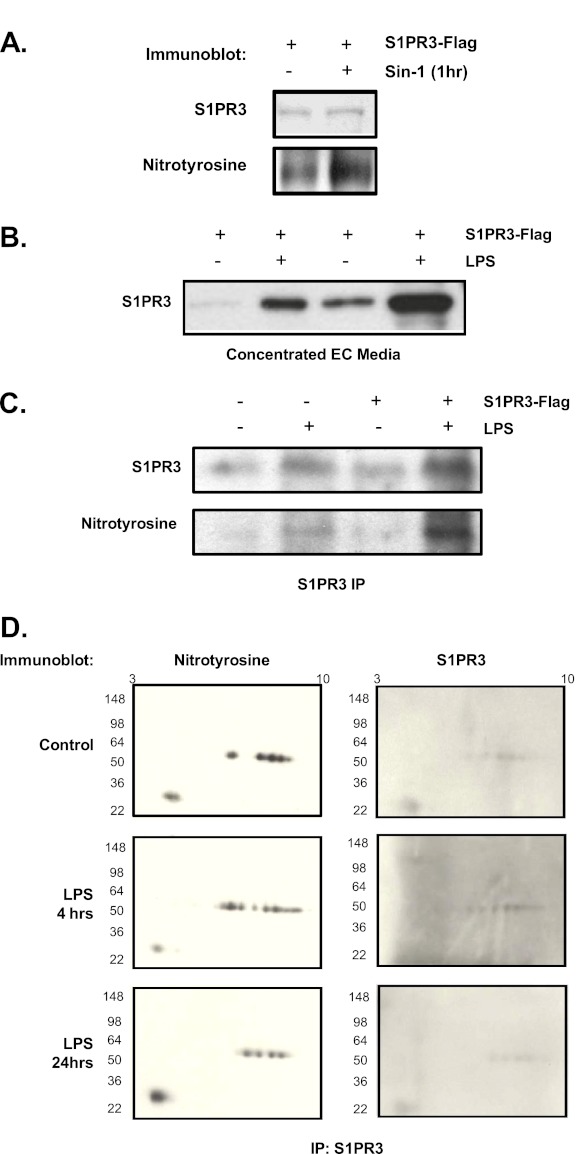
Figure 4.
Human endothelial cell S1PR3 undergoes tyrosine nitration and cellular release. (A) Flag-tagged S1PR3 was transfected and expressed in human pulmonary endothelial cells (HPAECs), and incubated with or without a nitrating agent (3-morpholinosydnonimine [SIN-1], 1 mM, 1 h) before sample collection. The S1PR3 was then immunoprecipitated from HPAEC homogenates by anti-Flag antibodies and immunoblotted with rabbit anti-S1PR3 and anti-nitrotyrosine antibodies. (B) HPAECs were transfected with Flag-tagged S1PR3 and stimulated with LPS (1 μg/ml, 24 h), and S1PR3 was detected in concentrated cell media by Western blotting with rabbit anti-S1PR3. EC, endothelial cells. (C) S1PR3 was immunoprecipitated by anti-Flag antibodies, and nitration status assessed by anti-nitrotyrosine antibodies. (D) Significant concentrations of released nitrated S1PR3 from HPAECs were detected in media after stimulation with LPS in vitro (80% increase, P < 0.05). Human lung microvascular endothelial cells treated by LPS (1 μg/ml) resulted in the detection of nitrated S1PR3 (indicated by expanded bands) by two-dimensional gel electrophoresis and immune precipitation with anti-S1PR3 and immunoblotting with anti-nitrotyrosine and anti-S1PR3 antibodies. Each experiment was repeated three times.
Barrier-Regulating Agonists Alter S1PR3 Nitration and Endothelial Release
In silico analysis identified 10 potential S1PR3 tyrosine nitration sites, including the peptide sequence identified by mass spectroscopy in the global identification of nitrated peptides. To confirm the nitration of S1PR3, we expressed a Flag-tagged S1PR3 in HPAECs, and exposed cells to the nitrating agent 3-morpholinosydnonimine (SIN-1), followed by immunoprecipitation with anti-Flag antibodies and detection with anti-nitrotyrosine antibodies. Figure 4A indicates that the expressed S1PR3 exhibits basal tyrosine nitration that is substantially increased by SIN-1 challenges. These data indicate that S1PR3 is a target for tyrosine nitration, which is consistent with the identification of nitrated S1PR3 in the plasma of murine models of ALI as well as human patients with clinically documented ALI.
