Abstract
Existing antipsychotic drugs are most effective at treating the positive symptoms of schizophrenia, but their relative efficacy is low and they are associated with considerable side effects. In this study deep brain stimulation of the ventral hippocampus was performed in a rodent model of schizophrenia (MAM-E17) in an attempt to alleviate one set of neurophysiological alterations observed in this disorder. Bipolar stimulating electrodes were fabricated and implanted, bilaterally, into the ventral hippocampus of rats. High frequency stimulation was delivered bilaterally via a custom-made stimulation device and both spectral analysis (power and coherence) of resting state local field potentials and amplitude of auditory evoked potential components during a standard inhibitory gating paradigm were examined. MAM rats exhibited alterations in specific components of the auditory evoked potential in the infralimbic cortex, the core of the nucleus accumbens, mediodorsal thalamic nucleus, and ventral hippocampus in the left hemisphere only. DBS was effective in reversing these evoked deficits in the infralimbic cortex and the mediodorsal thalamic nucleus of MAM-treated rats to levels similar to those observed in control animals. In contrast stimulation did not alter evoked potentials in control rats. No deficits or stimulation-induced alterations were observed in the prelimbic and orbitofrontal cortices, the shell of the nucleus accumbens or ventral tegmental area. These data indicate a normalization of deficits in generating auditory evoked potentials induced by a developmental disruption by acute high frequency, electrical stimulation of the ventral hippocampus.
Keywords: deep brain stimulation, ventral hippocampus, MAM, auditory evoked potential, local field potential, schizophrenia
1. Introduction
Deep brain stimulation (DBS) is accepted as an alternative therapy for patients refractory to conventional treatments in a variety of movement disorders and is finding increasing application in a large number of other neurological and psychiatric conditions. The most common DBS strategies find application in conditions with histories of successful treatment via surgical ablations leading to the “functional lesion” hypothesis to describe its mechanism of action. Schizophrenia, however, has no such successful history and consequently no immediately obvious stimulation target. As such, despite a desperate need to identify new treatment approaches, the use of DBS as a therapeutic approach in schizophrenia has as yet not been investigated.
Human studies implicate hyperactivity of the anterior hippocampus as a pathological factor in schizophrenia (Malaspina et al., 1999). Reductions in the levels of GAD65 and GAD67 have been reported in specific subfields of the hippocampus, most notably in layers of CA3/2 and CA1 normally associated with large numbers of GABA neurons (Benes et al., 2007, 2008). This diminished inhibitory control in the hippocampus is thought to contribute to the feed-forward excitation through the hippocampal circuit (Benes et al., 2008) resulting in subicular hyperactivity which may underpin the aberrant regulation of the dopamine system which is central to the manifestation of the positive symptoms associated with the disease (Lodge and Grace, 2007; Grace, 2012). Rodent studies using administration of the mitotoxin methylazoxymethanol acetate (MAM) on gestational day 17 in the rat, a validated animal model of this disorder (Moore et al., 2006; Lodge and Grace, 2009; Hradetzky et al., 2012), also demonstrate ventral hippocampal (the homologous rat brain region) disruption and hyperactivity presumably due to parvalbumin interneuron loss (Penschuck et al., 2006; Lodge et al., 2009). Moreover, the widespread projections of this region to cognitive (medial pre-frontal cortex, (Hoover and Vertes, 2007)) and affective (amygdala, (Canteras and Swanson, 1992)) regions suggest it may impact other symptom classes as well (Grace, 2012). Given the known inhibitory effects of hippocampal stimulation in patients with medial-temporal lobe epilepsy (Boon et al., 2007) it has been hypothesized that stimulation of this region may ameliorate the positive symptoms associated with schizophrenia (Mikell et al., 2009).
Amongst myriad symptom presentations, aberrant auditory processing has long been associated with schizophrenia. Decreased amplitudes in evoked response components with latencies between 50 and 300ms (Buchsbaum, 1977; Boutros et al., 1993; Ford et al., 1994) and decreased sensory gating (Freedman et al., 1991) are commonly reported. Abnormalities in evoked responses with latencies of approximately 50ms (P50) have been linked to deficits in gamma band activity in schizophrenia whereas deficits in components with latencies of approximately 100ms (N100) have been associated with theta activity (Brockhaus-Dumke et al., 2008). It has been suggested that the hippocampus plays a crucial role in the generation of these potentials (Freedman et al., 1991; Bickford-Wimer et al., 1990). Of these disrupted evoked components, it is believed that the longer latency components are due to disruption of attentional processes, whereas the earlier components are more resilient to attentional manipulation (Jerger et al., 1992). Disruption of the earlier components can be replicated in rats by administration of dopaminergic agonists which are reversed by the administration of first generation antipsychotic drugs such as haloperidol (Adler et al., 1986; Bickford-Wimer et al., 1990). These evidences implicate auditory evoked potentials as a valuable proxy measure of the hippocampal disruption and consequent dopamine hyperfunction observed in the MAM-E17 rat model of schizophrenia (Moxon et al., 2003).
In the present study the effects of acute high frequency electrical stimulation of the ventral hippocampus was explored in the MAM-E17 rodent model of schizophrenia. The effects of this stimulation were assessed through examination of auditory evoked potentials and spontaneous local field potentials recorded from multiple regions implicated in the pathophysiology of schizophrenia-like symptoms in the rat, including: the prelimbic-, infralimbicand orbitofrontal- cortices, the ventral striatum, the mediodorsal thalamic nucleus, the ventral tegmental area and the ventral hippocampus.
