Abstract
Rationale
Women are more sensitive than men to psychostimulants and progress from initial use to drug addiction more quickly. The mouse has been an under-utilized model to study sex differences in psychostimulant action. Mice could serve as an ideal genetically-tractable model for mechanistic studies into sex and hormone effects on psychostimulant behavior.
Objectives
To characterize psychostimulant effects in male and female mice with a combination of automated data collection and behavioral observation.
Methods
Male and female C57BL/6 mice (Charles River) were given a single dose or sequential ascending binge doses of d-amphetamine (AMPH) or cocaine (COC). Behavior was assessed in open field chambers using both automated photobeam interruptions and behavioral observations. Brain psychostimulant concentrations were determined at the time of maximum behavioral stimulation.
Results
Psychostimulants induced behavioral activation in mice including both increased locomotion as detected with an automated system and a sequence of behaviors progressing from stereotyped sniffing at low doses to patterned locomotion and rearing at high doses. Females exhibited more patterned locomotion and a shift towards higher behavior scores after either psychostimulant despite having lower AMPH and equivalent COC brain levels as males.
Conclusions
Female C57BL/6 mice exhibit enhanced psychostimulant-induced behavior compared to males, similar to reports in rats. The combination of automated behavioral measures and behavioral observation was essential for verifying the existence of these differences. These results indicate the importance of testing both sexes when characterizing genetically manipulated mice to control for potential sex-specific effects.
Keywords: Sex differences, Cocaine, Amphetamine, Psychostimulant, Metabolism, Behavior, Stereotypy, Mice, Male, Female
Introduction
Women are more sensitive to the effects of psychostimulants and progress from initial use to drug addiction more rapidly than males (Anker and Carroll 2011; Becker and Hu 2008; Lynch et al. 2002). These observed sex differences are due in part to ovarian hormones, as evidenced by fluctuations in reported subjective effects over the menstrual cycle (Becker and Hu 2008; Justice and de Wit 1999; Lynch et al. 2002). The behavioral responses of rats to psychostimulants exhibit similar sex differences. Relative to males, female rats have enhanced responses to cocaine (COC) (Hu et al. 2004; Kuhn et al. 2001; van Haaren and Meyer 1991; Walker et al. 2001; Walker et al. 2009), MDMA (Walker et al. 2007), methylphenidate (Chelaru et al. 2012; Dafny and Yang 2006) and amphetamine (AMPH) (Beatty and Holzer 1978; Brass and Glick 1981; Robinson et al. 1980; Stohr et al. 1998). Females work harder for psychostimulants, learn to self-administer them faster, and are more sensitive to their rewarding effects than males (Anker et al. 2011; Becker and Hu 2008; Davis et al. 2008; Russo et al. 2003b). These observations have made the rat the rodent model of choice in studies of sex differences in psychostimulant action.
Psychostimulants like AMPH and COC at low doses elicit increased locomotion in rats by raising extracellular dopamine (DA) in the nucleus accumbens (NuAcc) and at higher doses induce repetitive, in-place stereotyped behaviors through increased extracellular DA in the caudate putamen (CP) (Creese and Iversen 1975; Kelly and Iversen 1976; Kelly et al. 1975). Psychostimulants elicit both enhanced locomotion and more stereotyped behaviors in female rats compared to males (Becker et al. 2001; Bowman and Kuhn 1996; Kuhn et al. 2010; Parylak et al. 2008; Walker et al. 2001). These sex differences in psychostimulant-induced behaviors reflect multiple activational effects of ovarian hormones on DA function. Ovariectomy reduces the response to psychostimulants, while castration has little effect (Becker and Hu 2008; Kuhn et al. 2010; Kuhn et al. 2001; Parylak et al. 2008; Russo et al. 2003a; van Haaren and Meyer 1991; Walker et al. 2001; Walker et al. 2012). Estradiol replacement in ovariectomized animals increases psychostimulant-induced behavior, basal DA levels, and DA release (Becker 1990; Becker and Beer 1986; Becker and Ramirez 1981; Hu et al. 2004; Russo et al. 2003a; Thompson and Moss 1994). Estradiol also regulates DA system anatomy as females have more DA neurons than males and the number of DA neurons changes after gonadectomy (Johnson et al. 2010a; Johnson et al. 2010b; Kuhn et al. 2010; Leranth et al. 2000; Walker et al. 2012).
Most evidence indicates that the sex difference in the psychostimulant behavioral response is not mediated by differences in drug metabolism. Female rats have higher AMPH levels after injection compared to males (Meyer and Lytle 1978), but females still respond more than males when equivalent brain AMPH concentrations are present (Becker et al. 1982). The situation is even clearer with COC, as there is no sex difference in COC levels after administration in rats, primates, or humans (Bowman et al. 1999; Evans and Foltin 2010). Overall, sex differences in the metabolism of AMPH or COC do not explain the differences seen in psychostimulant-induced behavior.
A mouse model provides the advantage of genetic tractability for detailed studies of signaling mechanisms and anatomical circuitry, but it is first necessary to understand how normal male and female mice respond to psychostimulants. Studies of behavioral and neurochemical differences in genetically altered mice have shown that such effects can be sex-specific, so sexual dimorphisms must be carefully considered (Kuppers et al. 2008; Siuciak et al. 2007; van den Buuse et al. 2012). Previous studies have reported psychostimulant-induced stereotyped behavior in mice using behavioral scales developed in rats, but the topography of psychostimulant-induced behavior in mice has not been carefully characterized.(Quintero and Spano 2011; Quintero et al. 2008; Schlussman et al. 1998; Tolliver and Carney 1994).
The primary aim of this study was to utilize automatic behavior recording methods and a developed behavior rating scale to determine whether psychostimulant effects on behavior differ in male and female C57BL/6 mice as transgenic and knockout mice are frequently generated on this background. The few previous studies that focused on sex differences in psychostimulant-induced behavior in normal mice have yielded contradictory results providing evidence both for (Morse et al. 1993; Reith et al. 1991; Sershen et al. 1998) and against (Griffin and Middaugh 2006; Thomsen and Caine 2011) a sex difference. The present study shows that combining both automated and observational methods provides more complete characterization of behavioral activation and facilitates understanding of sex differences in these behaviors.