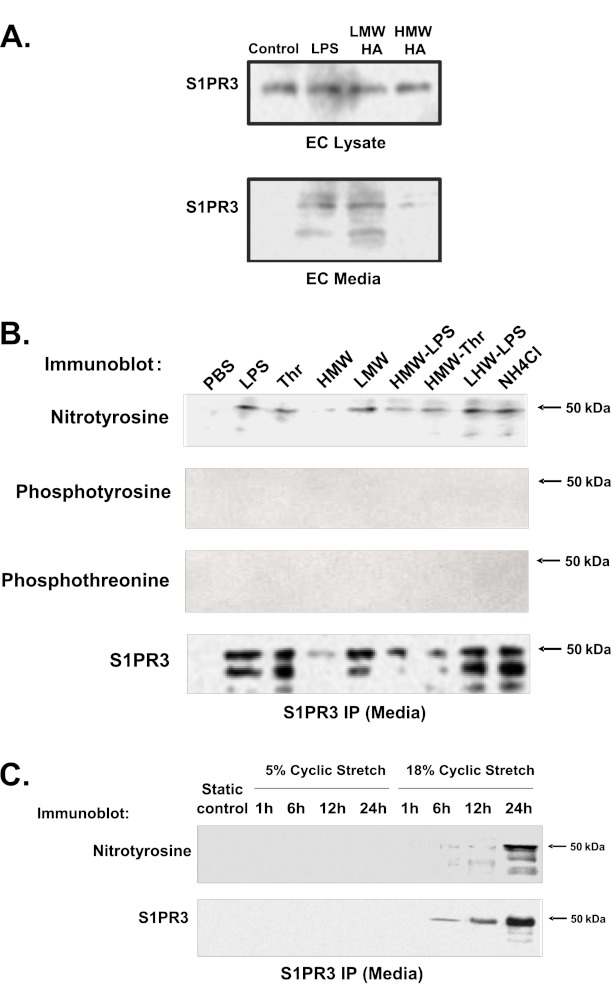
We next examined whether the endothelial cell (EC) barrier–disrupting agent LPS induces tyrosine nitration and alters S1PR3 localization. LPS challenge (24 h) resulted in the release by ECs of S1PR3 into the cellular media (Figure 4B), with extracellular S1PR3 exhibiting modification by tyrosine nitration (Figure 4C) without significant effects on cell viability (Figure E2; P > 0.05). Immunoprecipitation with anti-S1PR3 antibodies, followed by two-dimensional gel electrophoresis and Western blot analysis for nitrated proteins, further revealed LPS-induced increases in S1PR3 nitration in ECs (Figure 4D). In addition to LPS, Figure 5A confirms S1PR3 release after challenge with the barrier-disrupting low-molecular-weight hyaluronan (LMW-HA) (15) (24 h), whereas the EC barrier–enhancing high-molecular-weight hyaluronan (HMW-HA) (15) failed to induce S1PR3 release. Consistent with the regulation of S1PR3 release by EC barrier–disruptive agents, Figure 5B indicates that multiple barrier-disruptive agents LPS, thrombin, LMW-HA, and ammonium chloride induce S1PR3 tyrosine nitration and cellular release. These responses were attenuated by HMW-HA. However, in contrast to S1PR3 nitration, Figure 5B indicates that S1PR3 fails to exhibit tyrosine and threonine phosphorylation, the posttranslational modifications of S1PR3 we previously demonstrated after challenges with LPS and thrombin (16). Finally, because excessive mechanical stress is a well-recognized stimulus for lung inflammation and the loss of vascular integrity (20), the effects of excessive mechanical stress via cyclic stretch were examined in the EC release of S1PR3. Pathological barrier-disruptive cyclic stretch (18%) (24), but not nonpathological, barrier-preserving cyclic stretch (5%), induced S1PR3 shedding and tyrosine nitration (6–24 h) (Figure 5C).
