Abstract
Background
Gynecology clinic-based studies have consistently demonstrated that induced hypogonadism is accompanied by a decline in cognitive test performance. However, a recent study in healthy asymptomatic controls observed that neither induced hypogonadism nor estradiol replacement influenced cognitive performance. Thus the effects of induced hypogonadism on cognition might not be uniformly experienced across individual women. Moreover, discrepancies in the effects of hypogonadism on cognition also could suggest the existence of specific risk phenotypes that predict a woman’s symptomatic experience during the menopause. In this study, we examined the effects of induced hypogonadism and ovarian steroid replacement on cognitive performance in healthy premenopausal women.
Methods
Ovarian suppression was induced with a GnRH agonist (Lupron) and then physiologic levels of estradiol and progesterone were re-introduced in 23 women. Cognitive tests were administered during each hormone condition. To evaluate possible practice effects arising during repeated testing, an identical battery of tests was administered at the same time intervals in 11 untreated women.
Results
With the exception of an improved performance on mental rotation during estradiol, we observed no significant effects of estradiol or progesterone on measures of attention, concentration, or memory compared with hypogonadism.
Conclusions
In contrast to studies in which a decline in cognitive performance was observed in women receiving ovarian suppression therapy for an underlying gynecologic condition, we confirm a prior report demonstrating that short term changes in gonadal steroids have a limited effect on cognition in young, healthy, women. Differences in the clinical characteristics of the women receiving GnRH agonists could predict a risk for ovarian steroid-related changes in cognitive performance during induced, and possibly, natural menopause. Key Words: estradiol, hypogonadism, progesterone, cognition.
INTRODUCTION
The nature and magnitude of the effects of declining ovarian hormone secretion and the onset of menopause on cognitive performance remain controversial. The plausibility of the capacity of ovarian steroids to regulate cognitive performance in women is supported by two types of evidence: 1) a multitude of studies in animals documenting the manifold neuroregulatory actions of ovarian steroids (Diaz Brinton 2009; Dumitriu et al. 2010; Gibbs 2010; Kelly et al. 2009; Korol et al. 2002; McEwen 2010; Wise et al. 2005), and 2) neuroimaging studies in humans demonstrating the modulatory effects of ovarian steroids on brain activation patterns in regions implicated in the function of a wide range of cognitive domains (Berman et al. 1997; Eberling et al. 2000; Goldstein et al. 2005; Maki et al. 2000; Protopopescu et al. 2005; Rasgon et al. 2005; Resnick et al. 1998; van Wingen et al. 2007; Yaffe et al. 1998). Nonetheless, several recent randomized clinical trials, including the Women’s Health Initiative (WHI), have demonstrated that estrogen therapy has no direct effect on cognitive performance in older postmenopausal women (Espeland et al. 2004; Hogervorst et al. 2002; Lethaby et al. 2008; Low et al. 2006; Mulnard et al. 2000; Rapp et al. 2003; Yaffe et al. 2006). In fact, the WHI findings suggest that estradiol increases the risk of cognitive disorders (Shumaker et al. 2003; Shumaker et al. 2004). These findings have been replicated in younger perimenopausal women (Maki et al. 2007), who frequently report a decline in cognitive function during the perimenopause (Gold et al. 2000; Mitchell et al. 2001) (although actual performance deficits have not been observed to accompany these cognitive complaints in perimenopausal women (Fuh et al. 2006; Henderson et al. 2003; Kok et al. 2006)). In contrast to the randomized controlled trials of estrogen therapy, observational studies more consistently report that estrogen therapy in young perimenopausal women has long term beneficial effects on cognitive function and affords a small reduction in the risk of dementia (Bagger et al. 2005; Henderson et al. 2005; MacLennan et al. 2006; Whitmer et al. 2011; Yaffe et al. 1998; Zandi et al. 2002). The remaining evidence suggesting a significant effect of estradiol on cognitive function to a large degree is derived from clinic-based studies of hypogonadal women in whom surgical oophorectomy was performed (Phillips et al. 1992; Sherwin 1988) or a GnRH agonist was administered to suppress ovarian function as part of a treatment for an underlying gynecologic condition (Craig et al. 2007; Craig et al. 2008; Grigorova et al. 2006; Palomba et al. 2004; Sherwin et al. 1996; Varney et al. 1993). Although the specific cognitive function that is reported to decline during hypogonadism varies across studies, a decline in some aspect of cognitive performance is consistently observed during induced hypogonadism (and in some studies, a subsequent improvement in performance after estradiol therapy). Nonetheless, in contrast to the majority of clinic-based studies, a recent study by Owens (2002) reported that four months of GnRH agonist-induced ovarian suppression had no effect on cognitive performance measures in 16 asymptomatic healthy premenopausal women. Thus, despite employing an identical hormonal manipulation (i.e., GnRH agonist-induced hypogonadism) and administering similar cognitive tests, the findings in the Owens study (2002) are ostensibly at variance with numerous studies in which a decline in at least one aspect of cognitive performance was observed.
Several potential confounds could account for discrepancies across studies including the small effect sizes of the changes in cognitive outcomes measured, the presence of practice effects after repeated testing, and differences in baseline cognitive performance in the women prior to induced hypogonadism. Additionally, the clinical characteristics of the samples also obviously differed between a selected sample of healthy women in the Owens study compared with women receiving treatment for a gynecologic condition who constituted the majority of the clinic-based studies. The difference between the cognitive effects of induced hypogonadism in healthy women (observed by Owens (2002)) compared with those in studies of gynecological clinic-based samples, therefore, could suggest a substrate of risk for a woman to experience a cognitive decline during an induced or natural menopause. Moreover, confirmation of these differences could identify subgroups of women who are differentially sensitive to changes in ovarian steroids and inform animal studies in which the mechanisms underlying these differences could be explored.