Materials and Methods
Animals
Drug-naïve male and female C57BL/6 mice (9–10 weeks old, Charles River Laboratories, Raleigh, NC) were received 1 week before behavior tests. Mice were housed by sex in ventilated plastic cages with ad libitum food and water on a 12-hour light/dark cycle (lights on at 6:00 AM). Females were randomly cycling and not selected based on estrous cycle stage as repeated vaginal lavaging to monitor the cycle reduces cocaine-stimulated locomotion in female Sprague Dawley rats (Walker et al. 2002). Effects of the estrous cycle on psychostimulant-induced behavior in mice will be addressed in a future study. All animal studies were approved by the Duke University Institutional Animal Care and Use Committee and followed NIH guidelines.
Drugs
d-Amphetamine (AMPH) and cocaine HCL (COC) were provided by the Research Triangle Institute (Research Triangle Park, NC), courtesy of the National Institute of Drug Abuse. AMPH and COC were diluted in sterile saline and given as intraperitoneal injections (10 mL/kg).
Behavioral Measurements and Treatment Paradigms
Mice were placed into 40 × 40 cm open field locomotor boxes (Kinder Scientific, Inc., Poway, CA) for 2.5 hours of habituation (starting at 9:00 am) before injections. Photobeam interruptions were automatically recorded as horizontal (e.g. ambulations, fine movements) or vertical (e.g. rearing) movements. Behavior was monitored for 1–2 hours after saline and either a single dose or three sequential ascending binge doses of AMPH (experiments 1 and 2, respectively) or COC (experiments 3 and 4, respectively) according to Table 1. In the ascending binge paradigms, mice were given all four injections (saline + 3 drug doses) on the same day.
Table 1.
Psychostimulant treatment paradigms
The experiments and treatment paradigms used to compare psychostimulant-induced behavior in male and female C57BL/6 mice. Each mouse received each injection of saline and AMPH or COC for their paradigm in sequential order at the indicated times during the light cycle (lights on at 6:00 am). Mice in the single dose paradigms received just one dose of the psychostimulant while mice in the sequential ascending binge paradigms received three doses of increasing psychostimulant concentration. Mice were observed for 1–2 hours after each injection and behaviors were recorded.
| Expt. # | Paradigm | # mice per sex | Total # mice in expt. | Injection #1 (~11:30 am) | Injection #2 (~1:00 pm) | Injection #3 (~2:15 pm) | Injection #4 (~3:30 pm) |
|---|---|---|---|---|---|---|---|
| 1 | Single AMPH | 7–8 | 15 | Saline | 5 mg/kg | N/A | N/A |
| 2 | Asc AMPH | 8 | 16 | Saline | 1 mg/kg | 2.5 mg/kg | 5 mg/kg |
| 3 | Single COC | 8 M, 12 F | 20 | Saline | 30 mg/kg | N/A | N/A |
| 4 | Asc COC | 15–16 | 31 | Saline | 5 mg/kg | 15 mg/kg | 30 mg/kg |
M = males, F = females, Asc = ascending, N/A = not applicable
Behavior scoring
We adapted our mouse rating scale from two previous scales, one developed for rats (Ellinwood and Balster 1974) and another rat-based scale used in mice (Schlussman et al. 1998; Schlussman et al. 2003). A trained observer blind to experimental group monitored each mouse as locomotor behavior was being recorded. Every 5 minutes, each mouse was observed for two consecutive 15-second bins for a total of 24 observations/animal/hour. Each observed behavior was recorded, and every bin was later assigned a score (Table 2). Behaviors seen at lower AMPH doses and during the waning effects of the drug were assigned lower scores, while behaviors observed after higher AMPH doses and during maximal drug effects were scored higher. With this rating scale, increasing doses of AMPH cause a shift towards higher scores in the behavior distribution of both male and female mice.
Table 2.
The psychostimulant-induced behavior observation rating scale
The psychostimulant-induced behavior rating scale used in C57BL/6 mice. A higher score on this non-linear rating scale indicates a greater response and greater activation by a psychostimulant. Scores 1–3 are normal behaviors seen in mice after saline injections, while scores 4–9 are induced by psychostimulants.
| Score | Behavior Category | Included Behaviors |
|---|---|---|
| 1 | Asleep/Inactive | Asleep; Awake but still |
| 2 | Light in-place directed activity | Normal grooming; Light sniffing |
| 3 | Normal exploratory behavior | Slow intermittent locomotion; Occasional rearing; Sniffing or gnawing bedding |
| 4 | Fast exploratory behavior | Fast exploratory locomotion/sniffing; Increased rearing |
| 5 | In-place stereotyped behaviors | Continuous sniffing; Head bobbing/weaving; Circling/pivoting; Intense grooming/self-gnawing |
| 6 | Patterned locomotion | Running in a pattern (i.e. around periphery of box) with head up and erect tail |
| 7 | Patterned rearing | Continuous “up-and-down” rearing motion with continuous sniffing; Often along wall/in corner; Sometimes licking wall |
| 8 | Maintained rear | In a full rear for entire 15-second observation bin, with continuous sniffing |
| 9 | Dyskinetic/Sick | Splayed hindlimbs; Laying on side; Seizures |
Brain and serum psychostimulant levels
To compare potential sex differences in AMPH and COC metabolism, male and female mice were injected as above in either the single dose or ascending binge paradigms (AMPH, experiment 5; COC, experiment 6). Mice were anesthetized with isofluorane and decapitated at the time of maximal behavioral activation (45 min after AMPH, 15 min after COC). Blood was collected via cardiac puncture, placed into tubes (containing 10 uL saturated NaF for COC samples), and centrifuged at 4°C. Brains and sera were stored at −80°C. Samples were analyzed for AMPH or COC, benzoylecgonine (BE), ecgonine methyl ester (EME), and norcocaine (NC) using published protocols courtesy of Dr. David Moody, Dr. David Andrenyak, and Dr. Wenfang Fang at the University of Utah (Lin et al. 2001; Lin et al. 2003; Slawson et al. 2002).
Data Analysis
Automatically recorded behaviors are presented as 10- or 60-minute interval means ± SEM (Prism 5.0, GraphPad Software Inc.) and were analyzed in NCSS (NCSS, Kaysville, UT) for main effects of time, psychostimulant dose and/or sex using 2- and 3-way repeated-measures ANOVA with time and/or dose as the repeated measures. Habituation data from experiments 1–4 were pooled as all animals had been similarly handled until habituation (total n = 41 males, 44 females). The concentrations of AMPH or COC and metabolites are reported as ng/g brain tissue or ng/mL serum (mean ± SEM). Results were analyzed by 2-way ANOVA (NCSS) with sex and treatment paradigm as the between factors. Significant effects (p<0.05) were followed by post-hoc Tukey-Kramer tests to compare doses/sexes/treatment. Outliers were determined by a Grubbs statistical test.