Figure 5.
Nitrated S1PR3 is released from human lung endothelium after treatment with barrier regulatory agents and mechanical stress. (A) Human pulmonary artery ECs were treated with LPS (1 μg/ml), low-molecular-weight hyaluronan (LMWHA; 100 nM), or high-molecular weight hyaluronan (HMWHA; 100 nM) for 24 hours. S1PR3 in cell lysate and media were detected by anti-S1PR3. S1PR3 release from human pulmonary artery ECs into media increased after stimulation with LPS or LMWHA, and treatment with HMWHA reduced this release. (B) Human lung microvascular ECs were challenged with LPS 1 μg/ml, or thrombin (Thr) 1 U/ml, or HMWHA 100 nM, or LMWHA 100 nM, or HMWHA with LPS, or HMWHA with thrombin, or LMWHA with LPS, or ammonium chloride, or PBS for 24 hours. Tyrosine nitration and the tyrosine and threonine phosphorylation of S1PR3 released into media from human lung microvascular ECs were detected by immunoblotting with anti-nitrotyrosine, anti-phosphotyrosine, and anti-phosphothreonine, after S1PR3 immunoprecipitation with anti-S1PR3. (C) In addition, levels of S1PR3 tyrosine nitration and release into media from human pulmonary artery ECs were detected by immunoblotting with anti-nitrotyrosine after S1PR3 immunoprecipitation with anti-S1PR3 antibodies after 5% and 18% cyclic stretch of human pulmonary artery ECs for 1, 6, 12, and 24 hours. Data are representative of three independent experiments.
Microparticles Containing Nitrated S1PR3 Enhance EC Barrier Disruption In Vitro
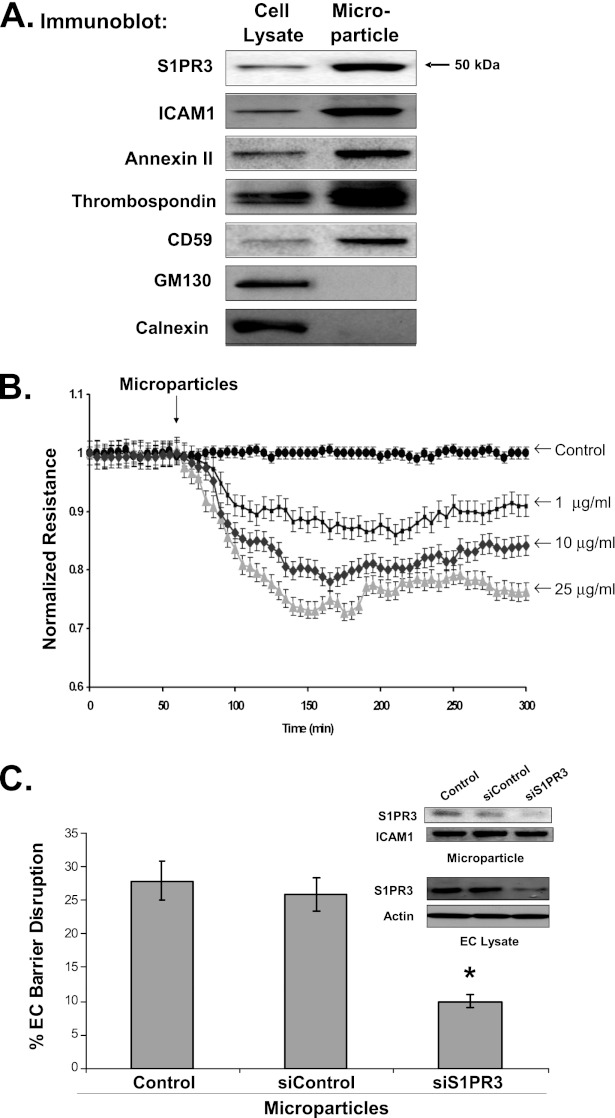
To examine the mechanism by which S1PR3 is released from ECs after exposure to barrier-disrupting agents, we isolated 10- to 1,000-nm microparticles released from LPS-challenged ECs containing intact plasma membrane proteins. Using differential centrifugations of LPS-challenged EC media (24 h), materials were isolated that contained known microparticle markers (intercellular adhesion molecule 1 [ICAM-1], annexin II, thrombospondin, and CD59), and that excluded nonmicroparticle markers (GM130 and calnexin) (Figure 6A). LPS-induced microparticles contained a significant amount of S1PR3, suggesting a potential mechanism for cellular release from plasma membranes.