2. Materials and methods
2.1. Animals and surgery
All experiments were performed in accordance with the United States Public Health Service “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Timed pregnant female Sprague-Dawley rats were obtained at gestational day 15 and housed individually with free access to food and water. A DNA methylating agent (methylazoxymethanol (MAM) in saline) or vehicle (saline) was administered (20mg/kg, 1ml/kg, i.p.) on gestational day 17. Male pups were weaned on post-natal day 21 and housed in groups of two or three with free access to food and water. Experiments occurred no earlier than 12 weeks of age.
Rats weighing 560-610g (mean = 583g, n(MAM) = 10, n(SAL) = 8, 18 total) were anesthetized with isoflurane (induction: 5%, maintenance: 2% in oxygen) and mounted in a sterotaxic frame. Carprofen (Rimaydyl™) was administered for analgesia (5mg/kg s.c.). Body temperature was maintained with a small heating pad (Fintronics Inc.). The scalp was incised, the skull exposed and burr holes drilled. LFP electrodes were implanted bilaterally into the prelimbic (RC3: +3.4, ML: ±0.7mm, DV: -4.0mm), infralimbic (RC: +3.4, ML: ±0.7mm, DV: -5.0mm) and orbitofrontal cortices (RC: +3.2, ML: ±3.4mm, DV: -5.0mm, PrL, IL and OFC respectively), the core (RC: +1.2, ML: ±2.0mm, DV: -7.2mm) and shell (RC: +1.2, ML: ±2.0mm, DV: -8.0mm) of the nucleus accumbens (AcbC, AcbSh respectively), the mediodorsal thalamic nucleus (RC: -3.3, ML: ±0.7mm, DV: -5.5mm, MD) and the ventral tegmental area (RC: -5.3, ML: ±0.7mm, DV: -8.5mm, VTA). Custom made dual recording/stimulating electrodes were implanted, bilaterally, into the ventral hippocampus (RC: -6.0, ML: ±4.5mm, DV: -8.2mm, vHipp). Two ground screws were affixed either side of lambda and 6 additional stainless steel screws fixed in the skull. All electrodes and connectors (E363, Plastics 1) were secured with dental cement and the incision sutured tightly around the implant. Animals were housed singly following surgery, given free access to rat chow softened with children's Tylenol for the first 2 days and allowed to recover for at least a week before beginning recordings.
2.2. Data acquisition
Local field potential (LFP) recording electrodes (125μm, polyimide insulated, stainless steel wire) and stainless steel screw electrodes were obtained from Plastics 1. Custom-made dual recording/stimulating bipolar-concentric electrodes were fabricated by feeding 125μm, polyimide insulated, stainless steel wire through 26G stainless steel tubing. A third 125μm LFP recording electrode was affixed to the outside of the tubing to allow simultaneous stimulation and recording from the same site. The stainless steel tubing was insulated with miniature heatshrink tubing (Plastics 1).
Animals were tethered to the recording system (RHA2000-EVAL board, Intan Technologies, LLC) via a custom-made 11 channel cable and commutator (Plastics 1). All recordings were made against the ground screws affixed above the cerebellum. LFPs were amplified (gain = 800), high pass filtered at 1Hz, low pass filtered at 7.5kHz and sampled at 25kHz. Animals were habituated to the recording apparatus over 3 days before beginning data acquisition. Auditory evoked potential event markers were recorded via one of the auxiliary inputs on the RHA2000-EVAL board. Recordings were made while animals were loosely restrained within a 4l glass jar. The jar was placed inside a sound attenuated chamber (SR lab, San Diego Instruments) and auditory stimuli presented via a speaker mounted in the roof of the chamber. Testing for each animal consisted of the presentation of 100 identical pairs of white noise auditory stimuli (15dB above background (60dB), 10ms duration, 500ms interstimulus interval, 10s intertrial interval) (Mears et al., 2009).
2.3. Stimulation
Custom designed stimulation devices were fabricated for the continuous delivery of chronic DBS in the rat. Devices were configured to stimulate with square, monophasic constant current 100μs pulses at a frequency of 130Hz delivered at an amplitude of 200μA. Stimulation began at the start of the testing session (5 minutes before the first stimulus presentation) and was delivered continuously throughout (approximately 20 minutes). Animals were randomly allocated by stimulation protocol (stimulation or sham) and time of day. They performed the inhibitory gating tests twice on consecutive days based on the double test cross-over design which is purported to have greater sensitivity (Leyland et al., 1979). Animals would either be receiving stimulation or sham-stimulation (attachment of devices and connectors but no delivery of current) in each of the two sessions in a counterbalanced design with half of the animals receiving stimulation on the first day of testing and sham-stimulation on the following day with these conditions reversed in the remaining animals.
2.4. Signal processing
Auditory evoked potentials were identified (via the event marker) and extracted for 0.3s preceding and 0.5s following the stimulus presentation. These signals were then processed to remove electrical artifacts caused by stimulation using custom written algorithms based on those developed by others (Erez et al., 2010). Briefly, the evoked potentials are averaged and subtracted from each stimulation period. The remaining artifact was removed by an offline sample and hold algorithm. The algorithm detects stimulus artifacts by thresholding the first derivative of the signal and deleting a user defined period which encompasses this artifact. This gap in the signal is then filled using a linear interpolation. To account for potential processing artifacts simulated artifacts were imposed on non-stimulated signals and then removed by the same algorithm. The artifact free AEPs were then averaged over the 100 trials to generate two AEPs per subject (CR is the response to the conditioning stimulus, TR is the response to the test stimulus).