As part of a larger study investigating the effects of ovarian steroids on brain function and behavior, we had the opportunity to evaluate cognitive performance in a sample of premenopausal women who were healthy and free of gynecologic disease. To further examine the effects of ovarian steroids on measures of cognitive function in these women, we administered a battery of cognitive tests under conditions of GnRH agonist-induced ovarian suppression, and then repeated testing after replacement with physiologic levels of estradiol (E) and progesterone (P), respectively. We, therefore, had the opportunity to ask the following questions: First, do some cognitive tasks elicit hormone-related changes in performance in healthy women. Second, do E and P mediate distinct changes in cognitive test performance in these women?
METHODS
Subject Selection
a) Lupron-treated group (GnRH agonist-induced hypogonadism and hormone replacement)
Subjects were 23 women (mean ± SD age = 35 ± 7 years) recruited through advertisements in the hospital newsletter. All were medication free, and all were screened for the absence of significant medical and gynecologic illness through history, physical examination, and laboratory tests. All subjects were administered the Structured Clinical Interview for DSM-III-R (Spitzer et al. 1990), to confirm the absence of current Axis I psychiatric illness. The protocol was approved by the NIMH Intramural Research Review Board, and written informed consents were obtained from all subjects. All women completed an average of 16.2 ± 1.8 years of education.
b) Untreated Control group
To examine for possible practice effects arising during the repeated testing that we performed in this protocol, we recruited a group of 11 healthy women who served as an un-medicated [i.e., eugonadal] comparison group (mean ± SD age = 33.8 ± 10.5). They received the same medical and psychiatric screening as the participants who received Lupron, and all were medication free and had no medical, gynecologic, or psychiatric illness, current or past. All women completed an average of 17.5 ± 1.6 years of education.
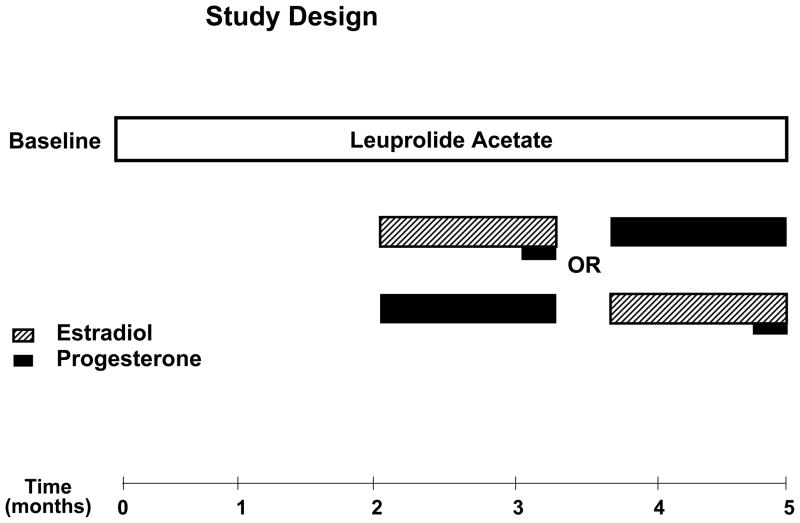
Protocol (Figure 1)
Figure 1.
All women received 3.75 mg of depot Lupron (leuprolide acetate, TAP Pharmaceuticals, Chicago, IL) by intramuscular injection every four weeks for five-six months. The first injection of Lupron was administered during the follicular phase between days 2–6 after the onset of menses. Lupron alone was administered for the first eight-12 weeks. All women then received, in addition to Lupron, 17 beta estradiol (0.1 mg/day) (E) by skin patch (Alora, Watson Pharmaceuticals, Salt Lake City, UT) or progesterone suppositories (200 mg b.i.d.) (P) (NIH Pharmacy, Bethesda, MD) for five weeks each. The two replacement regimens were separated by a two week washout period. Subjects were administered both patches and suppositories (active or placebo, depending upon the treatment phase) daily throughout the entire replacement period to ensure the double-blind was maintained. During the last week of E, all women received one week of active P suppositories in addition to E to precipitate menses. All women received prepackaged one week unit-dose supplies of suppositories that were formulated and coded (weeks 1–5) by the NIH Pharmacy Department.
a) Hormone manipulation group
Women received depot Lupron, (leuprolide acetate, TAP Pharmaceuticals, Chicago, IL) 3.75 mg by intramuscular (IM) injection every four weeks for five-six months. Lupron alone was administered for the first eight-twelve weeks. Subjects then received, in addition to Lupron, 17 beta E (0.1 mg/day) by skin patch (Ciba Geigy, Rariton, NJ) or P suppositories (200 mg b.i.d.) (NIH Pharmacy, Bethesda, MD) for five weeks each. The two “addback” regimens were separated by a one week washout period. Subjects were administered both patches and suppositories (active or placebo, depending upon the treatment phase) daily throughout the entire addback period to ensure the double-blind was maintained. The order of receiving E and P was randomly assigned and counter-balanced. During the last week of E all subjects additionally received one week of P suppositories to precipitate menses.
Cognitive testing was performed: at baseline prior to study (randomly across the menstrual cycle), after six weeks of Lupron alone (hypogonadal), and after three to four weeks of hormone replacement (Lupron plus E and Lupron plus P). Mood symptoms were monitored by the Beck Depression Inventory (BDI) (Beck et al. 1961), and the presence and severity of hot flushes were measured by a daily self-rating scale (Endicott et al. 1981). All women were paid for their participation according to NIH guidelines.
b) Untreated Control group
The women who served as controls for this study did not receive Lupron at any time point and did not receive any hormone therapy, but received the same battery of tests at similar time intervals as those in the main treatment protocol.
Blood samples were drawn at the time of testing in all women. The samples were centrifuged, and aliquots of serum or plasma were frozen at −20°C until the time of assay. Plasma levels of E and P (Abraham et al. 1971; Jiang et al. 1969) and total T (Furuyama et al. 1970) were analyzed by radioimmunoassay.
Cognitive Tests – Table 4
Table 4.
Cognitve tests administered to Lupron-treated women and the untreated control group.