Figure 6.
Nitrated S1PR3 released in microparticles contributes to endothelial cell barrier disruption. The cell lysates of human pulmonary artery endothelial cells (ECs) and microparticles from EC media were collected after stimulation with LPS (1 μg/ml). (A) The concentrations of S1PR3, microparticle membrane markers, and cellular markers were detected in cell lysates and microparticles by anti-S1PR3, anti–intercellular adhesion molecule 1 (ICAM-1), anti-annexin II, anti-thrombospondin, anti-CD59, anti-GM130, and anti-calnexin, respectively. (B) Collected microparticles were added to cell media of human artery ECs, and the transendothelial electrical resistance (TER) response to microparticles was measured. Microparticles induced dose-dependent reductions in EC barrier integrity (1, 10, and 25 μg/ml). (C) In addition, the reduction in TER was measured in response to microparticles collected from human pulmonary artery ECs incubated with 3 μM of S1PR3 siRNA or control siRNA. The microparticles generated from ECs, with previous reductions in S1PR3 expression via S1PR3 siRNA (inset), demonstrated a reduced capacity of these microparticles to decrease normalized resistance (*P < 0.05). Data are representative of three independent experiments.
Microparticles have been reported to cause vascular barrier disruption (25). The effects of microparticles isolated from LPS-challenged ECs were examined in EC barrier integrity (normalized electrical resistance) to assess whether S1PR3 in microparticles contributes to this process. The addition of isolated microparticles produces EC barrier disruption in a concentration-dependent manner (Figure 6B). Microparticles derived from LPS-stimulated ECs previously treated with S1PR3 siRNAs to reduce S1PR3 expression displayed a markedly reduced capacity to induce EC permeability, compared with microparticles from ECs exposed to silenced control samples (Figure 6C), indicating an important role for S1PR3 in microparticle-induced EC barrier disruption.
Discussion
Biomarkers are critical in the early detection of lung injury and in risk-stratification for clinical trials, and ultimately in tailoring specific therapies to individual patients (3). Multiple ALI biomarker candidates have evolved (26), with plasma concentrations of IL-6 and IL-8 well studied. ALI biomarkers exhibiting elevated concentrations in spontaneously ventilating patients with ALI before endotracheal intubation (27) are associated with ALI morbidity and mortality (28).
Increasing interest in ALI biomarkers that reflect vascular perturbation and injury has focused attention on endothelium-derived factors, such as VEGF, angiopoietin-2, vWF, ICAM-1, E-selectins, P-selectins, protein C, and thrombomodulin (3). Our efforts to identify novel plasma markers in ALI that are mechanistically linked with disease onset or progression have identified S1PR3, a critical G-protein–coupled receptor for the angiogenic factor sphingosine-1–phosphate (S1P) (17, 29), and a direct participant in the regulation of vascular permeability (16, 18). This report documents the increased expression of S1PR3 in pulmonary vascular endothelial cells with tyrosine nitration and release into the circulation within microparticles, an event associated with poor prognoses in ALI. Bacterial LPS increased total S1PR3 concentrations in murine lungs and plasma, as well as nitrated S1PR3. We also confirmed that S1PR3 nitration and release into media was increased in vitro by EC barrier–disruptive agents and by mechanical stress, and directly contributed to endothelial barrier disruption.