Following the end of the inhibitory gating paradigm 60s segments were selected for further spectral analysis. The artifact-free signals were processed by Fourier methods (Welch's modified periodogram method) to determine band power within regions and coherence (magnitude-squared) between regions for the whole epoch. Signal processing was performed using custom written scripts with GNU Octave.
2.5. Electrode localization, exclusions and statistical analysis
Animals were re-anesthetized with isoflurane and electrode locations marked by driving a 200μA constant current through each electrode for 10s. Brains were then removed and fixed in 8% paraformaldehyde with added potassium ferrocyanide. All animals with incorrectly placed stimulating electrodes were excluded from the analysis. Animals with incorrectly placed recording electrodes were excluded on a region by region basis leaving final group sizes of >7 per region, per treatment. Differences between evoked potential components were determined via a Student's t-test. The significance level was corrected using an FDR method (false discovery rate (Benjamini and Yekutieli, 2012)) for the 16 brain regions yielding a significance level of 0.01479. All analyses were performed using GNU Octave.
3. Results
3.1. Spectral analysis
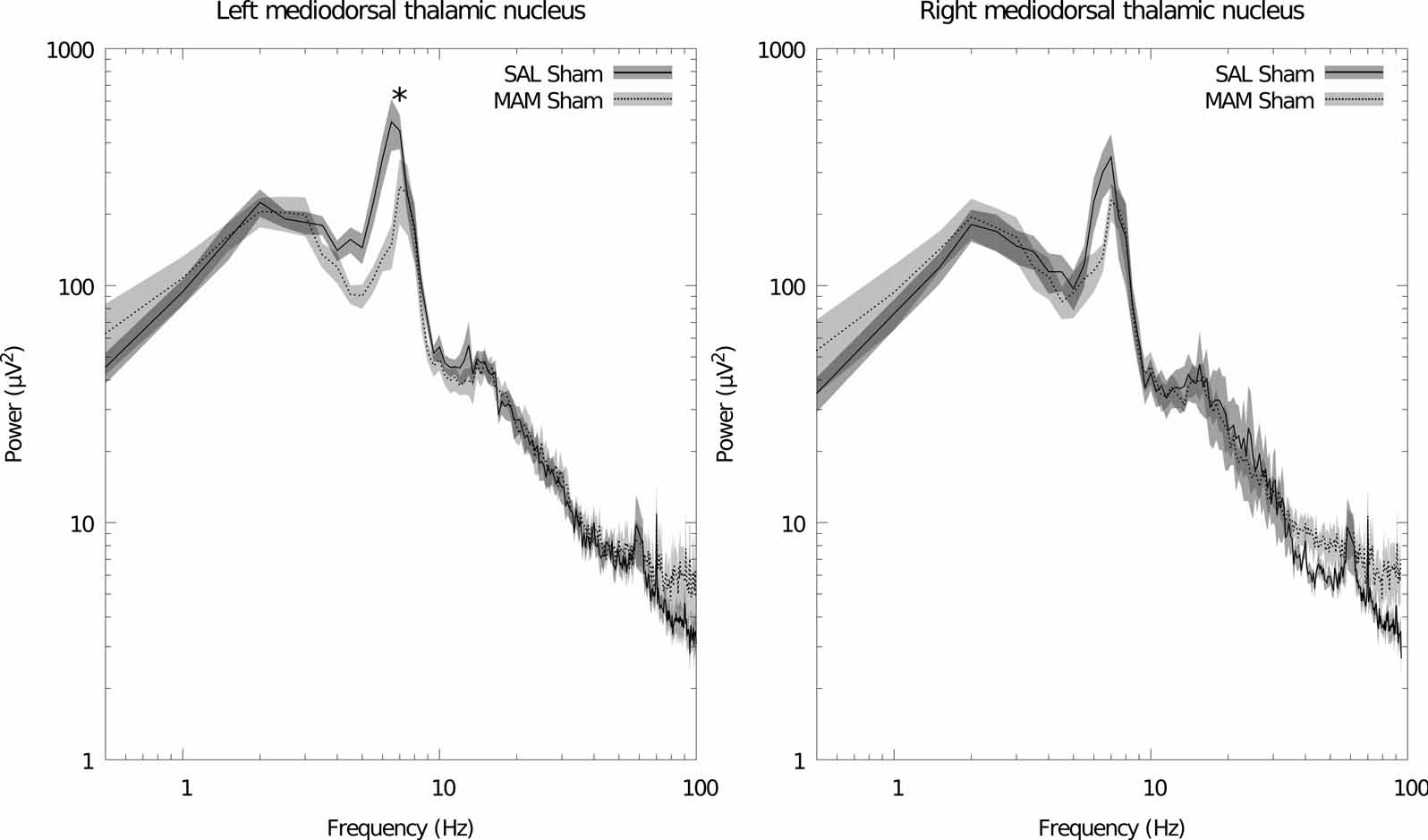
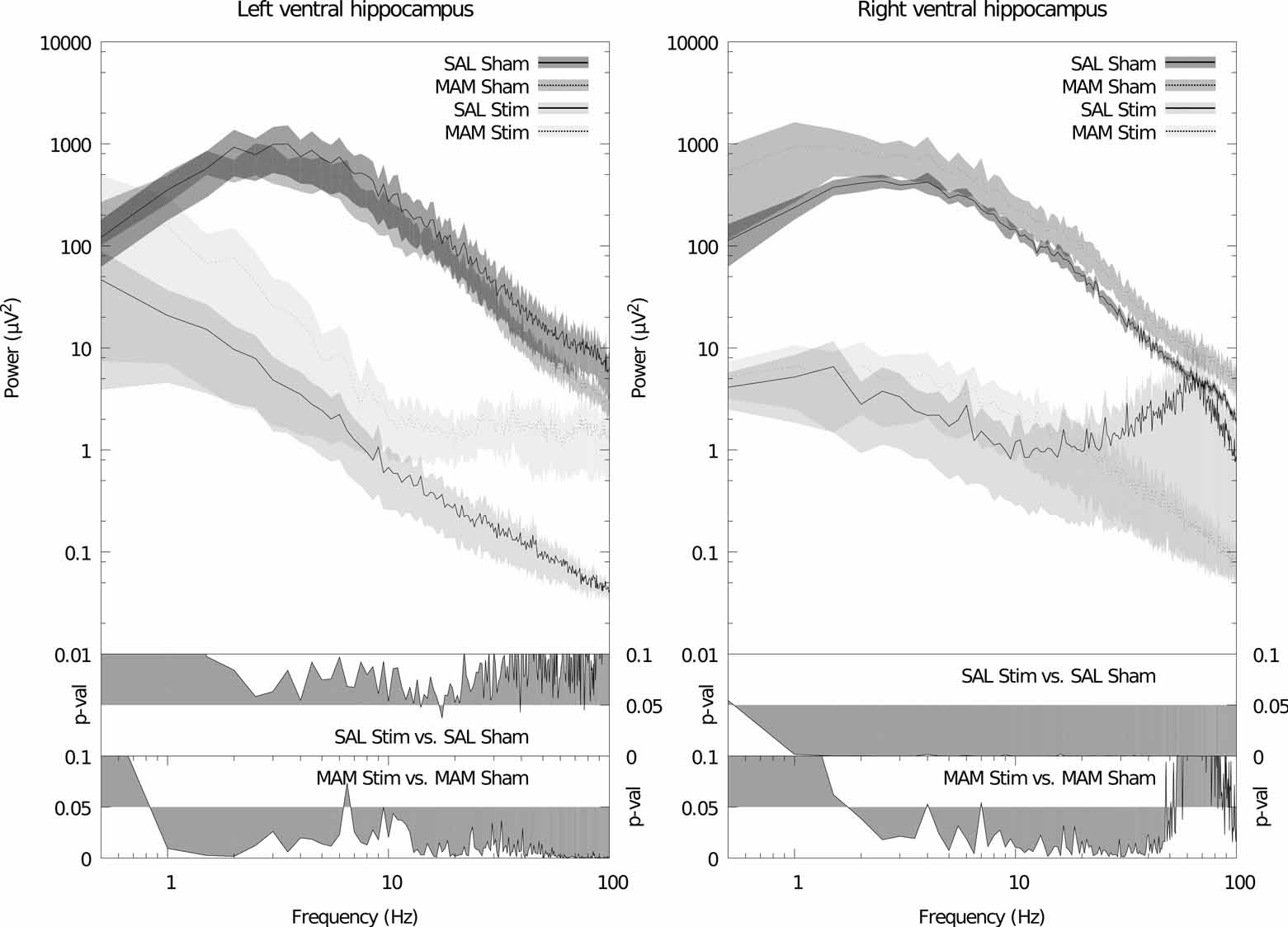
Maternal treatment with MAM induces significant deficits, in the adult offspring, in the power of theta band oscillations in the left mediodorsal thalamic nucleus (f = 6.5, p = 0.011, Fig. 1). Apparently similar reductions in thalamic theta power