Spatial Ability
Verbal Fluency and Articulation
Motor Speed and Dexterity
Attention/Concentration
|
Cognitive tests were selected to assess performance in the following cognitive domains: verbal and visual memory, visuospatial ability, verbal fluency and articulation, motor speed and dexterity, and attention and concentration. General ability was evaluated employing components of the WAIS-R (Wechsler 1981), and the WRAT-R (Jastak et al. 1984). The selection of cognitive tests was limited by the repeated measures design of this study, since subjects were tested on four separate occasions. We, therefore, selected cognitive tests (e.g., paragraph memory) in which at least four separate forms were available.
Statistical Analysis
First, data from the Lupron-treated women (i.e., hormone manipulation protocol) were analyzed by analysis of variance with repeated measures (ANOVA-R; Systat, SPSS, Chicago, IL). In these women, test scores at each time point were compared by ANOVA-R, with hormone condition (baseline vs. hypogonadal vs. E replacement vs. P replacement) as the within-subjects variable. Complete data sets were present for all tests except for four (i.e., Line Orientation, Digit span, Fragmented Pictures, and Mental Rotation) in which two women treated with Lupron in each test had unusable data during one hormone condition. If the ANOVAs were significant, we performed post-hoc comparisons of cognitive test performance during hormone replacement compared with hypogonadal and baseline conditions and, additionally, compared the cognitive performance during hypogonadism with baseline.
The principle focus of this study was whether significant changes in cognitive test performance could be observed during the pharmacologically-induced hormone conditions. The repeated testing in this protocol could give rise to practice effects that mistakenly could be attributed to an effect of a specific hormone condition. Additionally, the testing in this protocol occurred in a partially fixed order with the first and second testing sessions occurring during baseline and hypogonadism, respectively. Given the partially fixed order of our study, in a second analysis, we performed ANOVAs on the cognitive data from both the Lupron-treated women and the untreated controls, who were tested at the same approximate time intervals as in women receiving Lupron and hormone replacement. These data were analyzed with Group (Lupron-treated and untreated controls) as the between subjects factor and time (i.e., hormone condition in Lupron-treated women and time in the untreated women). The absence of a significant main or interaction between group and time/hormone condition would be consistent with a practice effect in that test measure. ANOVAs were performed on both the cognitive test scores at each test phase and the change in test scores between sessions. By so doing we could effectively identify many of the ostensible hormone condition-related changes in cognitive function that were simply a product of repeated testing (i.e., practice effects).
Several secondary analyses evaluated potential confounds in the data. First, the ANOVA-R in the women was repeated with each of the following as a between-subjects variable: 1) phase of menstrual cycle (i.e., follicular (n = 15) vs. luteal (n = 8) during baseline testing, and 2) hormonal milieu during baseline testing (i.e., plasma levels of P ≥ 2 ng/ml and/or E ≥ 70 pg/ml (n = 10) vs. plasma levels of P < 2 ng/ml and E < 70 pg/ml (n = 13)). These two analyses examined the potential impact of baseline hormonal status on the observed performance across the different hormone conditions. Second, eight women reported the presence of premenstrual symptoms (PMS) prior to study entry. Thus, ANOVA-R analyses were repeated in the asymptomatic women without PMS (n = 15) to ensure that the pattern of cognitive performance across each hormonal state was not confounded by the inclusion of women reporting PMS (either due to differences in symptomatology at baseline or differences in response characteristics during hormone replacement). Further, due to baseline differences in BDI scores between women with and without reported PMS, the ANOVA-R analyses were repeated with baseline BDI scores as a covariate. Third, in the Lupron-treated women we examined the effects of age as a covariate in the first set of ANOVAs. Fourth, in the Lupron-treated women we examined the effect on test scores of the order of receiving estradiol or progesterone first during the replacement phase of the study. Finally, several studies (LeBlanc et al. 2001; Maki et al. 2008; Yaffe et al. 1998) but not all (LeBlanc et al. 2007) suggest an interaction between E’s observed effects on verbal memory and hot flush-induced sleep disturbance. Thus, we examined differences (ANOVA-R) in the severity of self-ratings of hot flushes and disturbed sleep (on the days of cognitive testing) between women whose performance on selected tests improved and those whose performance declined, from baseline to hypogonadism as well as from hypogonadism to E replacement.
Age, baseline BDI scores and years of education in the Lupron-treated women and the untreated control group were compared with Student’s t-test.
RESULTS
Lupron-treated women did not differ in age or years of education from the untreated control group (p=ns). Baseline BDI scores were non-significantly higher in the Lupron-treated women compared with the untreated control group (Student’s t30=1.7, p=ns) due to the presence of several women with PMS in the Lupron-treated group (see below).
Effects of Hormone Condition on Cognitive Test Performance (Table 3 and Supplemental Table 1)
Table 3.
Cognitive performance scores in Lupron-treated women (n=23) at baseline (eugonadal), during hypogonadism, and during hormone replacement, and Untreated controls (n=11) tested at similar time intervals - (mean ± standard deviation). Reported scores are not adjusted for educational levels.