The relative frequency of each behavior score was calculated and depicted as a histogram (Prism 5.0). The scores for normal behaviors (1–3) were combined for statistical analysis due to their low frequencies after high psychostimulant doses. Significant main effects of dose/sex, as determined by chi square tests, were followed by post-hoc pairwise comparisons.
Results
Sex differences in novelty-induced locomotion
Female C57BL/6 mice responded more than males to the novel environment throughout habituation (main effect of sex on ambulations [F(1,1274)=28.77; p<0.001], (Fig. 1a), fine movements [F(1,1274)=9.88; p=0.002], (Fig. 1b), and rearing [F(1,1274)=11.02; p=0.001], (Fig. 1c)). Both sexes exhibited decreased levels of each behavior over time (main effect of time during habituation on ambulations [F(1,169)=731.36; p<0.001], fine movements [F(1,169)=367.58; p<0.001], and rearing [F(1,169)=227.02; p<0.001]). Analysis also yielded a sex x time interaction on the time course of ambulations [F(14,1274)=7.81; p<0.001] and on the 60-minute ambulation totals [F(1,169)=21.72, p<0.001]. Post-hoc tests confirmed enhanced behavior in female C57BL/6 mice versus males and decreased behavior in the second hour versus the first hour.
Fig. 1.
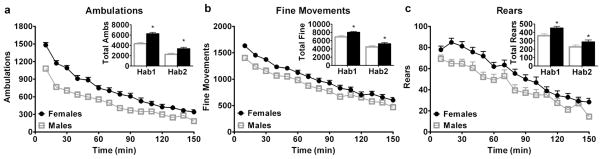
Female C57BL/6 mice respond more during habituation to a novel environment than males (pooled from experiments 1–4). 10-minute interval time course of ambulations (a), fine movements (b), and rearing (c) during habituation. Insets show the 60-minute totals of each behavior for the first and second hour of habituation. *Indicates different from males. Total n=41 males, 44 females
Sex differences in amphetamine-induced behavior
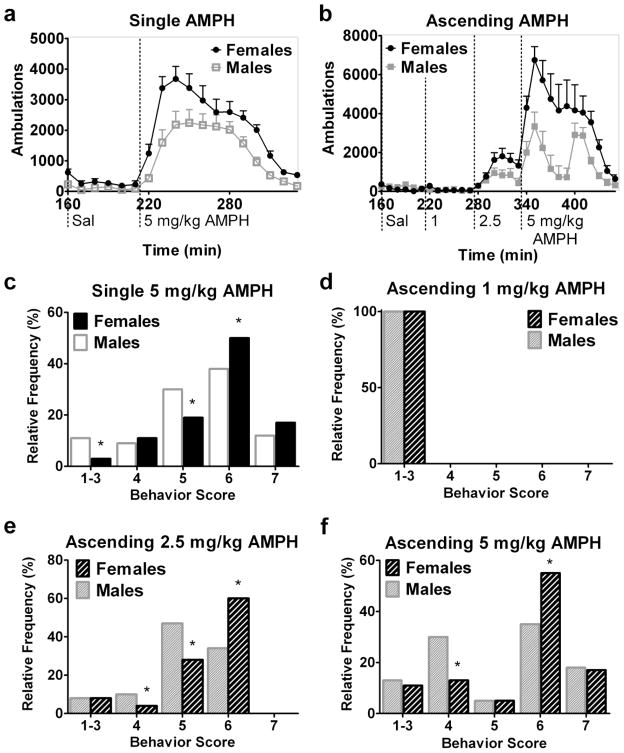
Figures 2 and 3 show automatically-recorded ambulations after all injections and the behavior distributions after AMPH or COC. Female C57BL/6 mice exhibited more ambulations after AMPH (experiment 1) than males, and all animals had more ambulations after AMPH than after saline (Fig. 2a). Main effects of sex [F(1,179)=7.90, p=0.02] and treatment (saline vs AMPH) [F(1,179)=106.97, p<0.001] and their interaction (sex x treatment [F(1, 179)=4.70, p=0.049]) on ambulations were observed. Post-hoc analyses confirmed that females had more ambulations than males after AMPH. No effect of sex on ambulations was observed after saline.
Fig. 2.
Female C57BL/6 mice exhibit more ambulations and patterned locomotion after AMPH than males. 10-minute interval time course of ambulations in male versus female mice after saline and a single dose (a, experiment 1) or ascending binge doses (b, experiment 2) of AMPH. Dotted vertical lines mark time of injection of saline (sal) or indicated dose of AMPH. The relative frequency distribution as a percentage of each behavior score after a single 5 mg/kg AMPH dose (c, experiment 1) or sequential ascending binge doses (d–f, experiment 2) of 1, 2.5, and 5 mg/kg AMPH. *Indicates different from males within score. n=7–8 per sex, total n=15 (a and c, experiment 1) or 16 (b and d–f, experiment 2)
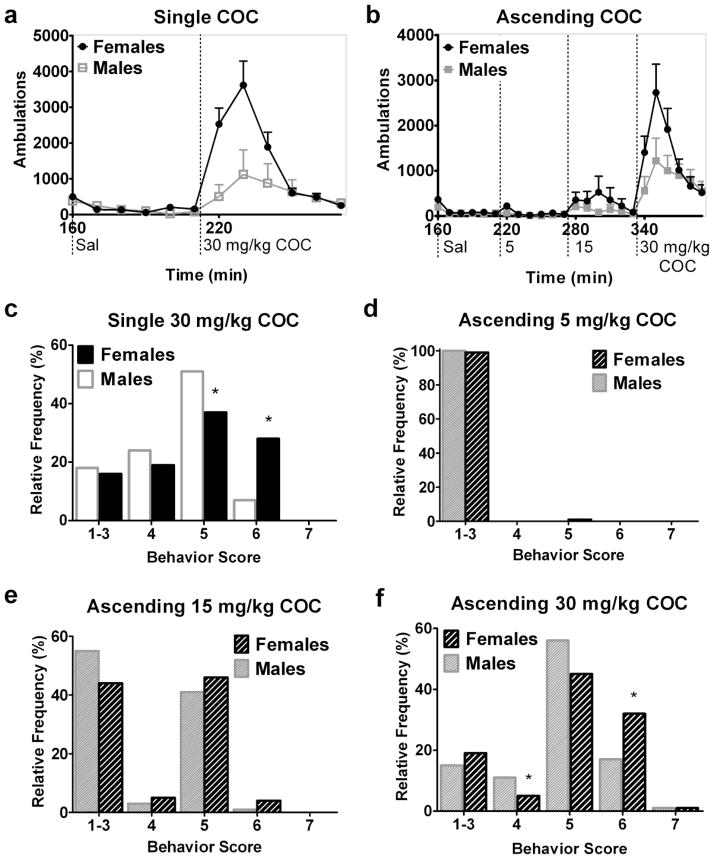
Fig. 3.