were also noted in the right hemisphere although these failed to attain significance (f = 6.5, p = 0.024, Fig. 1). No significant differences in power were found between MAM and SAL treated animals in the other brain regions. Stimulation failed to induce any further changes in all regions with the exception of the ventral hippocampus in which large broadband reductions in spectral power were observed in animals treated with both MAM and saline during gestation (Fig. 2). No significant changes were detected in coherence between regions as a consequence of either MAM treatment or DBS (data not shown).
Figure 1.
MAM treatment induces a deficit in mediodorsal thalamic nucleus theta power. This deficit is not restored by ventral hippocampal stimulation (data not shown). Left: grand averaged power spectra for the left hemisphere. Right: grand averaged power spectra for the right hemisphere. Data is plotted as the mean (solid/dashed line) ± SEM (shaded region). * indicates a significant difference between sham-stimulated SAL and MAM treated animals for that frequency bin.
Figure 2.
Acute high frequency electrical stimulation of the ventral hippocampus causes large broadband decreases in spectral power in the ventral hippocampus. Left: grand averaged power spectra for the left hemisphere. Right: grand averaged power spectra for the right hemisphere. Data is plotted as the mean (solid/dashed line) ± SEM (shaded region). Lower panels depicts a probability map identifying statistical significant differences in the power of the different frequency components of the spectrum for different treatment conditions.