| Testing Session: | Study Sjs: | Eugonadal | Hypogonadal | E-replaced | P-replaced |
|---|---|---|---|---|---|
| Controls: | Time #1 | Time #2 | Time #3 | Time #4 | |
| Memory: | |||||
| Paragraph Memory (Kramer et al. 1988; Lezak Deutsch 1995; Wechsler 1981) | |||||
| Immediate (% of paragraph) | Study Sjs | 57.0 (19.5) | 64.0 (16.1) | 64.3 (18.5) | 67.5 (12.7) |
| Controls | 62.4 (18.9) | 64.7 (17.3) | 72.4 (17.2) | 72.5 (15.8) | |
| Delay (% of paragraph) | Study Sjs## | 48.4 (18.6) | 57.3 (20.3) | 58.1 (18.5) | 63.0 (14.7) |
| Controls# | 53.7 (14.3) | 55.5 (15.5) | 68.5 (13.0) | 68.2 (17.2) | |
| Spatial Ability: | |||||
| Mental Rotation (Shepard et al. 1971; Vandenberg et al. 1978) | |||||
| Total (Number correct) | Study Sjs | 12.3 (4.5) | 11.7 (5.3) | 13.8 (4.9) | 13.8 (4.7) |
| Controls# | 9.2 (3.9) | 10.8 (4.4) | 13.4 (5.6) | 13.5 (5.8) | |
| Adjusted (Number correct) | Study Sjs# | 8.8 (5.5) | 8.3 (5.7)|| | 11.1 (6.1) | 10.4 (6.0) |
| Controls | 6.4 (4.6) | 7.5 (5.7) | 9.9 (7.7) | 9.3 (7.5) | |
| Money Road Map (Money 1976) | |||||
| Time up (seconds) | Study Sjs### | 23.0 (10.5) | 20.0 (6.0) | 17.3 (4.3) | 16.2 (3.4) |
| Controls# | 29.5 (16.5) | 26.2 (11.9) | 23.4 (12.7) | 22.5 (8.9) | |
| Time total (seconds) | Study Sjs### | 58.0 (23.9) | 50.3 (18.3) | 45.2 (12.8) | 41.3 (10.4) |
| Controls# | 69.2 (36.0) | 59.9 (28.5) | 55.0 (24.2) | 53.6 (22.4) | |
| Porteus Maze (Porteus 1959) | |||||
| Immediate (seconds) | Study Sjs# | 50.7 (29.2) | 40.4 (13.8) | 36.3 (14.9) ‡ | 37.0 (17.2)↕ |
| Controls | 52.4 (39.1) | 54.5 (42.1) | 39.9 (26.5) | 48.0 (33.5) | |
| Verbal Fluency: | |||||
| Verbal Fluency (Benton et al. 1976) | |||||
| Number of words generated | Study Sjs## | 40.4 (7.9) | 44.2 (9.9) | 44.2 (11.8) | 45.8 (10.4)↕ |
| Controls | 41.5 (9.5) | 42.2 (9.8) | 44.6 (11.9) | 44.0 (10.3) | |
| Stroop Color Naming (Stroop 1935) | |||||
| Number of words read | Study Sjs# | 52.2 (6.2) | 52.7 (6.3) | 53.2 (6.2) | 54.3 (5.4) |
| Controls# | 49.0 (5.7) | 48.0 (6.4) | 51.4 (5.9) | 50.5 (6.0) | |
| Motor Speed and Dexterity: | |||||
| Purdue Peg Board (Spreen et al. 1991) | |||||
| Dominant (Number of pegs) | Study Sjs# | 14.9 (1.2) | 15.4 (1.1) | 15.4 (1.2) | 15.9 (1.3)↕ |
| Controls | 15.2 (1.9) | 15.7 (1.7) | 15.8 (1.9) | 16.2 (0.9) | |
| Grooved Pegboard (Harley et al. 1980) | |||||
| Dominant Hand (seconds) | Study Sjs# | 56.9 (7.8) | 58.4 (7.0) | 58.1 (9.9) | 61.3 (5.5) |
| Controls# | 58.2 (8.7) | 60.3 (6.7) | 64.5 (6.2) | 61.9 (5.0) | |
| Finger Tapping (Kløve 1963; Reitan et al. 1993; Spreen et al. 1991) | |||||
| Dominant (Number of taps) | Study Sjs## | 55.7 (7.0) | 55.1 (7.3) | 55.9 (7.8) | 58.5 (7.2) |
| Controls# | 57.2 (6.5) | 55.6 (7.3) | 60.9 (8.5) | 61.6 (7.3) | |
| Attention and Concentration: | |||||
| Digit Span (Wechsler 1981) | |||||
| Number of correct responses (backward) | Study Sjs# | 5.4 (1.1) | 6.0 (1.2)† | 5.9 (1.2) | 5.9 (0.8) |
| Controls | 5.8 (1.6) | 6.1 (1.5) | 6.4 (1.2) | 6.5 (1.3) | |
| Trail Making (Kløve 1963; Reitan 1958; Reitan et al. 1993) | |||||
| Form A (seconds) | Study Sjs | 21.1 (6.2) | 19.2 (3.9) | 19.7 (4.3) | 19.1 (5.0) |
| Controls# | 26.4 (10.5) | 22.0 (6.3) | 21.8 (9.4) | 19.7 (7.4) | |
Analyses
-
-ANOVA: Hormone condition #p< 0.05, ##p < 0.01, ###p < 0.001;
-
-Bonferroni t: Eugonadal versus Hypogonadal †p < 0.05;
- Eugonadal versus E Replaced ‡p < 0.01;
- Eugonadal versus P Replaced 3p > 0.01;
- Hypogonadal versus E Replaced ||p < 0.05;
- Otherwise p = NS.
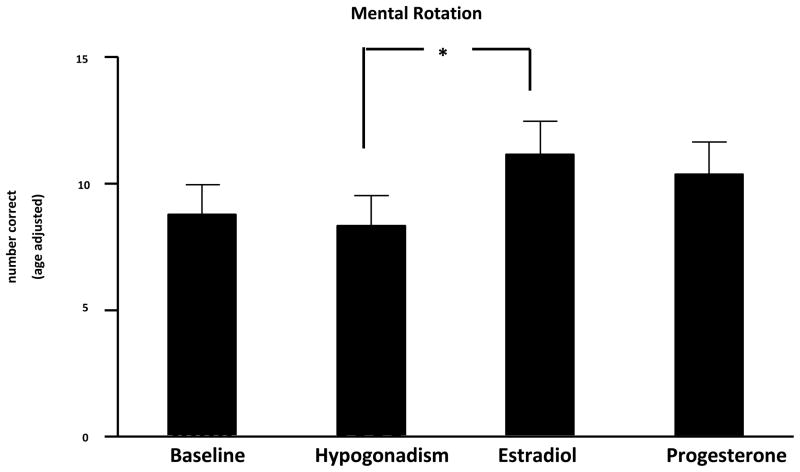
In the first analysis of only the data from the Lupron-treated women, ANOVA-Rs were significant for several cognitive tests (Table 3); however, with only a few exceptions (listed below) identical differences in test performances also were observed in the comparison subjects who remained eugonadal throughout the study. Thus, the potential differences across hormone conditions in the Lupron-treated women were in large part effects related to the repeated administration of these tests on performance scores. Significant effects across hormone conditions that were not also present in the comparison group were observed in the following tests: 1) mental rotation test (adjusted); 2) Porteus maze (immediate seconds); 3) verbal fluency (number of words); 4) Purdue peg board (dominant hand); and 5) digit span. Post-hoc testing examining these significant differences showed that performance in the adjusted mental rotation test was significantly better during E compared with hypogonadism. Other comparisons on this measure were not significant after correction for multiple comparisons (Figure 2). The remainder of the significant paired comparisons reflected significant differences between one of the hormone conditions and baseline. Specifically, performance scores were significantly better during P compared with baseline conditions in the Porteus maze, verbal fluency, and the Purdue peg board. Additionally, performance scores in the Porteus maze were significantly better during E compared with baseline. Finally, performance in the digit span was significantly better during hypogonadism compared with baseline.