Female C57BL/6 mice exhibit more ambulations and patterned locomotion after COC than males. 10-minute interval time course of ambulations in male versus female mice after saline and a single dose (a) or ascending binge doses (b) of COC. Dotted vertical lines mark time of injection of saline (sal) or indicated dose of COC. The relative frequency distribution as a percentage of each behavior score after a single 30 mg/kg COC dose (c, experiment 3) or ascending binge doses (d–f, experiment 4) of 5, 15, and 30 mg/kg COC. *Indicates different from males within score. n=8 males, 12 females (a and c, experiment 3); n=15–16/sex (b and d–f, experiment 4)
Sex differences in psychostimulant-induced behavior were also found during the ascending AMPH paradigm (experiment 2). ANOVA of ambulations after saline and three doses of AMPH showed main effects of sex [F(1,383)=9.39, p=0.008], dose (0, 1, 2.5, or 5 mg/kg AMPH) [F(3,383)=29.13, p<0.001], and a sex x dose interaction [F(3, 383)=8.00, p<0.001] (Fig. 2b). Within doses, there was a main effect of sex on ambulations only after the 5 mg/kg AMPH dose [F(1, 191)=8.13, p=0.01]. Sex x time interactions after 1 [F(5, 95)=3.49, p=0.007], 2.5 [F(5, 95)=3.02, p=0.02], and 5 mg/kg AMPH [F(11,191)=2.76, p=0.003] were also observed. Post hoc tests confirmed that females had more ambulations than males after 5 mg/kg AMPH.
Females also displayed a greater response than males based on observed behaviors. The behavior distribution of females was shifted towards higher scores after a single 5 mg/kg AMPH dose (experiment 1) compared to males (main effect of sex on the behavior distribution, [p=0.045]) (Fig. 2c). Females did more patterned locomotion (score of 6) and fewer in-place stereotyped behaviors (score of 5) and normal behaviors (scores 1–3) than males.
In the ascending binge paradigm of experiment 2, 1 mg/kg AMPH did not elicit a sex difference in the behavioral score distribution as both males and females exhibited normal behaviors (scores of 1–3) (Fig. 2d). After 2.5 mg/kg AMPH, the predominant behaviors were patterned locomotion and in-place stereotyped behaviors (scores of 6 and 5, respectively) (Fig. 2e). Females given 2.5 mg/kg AMPH exhibited more patterned locomotion (score of 6) and fewer in-place stereotyped and fast exploratory behaviors (scores of 5 and 4, respectively) than males (main effect of sex, [p<0.006]). The highest AMPH dose tested (5 mg/kg) induced patterned locomotion and patterned rearing (scores of 6 and 7) with some in-place stereotyped behaviors and fast exploratory locomotion as the effects waned. Females demonstrated a heightened response to 5 mg/kg AMPH relative to males (main effect of sex, [p=0.023]) (Fig. 2f). Post-hoc analysis revealed that females had more patterned locomotion and less fast exploratory behavior than males after 5 mg/kg AMPH.
Sex differences in cocaine-induced behavior
Similar sex differences in psychostimulant-induced behaviors were seen after COC. A single dose of 30 mg/kg COC (experiment 3) stimulated more ambulations than saline (main effect of treatment (saline versus COC), [F(1, 239)=18.96, p<0.001]). ANOVA revealed an effect of sex [F(1, 239)=5.10, p=0.04] and a sex x time interaction [F(5,119)=7.08, p<0.001] on ambulations after COC (Fig. 3a). Post-hoc tests confirmed that females ambulated more than males after COC.
The ascending COC paradigm (experiment 4) showed that higher COC doses induced more locomotion (main effect of dose on ambulations, [F(3, 743)=23.97, p<0.001]) (Fig. 3b). Females ambulated more than males after 5 mg/kg and 30 mg/kg COC. A main effect of sex [F(1, 185)=5.22, p=0.02] on ambulations after 5 mg/kg COC and sex x time interactions after 5 mg/kg [F(5,185)=3.88, p=0.002] and 30 mg/kg COC [F(5,185)=3.67, p=0.004] were observed.
Female C57BL/6 mice also displayed an increased response to COC compared to males based on the behavior observations. The behavior distribution of females was shifted towards higher scores after a single dose of 30 mg/kg COC (experiment 3) relative to males (effect of sex, (p=0.002)) (Fig. 3c). Post-hoc tests confirmed that females exhibited fewer in-place stereotyped behaviors and more patterned locomotion (scores of 5 and 6) than males.
Similar patterns of behavior were seen in the ascending COC protocol (experiment 4). Both male and female mice exhibited normal behaviors (scores 1–3) after 5 mg/kg COC (Fig. 3d), and 15 mg/kg COC induced predominantly in-place stereotyped behaviors (score of 5) (Fig. 3e). No effect of sex on the behavior distribution was observed after 5 or 15 mg/kg COC. After 30 mg/kg COC, the behavior distribution of females was shifted towards higher scores relative to males (effect of sex, p=0.049) (Fig. 3f). Females did less fast exploratory behavior but more patterned locomotion (scores of 4 and 6, respectively) than males after the 30 mg/kg dose. These results indicate that C57BL/6 females exhibit greater behavioral responses than males to AMPH and COC as shown by both automated and observational means.
Sex and treatment differences in brain but not serum AMPH levels
The possibility that sex differences in psychostimulant metabolism could cause the observed behavioral differences was explored in male and female C57BL/6 mice administered a single dose or sequential ascending binge doses of AMPH or COC. Post-hoc analyses of experiment 5 with AMPH showed that females had lower brain AMPH levels than males 45 minutes after injection (effect of sex, [F(1,53)=9.91, p=0.003]), and that mice which received the ascending AMPH treatment had higher levels than mice given a single dose (effect of treatment, [F(1,53)=14.24, p=0.0004]) (Fig. 4a). There was no sex x treatment interaction on brain levels, and serum AMPH levels did not show an effect of sex or treatment (Fig. 4b). These results indicate that the sex difference in the AMPH-stimulated behavior of C57BL/6 mice cannot be explained by a sex difference in AMPH metabolism.