3.2. Auditory evoked potentials
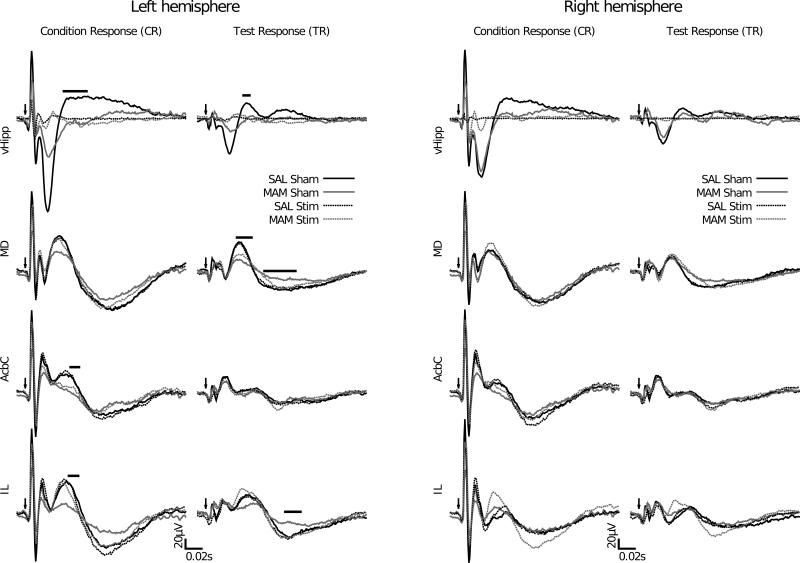
In the ventral hippocampus significant differences in the amplitudes of the P50 response to the conditioning tone (47ms < latency < 74ms, 0.0004 < p < 0.0146) and the test tone (47ms < latency < 53ms, 0.003 < p < 0.0111) were seen between sham-stimulated MAM and saline treated animals. Stimulation produced a large number of apparent effects. However, it seems apparent that these reflect a deficit in the propensity of the stimulated region's ability to generate an evoked response rather than a specific modulation of an evoked response (Fig. 3).
Figure 3.
MAM treatment induces deficits in auditory information processing in the ventral hippocampus, mediodorsal thalamic nucleus, core of the nucleus accumbens and infralimbic cortex in the left but not right hemisphere. Specifically significant differences are observed in MAM-treated rats in the P50 component of the ventral hippocampus evoked potentials to the conditioning and test stimuli, in the P50 and N100 components of the test response in the mediodorsal thalamic nucleus, in the P50 component of conditioning response in the core of the nucleus accumbens and in both the P50 of the conditioning response and the N100 of the test response in infralimbic cortex. These alterations in the MAM rats are restored to control level following ventral hippocampal stimulation. Left: grand averaged AEPs to the conditioning stimulus. Right: grand averaged AEPs to the test stimulus. The solid bar indicates a significant difference between sham-stimulated SAL and MAM treated animals for the duration of the bar. Arrows indicate the onset of the auditory stimulus.
In the mediodorsal thalamic nucleus significant differences in the amplitudes of the P50 response to the test tone (38ms < latency < 54ms, 3.8 × 10–5 < p < 0.0094) and the N100 response to the test tone (70ms < latency < 107ms, 0.0065 < p < 0.013) were seen between MAM and saline treated animals. These deficits were reversed by ventral hippocampal stimulation although the reversal in the P50 amplitude appears to be partial. Again, no effects of stimulation were detected in the saline treated group (Fig. 3).
In the core of the nucleus accumbens significant differences in the amplitudes of the P50 response to the conditioning tone (latency = 57ms, p = 0.014) were seen between sham-stimulated MAM and saline treated animals. This difference remained during stimulation (45ms < latency < 51ms, 0.0082 < p < 0.014). Again, no effects of stimulation were detected in the saline treated group (Fig. 3).
The infralimbic cortex exhibited evoked potentials that are qualitatively similar to those seen in the prelimbic cortex (Fig. 4). In the infralimbic cortex significant deficits were seen in the amplitudes of the P50 evoked response to the conditioning tone (53ms < latency < 64ms, 0.0017 < p < 0.0110) and the N100 response to the test tone (98ms < latency < 112ms, 0.0003 < p < 0.0101) between sham-stimulated MAM and saline treated animals. This deficit was reversed by ventral hippocampal stimulation. It is noteworthy that stimulation effects were only apparent in the MAM treated group (Fig. 3).
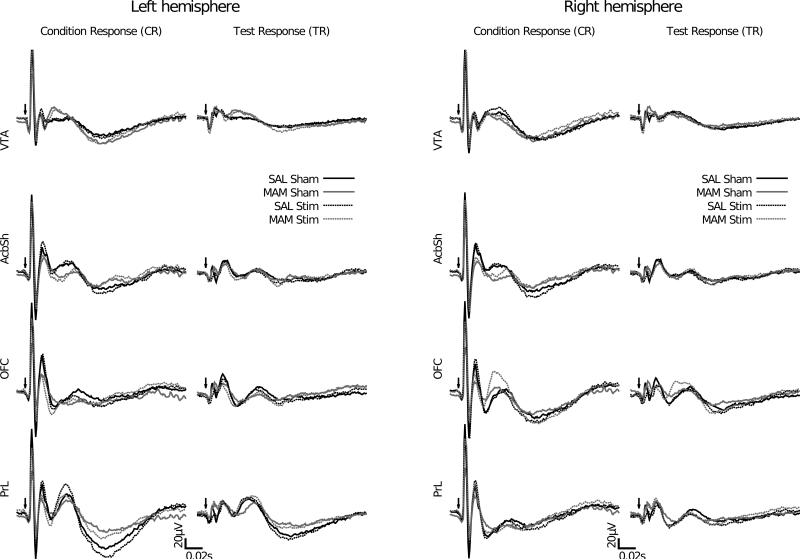
Figure 4.