S1PR3 was minimally detectable in the circulation of unchallenged mice, but was significantly elevated after a variety of inflammatory and vascular barrier–disruptive factors (LPS and excessive mechanical ventilatory stress). The clinical relevance of these findings was confirmed by the increased detection of S1PR3 in the plasma of humans with sepsis, trauma-induced ALI, or sepsis-induced ALI, compared with trauma and ICU cases without ALI or sepsis. Importantly, elevated plasma S1PR3 concentrations were significantly associated with increased mortality in both sepsis and ALI cases. These finding suggest that S1PR3 may serve as an informative biomarker of vascular injury in ALI.
Bioactive lipid S1P binds to plasma membrane heptahelical S1P receptors 1–5 (17, 30). Human ECs exhibit an elevated expression of S1PR1 and S1PR3. S1PR1 signaling is coupled to the Gi pathway and the activation of Ras-related C3 botulinum toxin substrate 1 (Rac-1), whereas S1PR3 signaling was coupled to the Gi, Gq/11, and G12/13 pathways that activate Ras homolog gene family, member A (RhoA) to a much greater extent than Rac1 (17). Activated RhoA binds to and activates the serine/threonine kinase, Rho-associated protein kinase (ROCK), involved in EC barrier disruption pathways (16, 17). Our previous data indicate that the S1PR1 receptor is crucial for endothelial barrier enhancement, whereas S1PR3 expression is critical for EC barrier disruption in vitro and in vivo (15, 31, 32). Our present experiments demonstrate that circulating plasma concentrations of S1PR3 are increased in mice with ALI, and decreasing S1PR3 is associated with attenuated vascular hyperpermeability in vivo. These studies confirm previous data indicating that S1PR3 is critical for EC barrier disruption (16). Consistent with our experiments, S1PR3 expression is increased in circulating neutrophils from patients with sepsis and pneumonia and heterodimerizes with chemokine (C-X-C motif) receptor 1 (CXCR1) (33), the receptor for IL-8 (also known as IL-8RA), a biomarker of inflammation (26), which also indicates that S1PR3 is a molecular target in sepsis-associated lung injury. Furthermore, in vivo studies of S1PR3 mutant mice demonstrate that proteinase-activated receptor 1 (PAR1) amplifies inflammation through sphingosine kinase 1 (SphK1)–S1PR3 signaling crosstalk, and promotes disseminated intravascular coagulation and lethality (34). These studies further underscore an essential role for S1PR3 in sepsis and ALI, implicating S1PR3 as a potential drug-discovery target (35).
Our work offers the novel observation that S1PR3 is nitrated on specific tyrosine residues and released as EC microparticles, packaged with other proteins. EC barrier–disruptive agents are known to increase the production of reactive oxygen and nitrogen species that induce specific posttranslational modifications of plasma membrane S1PR3 tyrosine and threonine phosphorylation (16). To the best of our knowledge, no previous reports described the nitration modification of S1PR3 and its mechanism or function. In our present study, only tyrosine nitration but not phosphorylation was detected in microparticles isolated from LPS-challenged ECs. This, to the best of our knowledge, is the first report of S1PR3 nitration in ALI. The pathophysiological function of S1PR3 nitration requires additional investigations. Endothelial microparticles are complex vesicular structures shed by activated or apoptotic ECs, and contain enzymes, transcription factors, and mRNA. Endothelial cells release microparticles after activation by a variety of inflammatory stimuli, such as TNF-α (36) and other inflammatory cytokines (37), LPS (37), and thrombin (38). Our experiments indicate that EC injury–producing agents, possibly through the inflammatory factors TNF-α and thrombin, induce EC S1PR3 nitration and shedding within microparticles. Our experiments further demonstrate that S1PR3-containing microparticles enhance EC barrier disruption in vitro, consistent with the effects of microparticles in sickle cell disease (39), which suggests microparticles as a measure of EC injury as well as a cellular source of vascular dysfunction–inciting agents. LPS, in combination with cytokines, has been observed to increase EC microparticle production (40). Microparticles can be detected in the plasma of healthy subjects, and increased concentrations under pathological conditions were associated with increased thrombotic risk and endothelial dysfunction (41). The signal transduction pathways and mechanisms related to the barrier-disruptive effects of S1PR3-containing EC microparticles and differences in the function of nitrated S1PR3 and non-nitrated S1PR3 will be the subjects of further study.