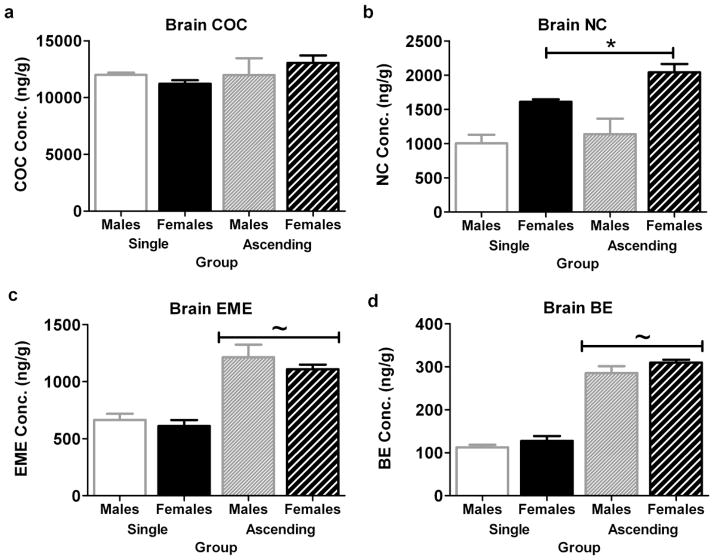
Fig. 4.
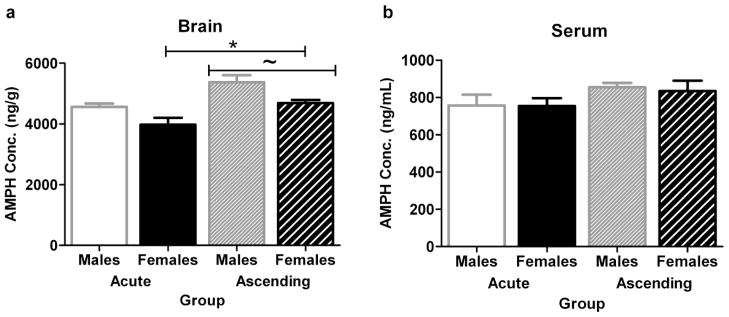
Female C57BL/6 mice have lower brain AMPH levels than males 45 minutes after injection (experiment 5). Levels of AMPH in brain (a) and serum (b) at the time corresponding to maximal behavioral activation after 5 mg/kg AMPH in the single dose and ascending binge paradigms. *Indicates females different from males, ~ ascending different from single dose. n=8–19 per group, total n=56
Sex and treatment differences in COC metabolism
Male and female mice had similar levels of brain COC 15 minutes after administration (experiment 6) (Fig. 5a). There was no effect of sex or treatment paradigm on brain COC levels. Females had more brain NC than males (effect of sex, [F(1,31)=26.98, p<0.002]) (Fig. 5b). EME (Fig. 5c) and BE (Fig. 5d) accumulated in the brains of both male and female mice after repeated COC dosing (effect of treatment paradigm, EME: ([F(1,31)=56.48, p<0.001], BE: [F(1,31)=270.54, p<0.001]). In serum, only BE showed an effect of sex or treatment (Table 3). Serum BE levels were higher in females than in males (effect of sex, [F(1,31)=30.29, p<0.0001]), and mice given ascending COC doses had more serum BE than singly-dosed mice (effect of treatment, [F(1,31)=13.87, p=0.0009]). These results demonstrate that the sex difference in the COC-stimulated behavior of C57BL/6 mice is not due to a differential in brain COC levels in male and female mice during behavior.
Fig. 5.
Female C57BL/6 mice have similar brain levels of COC but more NC than males 15 minutes after injection (experiment 6). Brain levels of (a) COC, (b) NC, (c) EME, and (d) BE at the time corresponding to maximal behavioral activation after 30 mg/kg COC in the single dose and ascending binge paradigms. *Indicates females different from males, ~ ascending different from single dose. n=8 per group, total n=32
Table 3.
Serum levels of COC and its metabolites
Serum levels of COC and its metabolites in male and female C57BL/6 mice 15 minutes after an injection of 30 mg/kg COC. Mice in the single dose paradigm received just one injection of 30 mg/kg COC while mice in the ascending binge paradigms received three sequential COC doses (5, 15, and 30 mg/kg). n=8 per group, total n=32. Mean ng/mL serum ± SEM.
| Single Dose | Ascending Binge Doses | |||
|---|---|---|---|---|
| Analyte | Males | Females | Males | Females |
| COC | 1111 ± 159 | 773 ± 102 | 1018 ± 149 | 945 ± 144 |
| NC | 241 ± 27 | 234 ± 11 | 208 ± 31 | 249 ± 31 |
| EME | 2053 ± 174 | 1923 ± 71 | 2056 ± 161 | 1908 ± 134 |
| BE | 2349 ± 120 | 3285 ± 176 * | 3010 ± 94 ~ | 3776 ± 204 *~ |
Indicates effect of sex (females greater than males),
indicates effect of treatment (ascending binge greater than single dose).
Discussion
A psychostimulant-induced behavior methodology and rating scale tailored to mice
This study utilized a methodology and observational rating scale that was tailored to mouse behavior to demonstrate a greater behavioral response of adult female than male C57BL/6 mice to psychostimulants. Automated counts yielded a systematic dose-related increase in behavior which behavioral observations demonstrate to reflect a changing behavioral topography with increasing dose. The sequence of behaviors began with in-place stereotyped behaviors (e.g. stereotyped sniffing with head weaving, circling/pivoting), progressed to patterned locomotion and then transitioned to patterned rearing with increasing behavioral activation. Therefore, mice which exhibit high ambulation counts by automatically recorded means could be exhibiting the fast exploratory locomotion (reflecting lower-level behavioral stimulation) or the more behaviorally-activated patterned locomotion. Observational methods are essential to detect qualitatively different behaviors that might yield identical automatically recorded data and can detect more subtle behavioral differences between groups than can automated methods (Antoniou et al. 1998). As an example, after 2.5 mg AMPH (Fig. 2) our female mice were more behaviorally activated than males based on observational data, but no significant effect of sex was found in the automatically-recorded ambulations.