In MAM treated rats, no alterations in auditory information processing in the prelimbic cortex, orbitofrontal cortex, shell of the nucleus accumbens or the ventral tegmental area were observed. In addition, the auditory evoked response is not modulated by DBS of the ventral hippocampus. Left: grand averaged AEPs to the conditioning stimulus. Right: grand averaged AEPs to the test stimulus. Arrows indicate the onset of the auditory stimulus.
In the adult offspring of pregnant dams treated with the anti-mitotic agent MAM there were no alterations in auditory information processing in the prelimbic and orbitofrontal cortices, the shell of the nucleus accumbens or the ventral tegmental area when compared with animals from saline treated dams. In addition DBS of the ventral hippocampus caused no significant modulation of potentials in these regions in either saline or MAM treated animals (Fig. 4).
4. Discussion
In rats that had been exposed to MAM during gestation, there were deficits in auditory information processing when compared with saline-treated controls. These deficits are selective for specific brain regions, namely the infralimbic cortex, the mediodorsal thalmic nucleus, the core of the nucleus accumbens and the ventral hippocampus, suggesting specific dysfunctions in this circuit. This is consistent with spectral analysis of spontaneous LFPs, with specific alterations in power of theta frequency oscillations observed in the mediodorsal thalamic nucleus. Thalamo-cortical dysfunction appears to be common to deficits in both spontaneous and evoked oscillatory activity and dysfunction within these nuclei have been reported to be deficits central to the pathology of schizophrenia (Lewis, 2000).
The mediodorsal thalamic nucleus is known to be influenced by a diverse array of brain regions, including the medial prefontal cortex, the dopamine system, and the ventral pallidum; all of which have been shown to be disrupted in the MAM-treated rat (Lavin et al., 2005; Lodge and Grace, 2007). Deficits in evoked activity in this region are reversed by acute DBS of the ventral hippocampus. Conversely, deficits in spontaneous LFP activity in the mediodorsal thalamic nucleus are not reversed by this stimulation. This finding may implicate distinct thalamic deficits beyond those that can be attributed to ventral hippocampal dysfunction which is consistent with the observed decrease in mediodorsal thalamic volume observed in the MAM rat (Moore et al., 2006) and in schizophrenia patients (Popken et al., 2000; Young et al., 2000; Byne et al., 2002; Danos et al., 2003; Kemether et al., 2003; Shimizu et al., 2008).
DBS of the ventral hippocampus produces significant reduction in the broadband power of spontaneous oscillations within the ventral hippocampus and appears to inhibit the ventral hippocampus’ ability to evoke a response to auditory stimuli. Taken together these data indicate a “shutting down” of the ventral hippocampus in response to DBS. In addition, these results suggest a reversal of decreased evoked auditory potentials in the infralimbic cortex and mediodorsal thalamic nucleus. Given that the ventral hippocampus has widespread projections to the prefrontal cortex, thalamus, and other structures, a modulation of a deficit in this structure is likely to affect activity across multiple brain regions. Furthermore, this may also impact deficits related to the hyper-dopaminergic state observed in schizophrenia since these auditory evoked potentials are modulated by dopaminergic agents (Adler et al., 1986; Bickford-Wimer et al., 1990). In addition, pharmacological inhibition of the ventral hippocampus is known to normalize the abnormal increase in tonic dopamine neuron firing and amphetamine induced hyperlocomotion in this animal model (Lodge and Grace, 2007).
These regionally selective stimulation effects were only apparent in MAM treated animals - the same stimulation in control animals produced no effects on evoked potentials in any of the brain regions outside of the stimulation target itself, where similar effects were observed in both groups. The common inhibitory effect within the stimulation target suggests that the mechanism underpinning the restoration in the MAM group is specific to the high ventral hippocampal activity in this animal model that is proposed to result from parvalbumin interneuron deficits (Lodge and Grace, 2007). In saline treated animals there is no hippocampal hyperactivity indicating that the effects are specific to systems in which an interneuron deficit is present. Auditory evoked potentials arise, as do all oscillations, as a consequence of synchronous activity of neural cell assemblies. Deficits in particular components in specific regions may arise as a consequence of uncoordinated neural activity due to the aberrant interneuron function seen in both the infralimbic cortex and ventral hippocampus in this model (Lodge et al., 2009). Thus the reversal of these deficits may implicate the activation of interneuron networks as a crucial target for the effects of DBS.
It may be of interest to note that the differences between MAM and saline treated animals and their reversal reported here were only apparent in the left hemisphere (Figs. 1 and 3). However, this is consistent with data collected in a similar animal model (Dickerson et al., 2010) and with reports indicating lower relative glucose metabolism in the left mediodorsal thalamic nucleus but not the right in schizophrenia (Hazlett et al., 2004). This finding mirrors the finding of reduced grey matter volume in the left dorsolateral prefrontal cortex (Gilbert et al., 2001). In addition, structural analysis of the hippocampus in schizophrenics indicates a marked exaggeration of the asymmetry between the left and right hippocampus seen in controls (Csernansky et al., 2002). This structural abnormality, rather than a volume reduction, may be more a deformity in the head of the hippocampus, the area in which the CA1 neurons are found. Neurons in the distal portion of the CA1 region project directly to the medial prefrontal cortex possibly indicating a mechanism by which hippocampal hyperactivity may additionally modulate cortical functions associated with cognition. The subiculum is ideally placed to underpin such laterality since, unlike the CA3 and CA1 regions, it does not give rise to commisural projections.
