Abstract
A method for the electrophoresis of intact bacteriophage T4D particles through polyacrylamide gels has been developed. It was found that phage particles will migrate through dilute polyacrylamide gels (less than 2.1%) in the presence of a low concentration of MgCl2. As few as 5 x 10(9) phage particles can be seen directly as a light-scattering band during the course of electrophoresis. The band can also be detected by scanning gels at 260 to 265 nm or by eluting viable phage particles from gel slices. A new mutant (eph1) has been identified on the basis of its decreased electrophoretic mobility compared with that of the wild type; mutant particles migrated 14% slower than the wild type particles at pH 8.3 and 35% slower at pH 5.0. The isoelectric points of both the wild type and eph1 mutant were found to be between pH 4.0 and 5.0. Particles of T4 with different head lengths were also studied. Petite particles (heads 20% shorter than normal) migrated at the same rate as normal-size particles. Giant particles, heterogenous with respect to head length (two to nine times normal), migrated faster than normal-size particles as a diffuse band. This diffuseness was due to separation within the band of particles having mobilities ranging from 8 to 35% faster than those of normal-size particles. These observations extend the useful range of polyacrylamide gel electrophoresis to include much larger particles than have previously been studied, including most viruses.
Full text
PDF



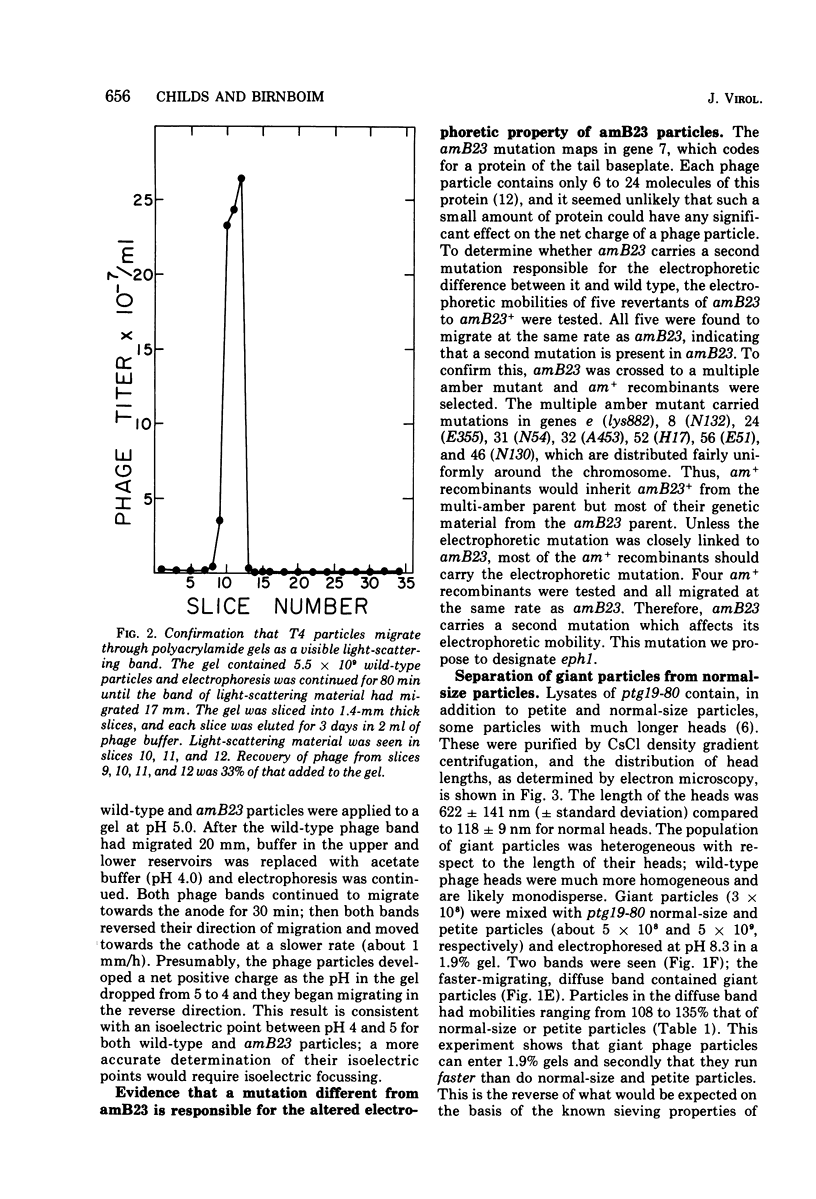
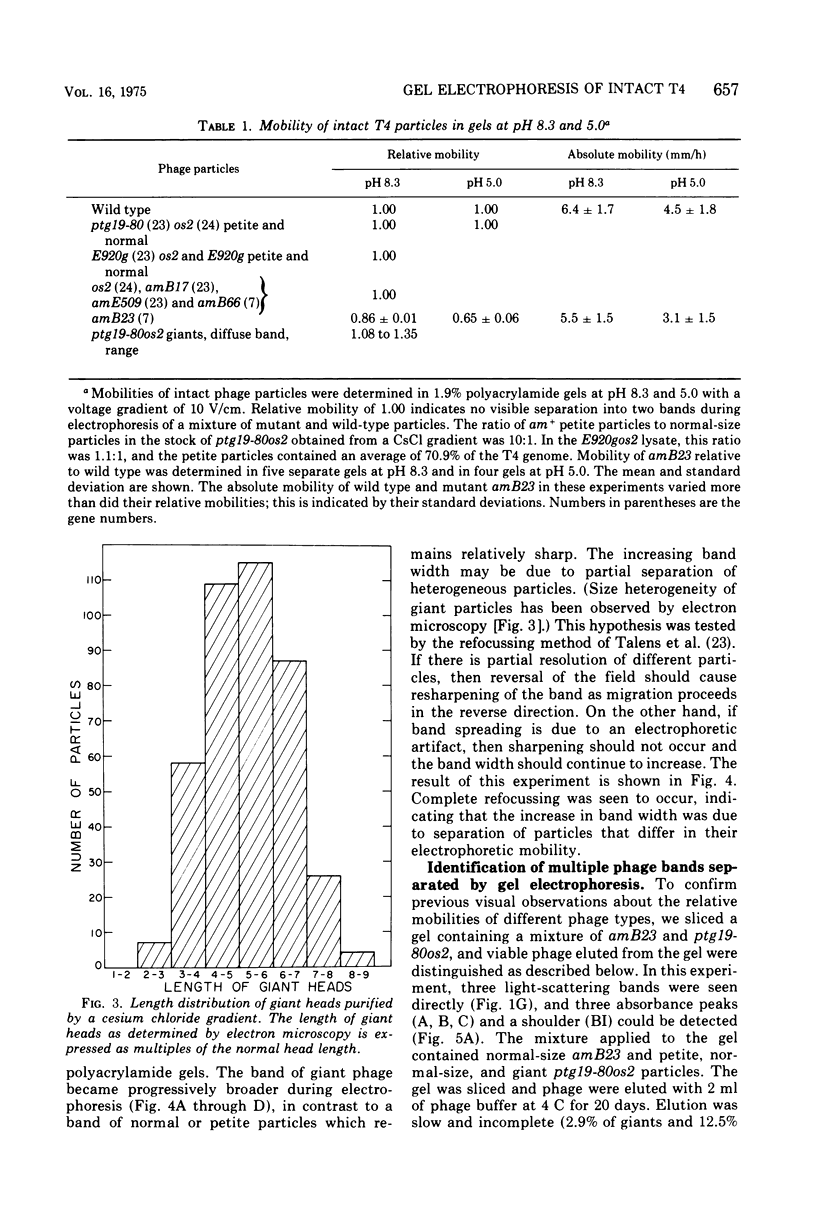

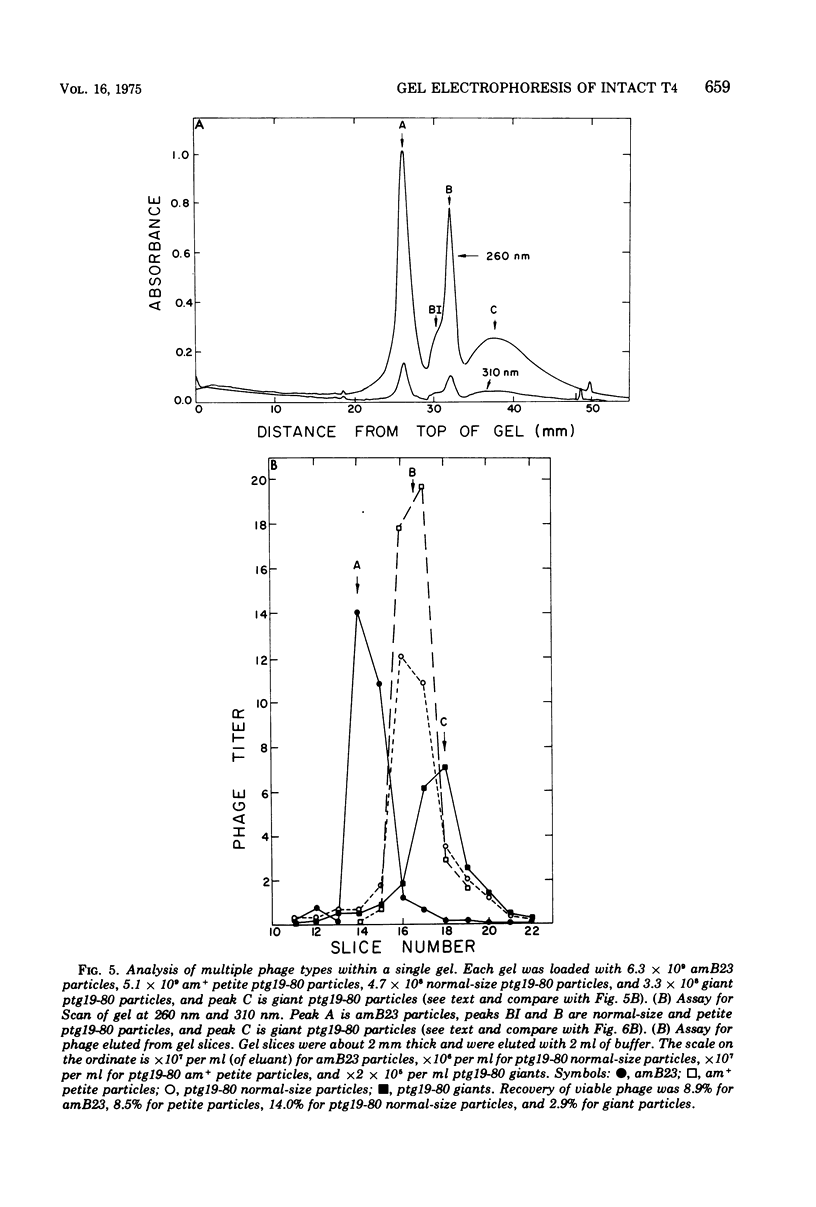


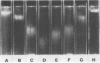