The brain levels of AMPH and the degree of activation observed in the present study are similar to those reported in rats and other mouse strains. Our data at 45 minutes after 5 mg/kg AMPH shows that C57BL/6 mice have 755 ± 34 SEM ng/mL AMPH in serum and 4226 ± 147 ng/g tissue in the brain. A previous study in Sprague Dawley rats found that 40 min after 5 mg/kg AMPH, AMPH concentrations were ~540 ng/mL in plasma and ~5400 ng/g tissue in the striatum (Melega et al. 1995). Another study that utilized the same dose of AMPH but a later timepoint of 1 hour reported whole brain AMPH levels of 2400 ± 240 ng/g tissue in male Wistar rats and 3030 ± 380 ng/g tissue in Swiss albino mice (Danielson et al. 1977). The time course of locomotion after psychostimulants in the present study is similar to that reported for Sprague Dawley rats: 5 mg/kg AMPH stimulated behavior for more than two hours in the present study and in Sprague Dawley rats (Kelly and Iversen 1976; Kelly et al. 1975). Thus, our C57BL/6 mice have AMPH concentrations in the brain that fall within the range previously reported in rats and exhibit a similar time course of stimulated behavior after AMPH as rats.
The mouse results obtained in the present study differed from those reported in rats in one aspect: our mice exhibited a slightly different sequence of behaviors after psychostimulants. Sprague Dawley, Wistar, and Long-Evans rats exhibit increased rearing and patterned locomotion after low psychostimulant doses and progress to in-place stereotyped behaviors after high doses (Antoniou et al. 1998; Ellinwood and Balster 1974; Kelly and Iversen 1976; Kelly et al. 1975; Sahakian et al. 1975). In contrast, this study found that C57BL/6 mice exhibit in-place stereotyped behaviors after low psychostimulant doses and progress into patterned locomotion and then patterned rearing after high doses. Using a rat-based scale in the present study with mice would have yielded females with lower scores than males and the opposite conclusion that female mice are less responsive than males to psychostimulants (Ellinwood and Balster 1974; Quintero and Spano 2011; Quintero et al. 2008; Schlussman et al. 1998; Schlussman et al. 2003).
Female mice respond more than males to novelty and psychostimulants
The present results provide evidence that female C57BL/6 mice exhibit greater behavioral responses to both novel environments and psychostimulants than males. A novel environment stimulated more behavior in female mice than males as shown by a main effect of sex and a sex x time interaction during habituation, similar to previous findings in rats (Beatty 1979; Hughes 1968; Hughes et al. 2004) and mice (Adriani and Laviola 2000; Beatty 1979; Goodrich and Lange 1986; Gray 1971; Siuciak et al. 2007). Female mice in this study responded more than males to AMPH and COC, observations that parallel the enhanced responses of female rats to psychostimulants compared to males (Beatty and Holzer 1978; Brass and Glick 1981; Chelaru et al. 2012; Dafny and Yang 2006; Hu et al. 2004; Kuhn et al. 2001; Parylak et al. 2008; Robinson et al. 1980; Stohr et al. 1998; van Haaren and Meyer 1991; Walker et al. 2001; Walker et al. 2009). The sex differences in behaviors observed here suggest that sex differences in the effects of novelty and psychostimulants on the DA system exist in C57BL/6 mice (Creese and Iversen 1975; Hooks and Kalivas 1995; Kelly and Iversen 1976; Kelly et al. 1975).
The present study adds important information to a small and conflicting literature about sex differences in the psychostimulant-induced behavior of mice by demonstrating that adult female C57BL/6 mice are more behaviorally activated by a single dose or sequential ascending doses of either AMPH or COC. One previous study showed that late adolescent female CFW mice had a greater response than males to 5 mg/kg AMPH (Goodrich and Lange 1986). Two studies in mice generated on C57BL/6N or C57BL/6J backgrounds showed increased responses in adult female mice compared to males after 3.5 and 5 mg/kg AMPH (Siuciak et al. 2007; van den Buuse et al. 2012). In contrast, one study in adult CD1 mice did not report an effect of sex after 2 or 10 mg/kg AMPH (Adriani and Laviola 2000).
A similar inconsistency exists in the COC literature as previous studies of COC-induced locomotion in mice have reported no sex difference in C57BL/6J mice (Griffin and Middaugh 2006; Thomsen and Caine 2011), enhanced locomotion in C57BL/6ByJ females (Reith et al. 1991; Sershen et al. 1998), or enhanced locomotion in C57BL/6J males (Morse et al. 1993). Differences in animal husbandry and handling or in behavioral methods could underlie these conflicting results. The omission of chronic vaginal lavaging may be critical for detecting sex differences in the psychostimulant-induced behaviors of mice. Previous mouse studies that reported females being significantly more active than males after psychostimulants did not chronically lavage their females (Goodrich and Lange 1986; Reith et al. 1991; Sershen et al. 1998; Siuciak et al. 2007; van den Buuse et al. 2012) while one study that did lavage their C57BL/6J females did not detect a sex difference in COC-stimulated locomotion (Griffin and Middaugh 2006). Repeated vaginal lavaging attenuates COC-stimulated behaviors in female Sprague Dawley rats, which can eliminate sex differences in COC-induced behavior that are robust enough to be detected with randomly cycling females (Parylak et al. 2008; Walker et al. 2001; Walker et al. 2002). Direct experimental testing of the effects of chronic vaginal lavage on behavior in female mice may be warranted in future studies.
Prolonged habituation may also be an important parameter which permits detection of sex differences in psychostimulant-induced behaviors in strains of mice with high exploration of novel environments like the C57BL/6. Previous studies which reported a greater psychostimulant response in female mice also habituated their animals for at least 1 hour, while studies reporting no differences or greater responses in males utilized little or no habituation (compare (Reith et al. 1991; Sershen et al. 1998; Siuciak et al. 2007; van den Buuse et al. 2012) to (Adriani and Laviola 2000; Griffin and Middaugh 2006; Morse et al. 1993; Thomsen and Caine 2011)). The decrease in behaviors between the first and second hour of habituation shows that our mice required at least 2 hours to completely habituate to the novel environment in the present study, which parallels previous studies in C57BL/6 mice (Siuciak et al. 2007; Thomsen and Caine 2011). An extended habituation protocol reduces behavior to a similar, low baseline level of activity across individuals and enables automated methods to detect even modest increases and group differences. Prolonged habituation may also enhance the response to psychostimulants when compared to shorter habituation periods, as reported with AMPH in Fisher and Lewis rats (Stohr et al. 1998). The use of an extended habituation and the omission of repeated vaginal lavaging in combination with an observational rating scale tailored to mouse behavior likely enhanced our ability to detect group differences.