While there were no overt behavioural effects noted during bilateral DBS, unilateral DBS may be equally efficacious, which would certainly be preferable in terms of device battery life. If indeed the major limbic output structure of the hippocampus is shut down, or at least potently down-modulated, there may be long-term consequences. This effect remains to be elucidated in future experiments although it seems plausible to suggest that only shutting down one side will be less likely to induce long-term side effects, providing it proves equally efficacious in the treatment of the symptoms.
4.1. Caveats
The data presented here describe the deficits in various components of the waveforms evoked by auditory stimuli presented in a standard inhibitory gating paradigm. Both the amplitude of the P50 evoked component and the sensory gating of this component are commonly reported as deficit in schizophrenia patients. However we have elected not to pursue the analysis of this inhibitory gating (the ratio of the evoked P50 responses to the first and second stimuli in each pair) since it appears clear from the data that any apparent deficit in inhibitory gating would arise as a consequence of the diminished amplitude of the P50 component in response to the conditioning stimulus rather than as an effect of gating the second response. Similar findings have been reported in humans (Clementz and Blumenfeld, 2001).
The ventral hippocampus comprises the ventral portions of CA1 and the subiculum. The subiculum is the major output structure of the hippocampus being the primary recipient of fibres from CA1 which in turn is the primary recipient of fibres from CA3. The GABAergic dysfunctions in the hippocampus have been localised to the CA3/CA2 and CA1 regions. Interestingly, dysfunction in CA1 appears to be specific to schizophrenia whereas CA3/CA2 dysfunction is common to both schizophrenia and bipolar disorder (Benes et al., 2008). Diminished inhibitory regulation throughout this hippocampal circuit likely leads to feed-forward excitation driving excessive hippocampal (subicular) output. Thus achieving a reduction in hippocampal output may plausibly be achieved by stimulation of CA3, CA1 or the subiculum. At this initial stage in this research we opted to target the ventral hippocampus since this region comprises the ventral portions of CA1 and the ventral subiculum. Subicular projections to the core of the nucleus accumbens originate predominantly in the dorsal subiculum whereas the ventral portion projects more heavily to the nucleus accumbens shell (Groenwegen et al., 1987). Thus by targeting the ventral hippocampus we aimed to selectively target the limbic output of the hippocampus.
4.2. Implications
Deep brain stimulation for neuropsychiatric disorders is a field of increasing interest due to its potential to treat intractable patients. Currently the most successful procedures are those in which stimulation is performed in regions known to generate therapeutic benefit when lesioned. No such successful lesion target exists in schizophrenia leaving it thus far unexplored as a candidate for deep brain stimulation. These data are the first to demonstrate that acute stimulation of the ventral hippocampus, a region that is dysfunctional and likely drives the dopamine hyperactivity in MAM rats, can potentially reverse the deficits in the processing of auditory information in regions known to be specifically affected in schizophrenia.
Schizophrenia requires lifelong medication and is primarily treated with antipsychotic drugs that block D2 receptors (Kapur et al., 2000). Moreover, these medications are far from reversing all symptom classes of schizophrenia and are all associated with undesirable side effects (Lieberman and Stroup, 2005). However, the dopamine system is likely not dysfunctional in schizophrenia, but instead is probably dysregulated by other systems (Grace, 2010), the primary among these being the ventral hippocampus. Moreover, the hyperactive hippocampal state may be related to deficits in parvalbumin interneurons. DBS has been proposed to exert its therapeutic action by augmenting interneuron function (McCracken and Grace, 2009; Ewing and Grace, 2012). Thus, by directly stimulating this dysfunctional region, DBS may be able to more directly reverse the core deficit in this disorder.
Acknowledgments
We thank N. Macmurdo for technical assistance. This work was supported by NIH grants MH086400 and MH57440.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
abbrev. RC, rostrocaudal; ML, mediolateral; DV, dorsoventral
Financial Disclosures
Dr. Ewing reports no conflicts of interest. Dr. Grace reports the following as potential conflicts of interest: Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche.