Figure 2.
Performance scores (number of correct responses adjusted for age) on the Mental Rotation Test during baseline (eugonadal), Lupron-induced hypogonadism, estradiol-, and progesterone-replaced conditions (mean + SEM).
ANOVA-R demonstrated a significant effect of hormone condition in the group receiving Lupron and hormone replacement that was not identified in the medication-free comparison group. Post-hoc testing identified a significant improvement in the performance on the Mental Rotation Test during estradiol replacement compared with hypogonadism (*p < .05). However, there were no significant differences between baseline (eugonadal) and hypogonadism or between progesterone and either hypogonadism or baseline conditions.
The analysis of change scores showed a similar pattern of effects with no significant treatment group by test session interactions, with the exception of two test scores. First, in the scores of both dominant and nondominant finger tapping, we identified a significant interaction that reflected an improved performance in the untreated controls during their second test compared with their first test that was not observed in women receiving Lupron. Second, in the symbol digit written test, the women receiving no medication performed better during test session 3 compared with the other times, whereas women receiving Lupron did not show substantial differences across time points.
When the women reporting PMS were excluded from the analyses a similar pattern of effects (both non-significant and significant) were observed with the 15 asymptomatic women, although fewer test scores showed significant differences due to the reduced sample size. Finally, in the Lupron-treated women, there were no significant main or interactive effects with age on any test score, nor was there a significant effect of the order of receiving estradiol or progesterone first during the replacement phase of the study.
Mood Symptoms and Hot Flushes
No significant changes in BDI scores were observed across hormonal conditions. However, since we observed higher BDI scores at baseline prior to study entry in the eight women reporting PMS compared to controls, baseline BDI scores were included as a covariate. The pattern of results did not differ from that observed in the original analysis. Women experienced a significant increase in the severity of hot flushes during the hypogonadal state: all women reported hot flushes during hypogonadism, and none of the women reported hot flushes at baseline. For those tests in which the literature suggests an effect of estradiol that could interact with symptoms (e.g., paragraph memory, MRT), differences in self-reports of the severity of hot flushes or disturbed sleep did not distinguish the women whose performance worsened from those whose performance improved during hypogonadism or hormone replacement.
DISCUSSION
In this study we did not replicate previous reports that GnRH agonist (Lupron)-induced ovarian suppression was accompanied by a decline in measures of cognitive performance. Nor did we observe an estradiol-related improvement in cognitive test performance (Grigorova et al. 2006; Kampen et al. 1994; Resnick et al. 1997; Sherwin et al. 1996). In particular, we observed no changes in measures of attention, concentration, or memory function (either verbal or visual). The majority of differences in cognitive performance in women also were observed in the untreated control subjects over time. These differences in cognition, then, are unlikely due to the effects of gonadal steroids but rather are “practice effects” due to the repeated administration of these tests. Indeed, significant differences across hormone conditions that were not mirrored in the comparison group reflected either improvements during E or P replacement compared with baseline (i.e., the first test session) with no improvement compared with hypogonadism (when both E and P levels were suppressed), or in the digit span test, a significant improvement during hypogonadism compared with baseline that was maintained during estradiol and progesterone replacement. One test, the digit span did show a difference between baseline and hypogonadism; however, the change in performance reflected an improvement not decline in performance scores during hypogonadism. Moreover, the improved scores during hypogonadism were virtually identical to those seen during gonadal steroid replacement, rendering a role for changing gonadal steroid levels in the performance scores un-compelling. An analysis of change scores across test sessions also failed to show specific effects of hormone condition, and differences from the untreated control group were observed in only two tests both of which reflected changes in the untreated controls not the women receiving Lupron. The only test scores that improved during E compared with hypogonadism (i.e., age adjusted scores of the mental rotation test) also were better (albeit not significantly so) during P compared with hypogonadism and during E compared with baseline. The better performance on mental rotation during E compared with baseline is not consistent with a specific effect of E on performance since similar plasma E levels were observed during E and baseline. These data, therefore, also suggest the absence of a specific effect of E levels on mental rotation performance. Nonetheless, a previous study reported a significant improvement in mental rotation performance scores in postmenopausal women after three weeks of estrogen therapy (Duka et al. 2000). Our findings are consistent with the one prior study in healthy asymptomatic young women, in whom cognitive testing was performed after four months of GnRH agonist-induced ovarian suppression (Owens et al. 2002). While the effects of E (or P) on cognitive function may vary with age (Rapp et al. 2002), our data and those of Owens et al (2002) suggest that changes in the secretion of neither E nor P substantially alter cognitive function in younger women, at least over the several weeks of exposure in this protocol.