Another important consideration in the analysis of psychostimulant-induced behavior is the timeframe over which behavior is examined. A recent study of COC-induced behavior in mice did not report a sex difference when data was collected over 3 hours, which would include times during which COC is no longer stimulating behavior (Thomsen and Caine 2011). The present study demonstrates that the sex differences in psychostimulant-induced behavior are robust when the behavioral effects of the drugs are maximal, and that these sex differences are detectable at the time of peak brain AMPH and COC levels. Our study also indicates that C57BL/6 mice exhibit individual variability in their behavioral responses to psychostimulants. Previous studies may have been underpowered to detect sex differences amidst high variability between individual animals (Thomsen and Caine 2011).
The type of housing, mouse strain/line used, and time of day between studies have been shown to alter psychostimulant-induced behavior (Abarca et al. 2002; Baird and Gauvin 2000; Kuzmin and Johansson 2000; Morse et al. 1993; Thomsen and Caine 2011; Tolliver and Carney 1994) but do not appear to underlie the inconsistent results regarding sex differences in the mouse literature. Most previous mouse studies utilized group-housing and light phase testing yet obtained conflicting results, suggesting that those elements of the present experimental design did not influence the results. However, studies using individual housing and dark phase testing did not report an effect of sex in the response to AMPH or COC, suggesting again that environmental circumstances in which baseline activity is high will obscure sex differences (Adriani and Laviola 2000; Griffin and Middaugh 2006). Differences between strains of mice also do not explain the inconsistent findings as most of the literature tested C57BL/6 mice, and studies that used the same mouse strain and supplier still disagreed (compare (Griffin and Middaugh 2006; Thomsen and Caine 2011) to (Morse et al. 1993)). Some previous reports assessed behavior after similar doses of COC (i.e. 20–40 mg/kg) to that used in the present study (30 mg/kg), indicating that the discrepancies in the literature are not simply due to the use of different doses (Griffin and Middaugh 2006; Thomsen and Caine 2011).
Observed sex differences in psychostimulant-induced behavior are not due to differential metabolism of amphetamine or cocaine
The enhanced behavior of female mice after AMPH did not reflect greater brain AMPH levels in females, as has been reported in rats (Groppetti and Costa 1969; Meyer and Lytle 1978). The lack of a sex difference in brain COC levels in our mice during maximal COC behavioral effects resembles previous findings in rats, primates and humans (Bowman et al. 1999; Evans and Foltin 2010; Mendelson et al. 1999). To our knowledge, this is the first study to compare serum and brain levels of AMPH or COC and its metabolites in male and female mice at behaviorally relevant time points. A previous study in CF-1 mice reported slower plasma elimination of COC in males than in females after several days of COC administration (Visalli et al 2005). This sex difference may be due to greater activity of COC metabolizing enzymes in females than in males, which has been reported for C57BL/6 and DBA/2 but not BALB/cJ or C57BL/6 x A/J crossed mice (Leibman et al. 1990; Thompson et al. 1984). These findings further support our conclusion that female mice are more responsive to psychostimulants than males as females had more locomotion and higher behavior ratings despite having similar or even lower brain AMPH or COC levels during maximum behavioral activation, when the sex differences in psychostimulant-induced behavior were robust.
Conclusions
Novelty and psychostimulants increase both locomotion and behavior ratings more in female C57BL/6 mice than males. The sex differences in psychostimulant-induced behavior are not explained by pharmacokinetics but may be due to sex differences in the DA system. Relatively few studies have investigated this possibility in mice (Becker and Hu 2008; Di Paolo 1994; Griffin and Middaugh 2006; Kuppers et al. 2008; van den Buuse et al. 2012). These results in mice parallel previous reports of an increased sensitivity of female rats and women to psychostimulants and should also facilitate the use of mice in mechanistic studies of sex and hormone effects on psychostimulant-induced behavior.
Acknowledgments
Funding: This work was supported by the National Institute of Drug Abuse [Contract N01DA-9-7767 with Dr. Moody at the University of Utah and Grant DA09079 to Dr. Kuhn].
The authors thank the lab of Dr. David Moody at the Center for Human Toxicology, University of Utah for the assessment of psychostimulant concentrations. We also thank Dr. Nicole Schramm-Sapyta, Andrew Arrant, Suzanne Frisbee, Caroline Biskup, and Alex Jaeger for assistance with this study. All experiments in this study comply with current United States laws.