References
- Adler LE, Rose G, Freedman R. Neurophysiological Studies of Sensory Gating in Rats : Effects of Amphetamine , Phencyclidine , and Haloperidol. Biol. Psychiat. 1986;(21):787–798. doi: 10.1016/0006-3223(86)90244-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Subburaju S, Walsh JP. Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. P. Natl. Acad. Sci. USA. 2008;105(52):20935–40. doi: 10.1073/pnas.0810153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. P. Natl. Acad. Sci. USA. 2007;104(24):10164–9. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics. 2012;29(4):1165–1188. [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biol. Psychiat. 1990;27(2):183–92. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, Van Zandijcke M, De Smedt T, Dewaele I, Achten R, Wadman W, Dewaele F, Caemaert J, Van Roost D. Deep Brain Stimulation in Patients with Refractory Temporal Lobe Epilepsy. Epilepsia. 2007;48(8):1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Boutros N, Zouridakis G, Rustin T, Peabody C. The P50 component of the auditory evoked potential and subtypes of schizophrenia. Psychiat. Res. 1993;47:243–254. doi: 10.1016/0165-1781(93)90082-r. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophr. Res. 2008;99(1-3):238–49. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS. The middle evoked response components and schizophrenia. Schizophrenia Bull. 1977;3(1):93–104. doi: 10.1093/schbul/3.1.93. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace L. a., Hazlett E. a., Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am. J. Psychiat. 2002;159(1):59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J. Comp Neurol. 1992;324(2):180–94. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Clementz B, Blumenfeld L. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp. Brain Res. 2001;139(4):377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, Miller JP, Miller MI. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am. J. Psychiat. 2002;159(12):2000–6. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- Danos P, Baumann B, Kra A, Stauch R, Krell D, Falkai P, Bogerts B. Volumes of association thalamic nuclei in schizophrenia : a postmortem study. Schizophr. Res. 2003;60:141–155. doi: 10.1016/s0920-9964(02)00307-9. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J. Neurosci. 2010;30(37):12424–31. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez Y, Tischler H, Moran A, Bar-Gad I. Generalized framework for stimulus artifact removal. J. Neuro. Meth. 2010;191(1):45–59. doi: 10.1016/j.jneumeth.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Ewing S, Grace A. Long-term high frequency deep brain stimulation of the nucleus accumbens drives time-dependent changes in functional connectivity in the rodent limbic system. Brain Stimul. 2012 doi: 10.1016/j.brs.2012.07.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J, White P, Lim K. Schizophrenics have fewer and smaller P300s: A single-trial analysis. Biol. Psychiat. 1994;35(2):96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunctions in schizophrenia. Schizophr. Res. 1991;4(2):233–43. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic Volumes in Patients With First-Episode Schizophrenia. Am. J. Psychiat. 2001;158(4):618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Grace AA. Ventral Hippocampus, Interneurons, and Schizophrenia: A New Understanding of the Pathophysiology of Schizophrenia and Its Implications for Treatment and Prevention. Curr. Dir. Psychol. Sci. 2010;19(4):232–237. [Google Scholar]
- Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62(3):1342–8. doi: 10.1016/j.neuropharm.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenwegen HJ, Vermeulen-van der Zee E, Kortschot TE, Witter MP. Organisation of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of phaseoulus vulgaris leucogglutinin. Neuroscience. 1987;23(I):103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Hazlett E. a., Buchsbaum MS, Kemether E, Bloom R, Platholi J, Brickman AM, Shihabuddin L, Tang C, Byne W. Abnormal glucose metabolism in the mediodorsal nucleus of the thalamus in schizophrenia. Am. J. Psychiat. 2004;161(2):305–14. doi: 10.1176/appi.ajp.161.2.305. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007 Sep.212(2):149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hradetzky E, Sanderson TM, Tsang TM, Sherwood JL, Fitzjohn SM, Lakics V, Malik N, Schoeffmann S, O'Neill MJ, Cheng TM, Harris LW, Rahmoune H, Guest PC, Sher E, Collingridge GL, Holmes E, Tricklebank MD, Bahn S. The Methylazoxymethanol Acetate (MAM-E17) Rat Model: Molecular and Functional Effects in the Hippocampus. Neuropsychopharmacol. 2012;37:364–377. doi: 10.1038/npp.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biol. Psychiat. 1992;31(4):365–77. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am. J. Psychiat. 2000;157(4):514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kemether E, Buchsbaum M, Byne W, Hazlett E, Haznedar M, Brick-man A, Platholi J, Bloom R. Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch. Gen. Psychiat. 2003;60(10):983. doi: 10.1001/archpsyc.60.9.983. [DOI] [PubMed] [Google Scholar]
- Lavin A, Moore HM, Grace AA. Prenatal disruption of neocortical development alters prefrontal cortical neuron responses to dopamine in adult rats. Neuropsychopharmacol. 2005;30(8):1426–35. doi: 10.1038/sj.npp.1300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Is There a Neuropathology of Schizophrenia? Recent Findings Converge on Altered Thalamic-Prefrontal Cortical Connectivity. The Neuroscientist. 2000;6(3):208–218. [Google Scholar]
- Leyland CM, Gwyther RJ, Ryiands JM. An improved method for detecting drug effects in the open field. Psychopharmacology. 1979;63(1):33–37. doi: 10.1007/BF00426918. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Stroup T. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl. J. Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci. 2009;29(8):2344–54. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007;27(42):11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav. Brain Res. 2009;204(2):306–12. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, Lignelli A, Gorman J, Van Heertum R. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol. Psychiat. 1999;46(1):89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J. Neurosci. 2009;29(16):5354–63. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears RP, Boutros NN, Cromwell HC. Reduction of prelimbic inhibitory gating of auditory evoked potentials after fear conditioning. Behav. Neurosci. 2009;123(2):315–27. doi: 10.1037/a0014364. [DOI] [PubMed] [Google Scholar]
- Mikell CB, McKhann GM, Segal S, McGovern RA, Wallenstein MB, Moore H. The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereot. Funct. Neuros. 2009;87(4):256–65. doi: 10.1159/000225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol. Psychiat. 2006;60(3):253–64. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon K. a., Gerhardt G. a., Adler LE. Dopaminergic modulation of the P50 auditory-evoked potential in a computer model of the CA3 region of the hippocampus: its relationship to sensory gating in schizophrenia. Biol. Cybern. 2003;88(4):265–75. doi: 10.1007/s00422-002-0372-8. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur. J. Neurosci. 2006;23(1):279–84. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Potkin SG, Jones EG. Subnucleusspecific loss of neurons in medial thalamus of schizophrenics. P. Natl. Acad. Sci. USA. 2000;97(16):9276–80. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Fujiwara H, Hirao K, Namiki C, Fukuyama H, Hayashi T, Murai T. Structural abnormalities of the adhesio interthalamica and mediodorsal nuclei of the thalamus in schizophrenia. Schizophr. Res. 2008;101(1-3):331–8. doi: 10.1016/j.schres.2007.12.486. [DOI] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol. Psychiat. 2000;47(11):944–53. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]