Previous studies examining the effects of E replacement in older hypogonadal (post-menopausal) women identified positive effects on measures of verbal and visual memory in some (Joffe et al. 2006; Kampen et al. 1994; Maki et al. 2001; Resnick et al. 1997) but not all studies (Berman et al. 1997; Keenan et al. 2001; LeBlanc et al. 2001; Shaywitz et al. 1999). Additionally, several neuroimaging studies have identified changes in brain activity corresponding to changes in ovarian steroid hormone secretion (Berman et al. 1997; Goldstein et al. 2005; Protopopescu et al. 2005; Shaywitz et al. 1999; Smith et al. 2006; van Wingen et al. 2007). We were unable to identify significant declines in performance scores during hypogonadism, in tests of attention or verbal or visual memory, nor improvements during E replacement in this study. Two meta analyses (LeBlanc et al. 2001; Yaffe et al. 1998) suggested that the beneficial effects of E on cognition are restricted to symptomatic hypogonadal women. Nonetheless, the severity of hot flushes and disturbed sleep did not distinguish those women in our study whose performance on the paragraph memory improved from hypogonadism to E replacement and those whose performance declined. Our finding that individual self-reports of neither hot flushes nor disturbed sleep impact cognitive performance are consistent with two recent reports in symptomatic peri- and postmenopausal women (LeBlanc et al. 2007; Maki et al. 2008); however, in one of these studies (Maki et al. 2008), objective measures of hot flushes suggested that the number of objective hot flushes (which exceeded the numbers recorded by self-report) was associated with declines in verbal memory performance. Thus it is possible that had we employed objective measures of the numbers of hot flushes experienced by each woman we might be able to better distinguish those women who experienced an improvement in verbal memory between the hypogonadal and estradiol replaced testing sessions. In our study, we also were limited in the choice of testing measures due to the repeated measures design (e.g., four separate test forms were not available for the CVLT) (Keenan et al. 2001). Alternate measures may have been more sensitive to differences in hormone-induced changes in memory function. However, several methodologic differences may explain the discrepancies between our findings and those reporting E-related memory improvements. First, cross-sectional studies in E users and non-users may reflect more enduring characteristics, such as education and general health, rather than the use of E replacement (Yaffe et al. 1998). Second, our younger sample permits no conclusion about potential age-related decline in verbal or working memory, which may be E-responsive (Rapp et al. 2002). Third, the healthy paid volunteers in this study were recruited from a local catchment area surrounding the hospital. Thus it is possible that our selection process could have introduced a bias that resulted in a more motivated group of women whose performances would be different from the scores of women studied in the clinic-based studies of treatment-seeking women. Finally, the duration of hypogonadism in postmenopausal women would be considerably longer than in our study and may be associated with a differing responsivity to E (Tinkler et al. 2002).
Several previous reports examined the effects of GnRH agonist-induced hypogonadism, and in some E replacement, on cognition in younger women receiving treatment for uterine fibroids or endometriosis (Grigorova et al. 2006; Sherwin et al. 1996). In contrast to our data, (Craig et al. 2007; Craig et al. 2008; Craig et al. 2008; Palomba et al. 2004; Varney et al. 1993) the majority of these studies observed a significant decline in cognitive performances during hypogonadism compared with baseline, including measures of verbal memory, working memory, and visual recognition, and an improvement in several of these measures (e.g., verbal memory) in those women receiving E (but not in those receiving placebo) while on GnRH agonist. Our findings are consistent with those of Owens (2002), who also studied asymptomatic healthy women at baseline and under conditions of GnRH agonist-induced ovarian suppression of four months duration and, in half of the women, after estradiol replacement. Both our study and that of Owens (2002) observed numerous practice effects after repeated testing but no specific effects of either hypogonadism or estradiol replacement despite administering similar test measures as those administered in several gynecologic clinic-based studies. Together, our data and those of Owens (2002) represent experience with over 30 healthy asymptomatic women (as well as 8 women reporting PMS who were otherwise gynecologically normal) showing a consistent lack of effect on cognitive performance after a decisive hormone intervention (i.e., ovarian suppression). These findings in healthy asymptomatic women also stand in remarkable contrast to the otherwise uniform findings in multiple studies of a decline in cognitive performance in women treated with ovarian suppression for gynecologic illness.
These ostensibly discrepant findings could reflect important differences in the clinical characteristics of the samples that are associated with or are predictive of a decline in cognitive function during either the natural or induced menopause. Epidemiologic studies in women during the perimenopause would suggest that complaints of cognitive decline are not uniformly reported by women (Gold et al. 2000; Mitchell et al. 2001), and some women, therefore, might be differentially vulnerable to the cognitive-impairing effects of declining ovarian steroids. For example, the presence of endometriosis or fibroids could be associated with a greater risk for cognitive decline under conditions of ovarian steroid withdrawal or suppression. Abnormalities of estrogen receptor function have been suggested to play a role in the pathophysiology of both of these conditions (Cavallini et al. 2011; Huhtinen et al. 2011; Li et al. 2001; Wei et al. 2007), and obviously both are treated in part by induced hypogonadism. Thus it is possible that in women with endometriosis or uterine fibroids, estrogen signaling is abnormal at other tissue sites including the brain, perhaps accounting for the otherwise discrepant cognitive findings during GnRH agonist therapy. Alternately, the symptoms of a longstanding gynecologic condition could be accompanied by increased stress in these women. Chronic stress, in turn, could either diminish cognitive reserve or impair ovarian estradiol secretion prior to treatment sufficient to amplify the effects of GnRH agonist-induced hypogonadism on cognitive performance. Clearly other factors could account for differences observed across studies including the baseline levels of cognitive performance prior to starting GnRH agonist. Finally, mood symptoms may impair performance and confound the effects of gonadal steroids on cognition. Mood symptoms, however, did not significantly differ between hypogonadal and hormone-replaced conditions. We did observe mood symptoms in women who reported PMS at baseline. Additional potential confounds related to the inclusion of women with PMS in this study include the documented efficacy of GnRH agonist treatment in PMS and observations of deficits in the retrieval of learned information that is menstrual cycle phase independent in women with PMS (Keenan et al. 1992; Keenan et al. 1995). However, a similar pattern of results was observed in the asymptomatic women without PMS who were analyzed separately.