Footnotes
Conflict of interest: The authors have no conflicts to declare.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–30. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–46. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011;215:785–99. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–96. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav. 2000;65:289–99. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Horm Behav. 1979;12:112–63. doi: 10.1016/0018-506x(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Holzer GA. Sex differences in stereotyped behavior in the rat. Pharmacol Biochem Behav. 1978;9:777–83. doi: 10.1016/0091-3057(78)90356-8. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–64. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204:361–72. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Kuhn CM. Age-related differences in the chronic and acute response to cocaine in the rat. Dev Psychobiol. 1996;29:597–611. doi: 10.1002/(SICI)1098-2302(199611)29:7<597::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther. 1999;290:1316–23. [PubMed] [Google Scholar]
- Brass CA, Glick SD. Sex differences in drug-induced rotation in two strains of rats. Brain Res. 1981;223:229–34. doi: 10.1016/0006-8993(81)90830-1. [DOI] [PubMed] [Google Scholar]
- Chelaru MI, Yang PB, Dafny N. Sex differences in the behavioral response to methylphenidate in three adolescent rat strains (WKY, SHR, SD) Behav Brain Res. 2012;226:8–17. doi: 10.1016/j.bbr.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–36. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Danielson TJ, Davis BA, Boulton AA. Species variation with respect to the metabolism and excretion of d-amphetamine and d,l-N-hydroxyamphetamine succinate. Can J Physiol Pharmacol. 1977;55:439–43. doi: 10.1139/y77-062. [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–8. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Jr, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich C, Lange J. A differential sex effect of amphetamine on exploratory behavior in maturing mice. Physiol Behav. 1986;38:663–6. doi: 10.1016/0031-9384(86)90261-1. [DOI] [PubMed] [Google Scholar]
- Gray JA. Sex differences in emotional behaviour in mammals including man: endocrine bases. Acta Psychol (Amst) 1971;35:29–46. doi: 10.1016/0001-6918(71)90029-1. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. The influence of sex on extracellular dopamine and locomotor activity in C57BL/6J mice before and after acute cocaine challenge. Synapse. 2006;59:74–81. doi: 10.1002/syn.20218. [DOI] [PubMed] [Google Scholar]
- Groppetti A, Costa E. Factors affecting the rate of disappearance of amphetamine in rats. Int J Neuropharmacol. 1969;8:209–15. doi: 10.1016/0028-3908(69)90041-0. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. The role of mesoaccumbens--pallidal circuitry in novelty-induced behavioral activation. Neuroscience. 1995;64:587–97. doi: 10.1016/0306-4522(94)00409-x. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Behaviour of male and female rats with free choice of two environments differing in novelty. Anim Behav. 1968;16:92–6. doi: 10.1016/0003-3472(68)90116-4. [DOI] [PubMed] [Google Scholar]
- Hughes RN, Desmond CS, Fisher LC. Room novelty, sex, scopolamine and their interactions as determinants of general activity and rearing, and light-dark preferences in rats. Behav Processes. 2004;67:173–81. doi: 10.1016/j.beproc.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Day AE, Ho CC, Walker QD, Francis R, Kuhn CM. Androgen decreases dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010a;22:238–47. doi: 10.1111/j.1365-2826.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010b;22:226–37. doi: 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–22. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58:122–37. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CM, Walker QD, Kaplan KA, Li ST. Sex, steroids, and stimulant sensitivity. Ann N Y Acad Sci. 2001;937:188–201. doi: 10.1111/j.1749-6632.2001.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Kuppers E, Krust A, Chambon P, Beyer C. Functional alterations of the nigrostriatal dopamine system in estrogen receptor-alpha knockout (ERKO) mice. Psychoneuroendocrinology. 2008;33:832–8. doi: 10.1016/j.psyneuen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol Biochem Behav. 2000;65:399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Leibman D, Smolen A, Smolen TN. Strain, sex and developmental profiles of cocaine metabolizing enzymes in mice. Pharmacol Biochem Behav. 1990;37:161–5. doi: 10.1016/0091-3057(90)90057-o. [DOI] [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci. 2000;20:8604–9. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SN, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J Anal Toxicol. 2001;25:497–503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- Lin SN, Walsh SL, Moody DE, Foltz RL. Detection and time course of cocaine N-oxide and other cocaine metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Anal Chem. 2003;75:4335–40. doi: 10.1021/ac030037c. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–6. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Jr, Lytle LD. Sex related differences in the physiological disposition of amphetamine and its metabolites in the rat. Proc West Pharmacol Soc. 1978;21:313–6. [PubMed] [Google Scholar]
- Morse AC, Erwin VG, Jones BC. Strain and housing affect cocaine self-selection and open-field locomotor activity in mice. Pharmacol Biochem Behav. 1993;45:905–12. doi: 10.1016/0091-3057(93)90138-j. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–23. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero GC, Spano D. Exploration of sex differences in Rhes effects in dopamine mediated behaviors. Neuropsychiatr Dis Treat. 2011;7:697–706. doi: 10.2147/NDT.S25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero GC, Spano D, Lahoste GJ, Harrison LM. The Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. Neuroreport. 2008;19:1563–6. doi: 10.1097/WNR.0b013e3283118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Wiener HL, Fischette CT. Sertraline and cocaine-induced locomotion in mice. I. Acute studies. Psychopharmacology. 1991;103:297–305. doi: 10.1007/BF02244282. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Ramirez VD. Sex differences in amphetamine-elicited rotational behavior and the lateralization of striatal dopamine in rats. Brain Res Bull. 1980;5:539–45. doi: 10.1016/0361-9230(80)90260-9. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003a;120:523–33. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003b;970:214–20. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain Res. 1975;84:195–205. doi: 10.1016/0006-8993(75)90975-0. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 1998;60:593–9. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2003;75:123–31. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A. Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release. Brain Res. 1998;801:67–71. doi: 10.1016/s0006-8993(98)00546-0. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Reed TM, Vorhees CV, Repaske DR. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology. 2007;53:113–24. doi: 10.1016/j.neuropharm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Slawson MH, Taccogno JL, Foltz RL, Moody DE. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine, and amphetamine) in human plasma by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Anal Toxicol. 2002;26:430–7. doi: 10.1093/jat/26.7.430. [DOI] [PubMed] [Google Scholar]
- Stohr T, Schulte Wermeling D, Weiner I, Feldon J. Rat strain differences in open-field behavior and the locomotor stimulating and rewarding effects of amphetamine. Pharmacol Biochem Behav. 1998;59:813–8. doi: 10.1016/s0091-3057(97)00542-x. [DOI] [PubMed] [Google Scholar]
- Thompson ML, Shuster L, Casey E, Kanel GC. Sex and strain differences in response to cocaine. Biochem Pharmacol. 1984;33:1299–307. doi: 10.1016/0006-2952(84)90184-9. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–6. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol. 2011;19:321–41. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver BK, Carney JM. Sensitization to stereotypy in DBA/2J but not C57BL/6J mice with repeated cocaine. Pharmacol Biochem Behav. 1994;48:169–73. doi: 10.1016/0091-3057(94)90513-4. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Halley P, Hill R, Labots M, Martin S. Altered N-methyl-d-aspartate receptor function in reelin heterozygous mice: Male-female differences and comparison with dopaminergic activity. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.02.005. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–7. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–30. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Walker QD, Johnson ML, Van Swearingen AE, Arrant AE, Caster JM, Kuhn CM. Individual differences in psychostimulant responses of female rats are associated with ovarian hormones and dopamine neuroanatomy. Neuropharmacology. 2012;62:2266–76. doi: 10.1016/j.neuropharm.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–52. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Walker QD, Schramm-Sapyta NL, Caster JM, Waller ST, Brooks MP, Kuhn CM. Novelty-induced locomotion is positively associated with cocaine ingestion in adolescent rats; anxiety is correlated in adults. Pharmacol Biochem Behav. 2009;91:398–408. doi: 10.1016/j.pbb.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Williams CN, Jotwani RP, Waller ST, Francis R, Kuhn CM. Sex differences in the neurochemical and functional effects of MDMA in Sprague-Dawley rats. Psychopharmacology. 2007;189:435–45. doi: 10.1007/s00213-006-0531-z. [DOI] [PubMed] [Google Scholar]