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudoin J., Henry T. J., Pratt D. Purification of single- and double-length M13 virions by polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):470–477. doi: 10.1128/jvi.13.2.470-477.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. Semiautomatic fractionation of dilute polyacrylamide gels. Anal Biochem. 1969 Jun;29(3):498–504. doi: 10.1016/0003-2697(69)90334-0. [DOI] [PubMed] [Google Scholar]
- Childs J. D. A map of molecular distances between mutations of bacteriophage T4D. Genetics. 1971 Apr;67(4):455–468. doi: 10.1093/genetics/67.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs J. D. Superinfection exclusion by incomplete genomes of bacteriophage T4. J Virol. 1973 Jan;11(1):1–8. doi: 10.1128/jvi.11.1.1-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S., Couse N. L. Structural aberrations in T-even bacteriophage. 3. Induction of "lollipops" and their partial characterization. Virology. 1973 Jul;54(1):245–261. doi: 10.1016/0042-6822(73)90134-7. [DOI] [PubMed] [Google Scholar]
- Doermann A. H., Eiserling F. A., Boehner L. Genetic control of capsid length in bacteriophage T4. I. Isolation and preliminary description of four new mutants. J Virol. 1973 Aug;12(2):374–385. doi: 10.1128/jvi.12.2.374-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin S. B., Benedek G. B., Bancroft F. C., Freifelder D. Molecular weights of coliphages and colip- hage DNA. II. Measurement of diffusion coefficients using optical mixing spectroscopy, and measurement of sedimentation coefficients. J Mol Biol. 1970 Dec 28;54(3):547–556. doi: 10.1016/0022-2836(70)90125-7. [DOI] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Edgell M. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XII. Phenotypic mixing between electrophoretic mutants of phi-X174. J Mol Biol. 1967 Feb 14;23(3):553–575. doi: 10.1016/s0022-2836(67)80125-6. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Bacteriophage T4 tail assembly: structural proteins and their genetic identification. J Mol Biol. 1973 Apr 5;75(2):315–337. doi: 10.1016/0022-2836(73)90024-7. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. A map of distances along the DNA molecule of phage T4. Genetics. 1968 Jun;59(2):137–151. doi: 10.1093/genetics/59.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G., Carnighan J. R., Bibring J. B., Cole R., Bock H. G., Bock S. Coordinate variation in lengths of deoxyribonucleic acid molecules and head lengths in morphological variants of bacteriophage T4. J Virol. 1972 May;9(5):857–871. doi: 10.1128/jvi.9.5.857-871.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma D. H. The structure of genomes of individual petit particles of the bacteriophage T4D mutant E920/96/41. Genetics. 1969 Oct;63(2):247–261. doi: 10.1093/genetics/63.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Horst J. Isoelectric focusing of viruses in polyacrylamide gels. Virology. 1972 Aug;49(2):602–604. doi: 10.1016/0042-6822(72)90512-0. [DOI] [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- Semancik J. S. Studies on electrophoretic heterogeneity in isometric plant viruses. Virology. 1966 Dec;30(4):698–704. doi: 10.1016/0042-6822(66)90174-7. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Kaesberg P. Acrylamide gel electrophoresis of bacteriophage Q beta: electrophoresis of the intact virions and of the viral proteins. Virology. 1970 Oct;42(2):437–452. doi: 10.1016/0042-6822(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Talens A., van Diggelen O. P., Brongers M., Popa L. M., Bosch L. Electrophoretic separation of Escherichia coli ribosomal particles on polyacrylamide gels. Eur J Biochem. 1973 Aug 1;37(1):121–133. doi: 10.1111/j.1432-1033.1973.tb02966.x. [DOI] [PubMed] [Google Scholar]
- Uhlenhopp E. L., Zimm B. H., Cummings D. J. Structural aberrations in T-even bacteriophage. VI. Molecular weight of DNA from giant heads. J Mol Biol. 1974 Nov 15;89(4):689–702. doi: 10.1016/0022-2836(74)90045-x. [DOI] [PubMed] [Google Scholar]