CONCLUSIONS
We did not find evidence that gonadal steroids regulate measures of cognitive function including verbal memory or visuospatial abilities in younger healthy women. Our data in women suggest that short term changes in either E or P do not substantially alter cognitive test performance in any of the domains measured, including those previously reported to change in the context of short term E therapy (Kampen et al. 1994) or across the menstrual cycle (Hampson et al. 1988). However, the discrepancies between data collected from gynecologic clinic-based samples and that from our study and that of Owens (2002) suggest that, not surprisingly, the effects of E on the brain are not uniform across individuals. Such differences further suggest the existence of as yet undefined risk phenotypes that might predict an individual woman’s symptomatic experience during the menopause and her possible response to estradiol therapy.
Supplementary Material
Table 1.
Baseline Demographics in Women treated with Lupron (n=23) and Untreated Controls (n=11)
| Lupron-treated Group | Untreated Control Group | |
|---|---|---|
| Age# | 35.0 (6.7) | 33.8 (10.5) |
| No. of Years of Education# | 16.2 (1.8) | 17.5 (1.6) |
| Racial Distribution | 21W/2AA | 6W/1AA/4A |
| MC Phase during First Test Session | 9Follicular/14Luteal | 5Follicular/6Luteal |
| BDI Scores# | 5.4 (9.4) | 0.3 (0.5) |
Values are Mean (SD) unless otherwise indicated.
Lupron-treated women did not differ in age, years of education, or baseline BDI scores from the untreated control group (p=ns for all comparisons).
Table 2.
Plasma Hormone Levels in Women treated with Lupron (n=23) and Untreated Controls (n=11)
| Lupron-treated Gp. | Baseline | Hypogonadal | E Replaced | P Replaced |
|---|---|---|---|---|
| Estradiol (pg/ml) | 84.3 (64.1) | 18.9 (9.7) | 108.9 (64.9) | 16.4 (6.0) |
| Progesterone (ng/ml) | 2.8 (4.3) | 0.4 (0.2) | 0.4 (0.3) | 14.1 (7.6) |
| Untreated Control Gp | Test # 1 | Test # 2 | Test # 3 | Test # 4 |
| Estradiol (pg/ml) | 115.2 (72.1) | 114.7 (77.6) | 88.6 (57.7) | 112.1 (75.8) |
| Progesterone (ng/ml) | 2.2 (3.8) | 3.9 (4.3) | 4.8 (6.1) | 4.0 (6.1) |
Conversions - E: pg/ml × 3.67 = pmol/L; P: ng/ml × 3.18 = nmol/L.
Acknowledgments
Dr. Schmidt had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was funded by the Intramural Research Program of the National Institute of Mental Health. Dr. Keenan is a full salaried employee of Johnson and Johnson.
This work was written as part of Dr. Schmidt’s official duties as a Government employee. The views expressed in this article do not necessarily represent the views of the NIMH, NIH, HHS, or the United States Government.
This work was supported by the National Institute of Mental Health Intramural Research Program.
Footnotes
None of the Authors report any potential conflicts of interest relevant to the information contained within this manuscript.
References
- Abraham GE, Swerdloff R, Tulchinsky D, Odell WD. Radioimmunoassay of plasma progesterone. J Clin Endocrinol Metab. 1971;32:619–624. doi: 10.1210/jcem-32-5-619. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher Kd. Multilingual aphasia examination. AJA Associates; Iowa City, IA: 1976. [Google Scholar]
- Benton AL, Hamsher Kd, Varney NR, Spreen O. Contributions to neuropsychological assessment. Oxford University Press; New York: 1983. [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini A, Resta L, Caringella AM, Dinaro E, Lippolis C, Loverro G. Involvement of estrogen receptor-related receptors in human ovarian endometriosis. Fertil Steril. 2011;96:102–106. doi: 10.1016/j.fertnstert.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Corwin J, Bylsma FW. Translations of excerpts from André Rey’s psychological examination of traumatic encephalopathy and P.A. Osterrieth’s the complex figure copy test. The Clinical Neuropsychologist. 1993;7:3–21. [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Picchioni MM, Brammer M, Giampietro V, Rymer J, McGuire PK, Maki PM, Murphy DGM. A study of visuospatial working memory pre- and post-gonadotropin hormone releasing hormone agonists (GnRHa) in young women. Horm Behav. 2008;54:47–59. doi: 10.1016/j.yhbeh.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Maki PM, Murphy DGM. Reversibility of the effects of acute ovarian hormone suppression on verbal memory and prefrontal function in pre-menopausal women. Psychoneuroendocrinology. 2008;33:1426–1431. doi: 10.1016/j.psyneuen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Cutter WJ, Brammer M, Giampietro V, Wickham H, Maki PM, Murphy DGM. Gonadotropin hormone releasing hormone agonists alter prefrontal function during verbal encoding in young women. Psychoneuroendocrinology. 2007;32:1116–1127. doi: 10.1016/j.psyneuen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R. Estrogen-induced plasticity from cells to circuits: predications for cognitive function. Trends Pharmacol Sci. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Reed BR, Coleman JE, Jagust WJ. Effect of estrogen on cerebral glucose metabolism in postmenopausal women. Neurology. 2000;55:875–877. doi: 10.1212/wnl.55.6.875. [DOI] [PubMed] [Google Scholar]
- Endicott J, Halbreich U, Schacht S, Nee J. Premenstrual changes and affective disorders. Psychosom Med. 1981;43:519–529. doi: 10.1097/00006842-198112000-00008. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: women’s health initiative memory study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447–453. doi: 10.1016/j.maturitas.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Furuyama S, Mayes DM, Nugent CA. A radioimmunoassay for plasma testosterone. Steroids. 1970;16:415–428. doi: 10.1016/s0039-128x(70)80124-6. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EG, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, Salamone L, Stellato R. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, Kramer E. Auditory comprehension tests. Auditory Comprehension Tests, Ltd; Edmonton, Canada: 1983. [Google Scholar]
- Grigorova M, Sherwin BB, Tulandi T. Effects of treatment with leuprolide acetate depot on working memory with executive functions in young premenopausal women. Psychoneuroendocrinology. 2006;31:935–947. doi: 10.1016/j.psyneuen.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Hampson E, Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci. 1988;102:456–459. doi: 10.1037//0735-7044.102.3.456. [DOI] [PubMed] [Google Scholar]