Current clinical scoring systems such as APACHE II and APACHE III have been validated, and provide important prognostic information for ICU patients (42). We confirmed that both APACHE II scores and elevated S1PR3 concentrations are significantly associated with increased ALI mortality, with a higher accuracy of prediction for S1PR3 (Figure E4). On the other hand, S1PR3 concentrations, but not APACHE II scores, significantly increased in sepsis-induced ALI cases, compared with cases of sepsis alone (P < 0.05). The combination of ALI biomarkers (43) with clinical indices such as APACHE II (44) may significantly increase the accuracy of evaluating the severity and prognosis of ALI. However, given our relatively limited sample sizes, these results need to be validated in a larger cohort.
In conclusion, using both in vitro and in vivo models of lung injury and pulmonary vascular permeability, we have demonstrated that S1PR3, the permeability-producing receptor for the angiogenic factor S1P, is tyrosine-nitrated and released in microparticles after exposure to agents that induce lung injury and vascular barrier disruption. In addition, increased circulating plasma concentrations of S1PR3 and of tyrosine-nitrated S1PR3 were observed in mice and humans with ALI, with elevated concentrations conferring risk for ALI morbidity and mortality. These experiments indicate that S1PR3 is potentially involved in ALI pathophysiology, and may serve as a novel ALI biomarker and therapeutic target in the management of ALI.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Lakshmi Natarajan for expert technical assistance.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute grants P01HL58064, HL91889, and P01HL98050 from the National Institutes of Health (J.G.N.G.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0048OC on July 5, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 2009;37:1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 2009;179:220–227 [DOI] [PubMed] [Google Scholar]
- 3.Levitt JE, Gould MK, Ware LB, Matthay MA. The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med 2009;24:151–167 [DOI] [PubMed] [Google Scholar]
- 4.Cribbs SK, Martin GS. Biomarkers in acute lung injury: are we making progress? Crit Care Med 2008;36:2457–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta–dependent mechanism. Am J Respir Crit Care Med 2001;163:1384–1388 [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003;285:L514–L521 [DOI] [PubMed] [Google Scholar]
- 7.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008;63:903–909 [DOI] [PubMed] [Google Scholar]
- 8.Garcia JG. Focusing on the flood: targeting functional polymorphisms in ALI permeability pathways. Am J Respir Crit Care Med 2011;183:1287–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamp R, Sun X, Garcia JG. Making genomics functional: deciphering the genetics of acute lung injury. Proc Am Thorac Soc 2008;5:348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:503–510 [DOI] [PubMed] [Google Scholar]
- 11.Parastatidis I, Thomson L, Burke A, Chernysh I, Nagaswami C, Visser J, Stamer S, Liebler DC, Koliakos G, Heijnen HF, et al. Fibrinogen beta-chain tyrosine nitration is a prothrombotic risk factor. J Biol Chem 2008;283:33846–33853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson L, Christie J, Vadseth C, Lanken PN, Fu X, Hazen SL, Ischiropoulos H. Identification of immunoglobulins that recognize 3-nitrotyrosine in patients with acute lung injury after major trauma. Am J Respir Cell Mol Biol 2007;36:152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, III, Finkel B, Lanken PN, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2000;278:L961–L967 [DOI] [PubMed] [Google Scholar]
- 14.Ghesquiere B, Colaert N, Helsens K, Dejager L, Vanhaute C, Verleysen K, Kas K, Timmerman E, Goethals M, Libert C, et al. In vitro and in vivo protein-bound tyrosine nitration characterized by diagonal chromatography. Mol Cell Proteomics 2009;8:2642–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1–phosphate receptors is essential for vascular barrier regulation: novel role for hyaluronan and CD44 receptor family. J Biol Chem 2006;281:34381–34393 [DOI] [PubMed] [Google Scholar]
- 16.Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of vascular permeability by methylnaltrexone: role of MOP-R and S1P3 transactivation. Am J Respir Cell Mol Biol 2007;37:222–231 [DOI] [PubMed] [Google Scholar]