- Harley JP, Leuthold CA, Matthews CG, Bergs LE. Wisconsin neuropsychological test battery T-score norms for older veterans administration medical center patients. Department of Neurology, University of Wisconsin Medical School; Madison, WI: 1980. [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60:1369–1371. doi: 10.1212/01.wnl.0000059413.75888.be. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2002:CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtinen K, Stahle M, Perheentyupa A, Poutanen M. Estrogen biosynthesis and signaling in endometriosis. Mol Cell Endocrinol. doi: 10.1016/j.mce.2011.08.022. in press. [DOI] [PubMed] [Google Scholar]
- Jastak S, Wilkinson GS. Wide range achievement test-revised. Jastak Assessment Systems; Wilmington, DE: 1984. [Google Scholar]
- Jiang N-S, Ryan PJ. Radioimmunoassay for estrogens: a preliminary communication. Mayo Clin Proc. 1969;44:461–465. [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kampen DL, Sherwin BB. Estrogen use and verbal memory in healthy postmenopausal women. Obstet Gynecol. 1994;83:979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Lindamer LA, Jong SK. Menstrual phase-independent retrieval deficit in women with PMS. Biol Psychiatry. 1995;38:369–377. doi: 10.1016/0006-3223(94)00303-K. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Stern RA, Janowsky DS, Pedersen CA. Psychological aspects of premenstrual syndrome I: cognition and memory. Psychoneuroendocrinology. 1992;17:179–187. doi: 10.1016/0306-4530(92)90056-d. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kløve H. Clinical neuropsychology. In: Forster FM, editor. The medical clinics of North America. Saunders; New York: 1963. pp. 1647–1658. [PubMed] [Google Scholar]
- Kok HS, Kuh D, Cooper R, van der Schouw YT, Grobbee DE, Wadsworth MEJ, Richards M. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Blusewicz MJ, Brandt J, Ober BA, Strauss M. Verbal memory errors in Alzheimer’s and Huntington’s dementias. Dev Neuropsychol. 1988;4:1–15. [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause. 2007;14:191–202. doi: 10.1097/01.gme.0000230347.28616.1c. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women (review) Cochrane Database Syst Rev. 2008;1:CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak Deutsch M. Neuropsychological assessment. 3. Oxford University Press; New York: 1995. [Google Scholar]
- Li S, McLachlan JA. Estrogen-associated genes in uterine leiomyoma. Ann N Y Acad Sci. 2001;948:112–120. doi: 10.1111/j.1749-6632.2001.tb03992.x. [DOI] [PubMed] [Google Scholar]
- Low L-F, Anstey KJ. Hormone replacement therapy and cognitive performance in postmenopausal women - a review by cognitive domain. Neurosci Biobehav Rev. 2006;30:66–84. doi: 10.1016/j.neubiorev.2005.05.003. [DOI] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848–856. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158:277–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204:E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ES, Woods NF. Midlife women’s attributions about perceived memory changes: observations from the Seattle midlife women’s health study. J Women’s Health Gend Based Med. 2001;10:351–362. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- Money J. Manual. Academic Therapy Publications; San Rafael, CA: 1976. A standardized road map test of direction sense. [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Owens JF, Matthews KA, Everson SA. Cognitive function effects of suppressing ovarian hormones in young women. Menopause. 2002;9:227–235. doi: 10.1097/00042192-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Palomba S, Orio F, Jr, Russo T, Falbo A, Amati A, Zullo F. Gonadotropin-releasing hormone agonist with or without raloxifene: effects on cognition, mood, and quality of life. Fertil Steril. 2004;82:480–482. doi: 10.1016/j.fertnstert.2003.11.061. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Porteus SD. The maze test and clinical psychology. Pacific Books; Palo Alto, CA: 1959. [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Silbersweig D, Stern E. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, McKay H, Kansky MT, Roberts JA. Ovarian hormone status modulates the cognitive outcome of aging in the rhesus monkey. Abstr Soc Neurosci 2002 [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang SC, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol Aging. 2005;26:229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171:701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: women’s health initiative memory study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol digit modalities test (SDMT) manual (revised) Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab. 2006;91:4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured clinical interview for DSM-III-R, patient edition. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1990. [Google Scholar]
- Spreen O, Benton AL. Embedded figures test. Neuropsychological Laboratory, University of Victoria; Victoria, Canada: 1969. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. Oxford University Press; New York: 1991. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Talland GA. Deranged memory: a psychonomic study of the amnesic syndrome. Academic Press; New York: 1965. Reproduction and distortion of narrative texts; pp. 235–243. [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Cholinergic neurons in the nucleus basalis following two-year estrogen loss or replacement in surgically menopausal monkeys. Abstr Soc Neurosci 2002 [Google Scholar]
- van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar J, Fernandez G. How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci. 2007;27:11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualization. Percept Mot Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Varney NR, Syrop C, Kubu CS, Struchen M, Hahn S, Franzen K. Neuropsychological dysfunction in women following leuprolide acetate induction of hypoestrogenism. J Assist Reprod Genet. 1993;10:53–57. doi: 10.1007/BF01204441. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale - revised. The Psychological Corporation, Harcourt Brace Jovanovich, Inc; San Antonio: 1981. [Google Scholar]
- Wechsler D. Wechsler memory scale-revised manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Wei T, Geiser AG, Qian HR, Su C, Helvering LM, Kulkarini NH, Shou J, N’Cho M, Bryant HU, Onyia JE. DNA microarray data integration by ortholog gene analysis reveals potential molecular mechanisms of estrogen-dependent growth of human uterine fibroids. BMC Womens Health. 2007;7:5. doi: 10.1186/1472-6874-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Quesenberry CP, Jr, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the women’s health initiative. Endocr Rev. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, Grady D. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol. 2006;63:945–950. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.