- 17.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1–phosphate promotes endothelial cell barrier integrity by EDG-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, et al. Differential effects of sphingosine 1–phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol 2010;43:394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol 2011;44:40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, Ma SF, Mirzapoiazova T, Evenoski C, Reeves RR, et al. Essential role of pre–B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med 2008;178:605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J, Singleton PA, Brown ME, Dudek SM, Garcia JG. Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1–phosphate–stimulated human endothelium: role in barrier enhancement. Cell Signal 2009;21:1945–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol 2010;299:L639–L651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christie JD, Ma SF, Aplenc R, Li M, Lanken PN, Shah CV, Fuchs B, Albelda SM, Flores C, Garcia JG. Variation in the myosin light chain kinase gene is associated with development of acute lung injury after major trauma. Crit Care Med 2008;36:2794–2800 [DOI] [PubMed] [Google Scholar]
- 24.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 2003;285:L785–L797 [DOI] [PubMed] [Google Scholar]
- 25.Martin S, Tesse A, Hugel B, Martinez MC, Morel O, Freyssinet JM, Andriantsitohaina R. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation 2004;109:1653–1659 [DOI] [PubMed] [Google Scholar]
- 26.McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care 2008;12:R41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cepkova M, Brady S, Sapru A, Matthay MA, Church G. Biological markers of lung injury before and after the institution of positive pressure ventilation in patients with acute lung injury. Crit Care 2006;10:R126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33:1–6, 230–232 [DOI] [PubMed] [Google Scholar]
- 29.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1–phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J 2000;14:2255–2265 [DOI] [PubMed] [Google Scholar]
- 30.Pyne S, Pyne N. Sphingosine 1–phosphate signalling via the endothelial differentiation gene family of G-protein–coupled receptors. Pharmacol Ther 2000;88:115–131 [DOI] [PubMed] [Google Scholar]
- 31.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1–phosphate–induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, TIAM1/RAC1, and alpha-actinin. FASEB J 2005;19:1646–1656 [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Ma SF, Wade MS, Flores C, Pino-Yanes M, Moitra J, Ober C, Kittles R, Husain AN, Ford JG, et al. Functional variants of the sphingosine-1–phosphate receptor 1 gene associate with asthma susceptibility. J Allergy Clin Immunol 2010;126:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahaman M, Costello RW, Belmonte KE, Gendy SS, Walsh MT. Neutrophil sphingosine 1–phosphate and lysophosphatidic acid receptors in pneumonia. Am J Respir Cell Mol Biol 2006;34:233–241 [DOI] [PubMed] [Google Scholar]
- 34.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature 2008;452:654–658 [DOI] [PubMed] [Google Scholar]
- 35.Marsolais D, Rosen H. Chemical modulators of sphingosine-1–phosphate receptors as barrier-oriented therapeutic molecules. Nat Rev Drug Discov 2009;8:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 1999;104:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szotowski B, Antoniak S, Goldin-Lang P, Tran QV, Pels K, Rosenthal P, Bogdanov VY, Borchert HH, Schultheiss HP, Rauch U. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation- and cytokine-induced endothelial cells. Cardiovasc Res 2007;73:806–812 [DOI] [PubMed] [Google Scholar]
- 38.Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 2006;108:1868–1876 [DOI] [PubMed] [Google Scholar]
- 39.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor–positive microparticles derived from endothelial cells and monocytes. Blood 2003;102:2678–2683 [DOI] [PubMed] [Google Scholar]
- 40.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003;101:3765–3777 [DOI] [PubMed] [Google Scholar]
- 41.Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, et al. Endothelial-derived microparticles: biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost 2010;104:456–463 [DOI] [PubMed] [Google Scholar]
- 42.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–829 [PubMed] [Google Scholar]
- 43.Calfee CS, Ware LB, Glidden DV, Eisner MD, Parsons PE, Thompson BT, Matthay MA. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med 2011;39:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 2010;137:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.