Abstract
Rationale
Negative emotional states during drug withdrawal may contribute to compulsive drug intake and seeking in humans. Studies suggest that extended access to methamphetamine induces compulsive drug intake in rats.
Objective
The present study tested the hypothesis that compulsive methamphetamine intake in rats with extended access is associated with negative emotional states during drug withdrawal.
Methods
Rats with short (1 h, ShA) and extended access (6 h, LgA) to methamphetamine self-administration (0.05 mg/kg/infusion) were tested for reward thresholds using intracranial self-stimulation (ICSS). Different groups of ShA and LgA rats were examined for depression-like and anxiety-like states in the novelty-suppressed feeding, open field, defensive burying, and forced swim tests.
Results
With extended access, ICSS thresholds gradually increased, which was correlated with the increase of drug intake. During drug withdrawal, the increased ICSS thresholds returned to levels observed before exposure to extended access to methamphetamine. Upon re-exposure to extended access to methamphetamine, ICSS thresholds showed a more rapid escalation than during the initial exposure. LgA rats showed a longer latency to approach chow in the center of a novel field and remained immobile longer in the forced swim test than ShA rats did during early withdrawal. In contrast, ShA rats actively buried an aversive shock probe whereas LgA rats remained immobile in the defensive burying test.
Conclusion
The data suggest that extended access to methamphetamine produces a more depressive-like state than anxiety-like state in rats during early withdrawal.
Keywords: Methamphetamine, Addiction, Withdrawal, Rats, Self-administration, Intracranial self-stimulation
Introduction
Addiction is a chronic relapsing disorder (Epstein et al. 2006; O’Brien 1997). One hypothesis underlying drug addition is that the development of negative emotional states with repeated drug use drives drug seeking and relapse to drug use leading to addiction in humans (Koob and Le Moal 1997). For example, methamphetamine abusers reported a significantly higher level of depression and anxiety than shown in control subjects (London et al. 2004; Thompson et al. 2004). Indeed, one of the most frequent determinants of relapse in compulsive drinking, heroin addiction, and binge eating was reported to be the presence of negative emotional states (Marlatt 1985). Therefore, characterizing reward function during methamphetamine withdrawal from compulsive drug taking within the context of an animal model of compulsive methamphetamine intake may help with understanding the neurobiology of drug addiction.
Over a decade ago, researchers established a rodent model of drug self-administration with extended access, which is suggested to mimic compulsive drug intake and seeking in dependent humans (Ahmed and Koob 1998; Ahmed et al. 2000; Kitamura et al. 2006). In this model, an increase in cocaine intake in rats with extended access was correlated with an increase of intracranial self-stimulation (ICSS) thresholds, suggesting a dysphoric-like state in rats with extended access (Ahmed et al. 2002). Additionally, rats with extended access to cocaine, compared to rats with short access, showed an increased anxiety-like state as measured in a defensive burying test during cocaine withdrawal (Aujla et al. 2008). Consistent with these findings, increased cocaine intake in rats with extended access was more sensitive to attenuation by blockade of corticotropin-releasing factor (CRF) type 1 receptors, compared with nonescalated cocaine intake in rats with short access (Specio et al. 2008), implying a relationship between increased cocaine intake with extended access and enhanced stress system function. Therefore, cocaine self-administration with extended access may induce negative emotional-like states in rats, which are associated with increases in drug intake.
National surveys indicate that approximately 13.0 million Americans aged 12 or older (5.1 % of the population) are reported to have used methamphetamine during their lifetimes (Substance Abuse and Mental Health Services Administration 2011) suggesting a persistent rate of methamphetamine abuse. Moreover, methamphetamine has consistently been the third popular substance of abuse for a decade in adolescents aged 12 to 17 compared with young adult nonusers. Methamphetamine not only has a high abuse potential but also engenders neurotoxicity in laboratory animals as well as in humans (Imam et al. 2001; Pasquali et al. 2008; Seiden et al. 1988).
Similar to cocaine, rats with extended access to methamphetamine exhibit increased methamphetamine intake with upward-shifted dose–response functions under fixed-ratio and progressive-ratio schedules, compared with rats under short access (Kitamura et al. 2006; Wee et al. 2007b), which suggests increased motivation for methamphetamine in rats with extended access and appears to support some face validity for compulsive drug intake and drug seeking by dependent humans. Based upon previous findings, therefore, our hypothesis is that the increase of methamphetamine intake in rats with extended access is associated with the development of negative emotional-like states during drug withdrawal This hypothesis was tested in the present study by measuring brain reward thresholds, behavior in the forced swim test, open-field behavior, novelty suppressed feeding, and the defensive burying response.
Methods
All animal use procedures were approved by The Scripps Research Institute Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines (NIH Publication No. 85-23, revised 1996).
ICSS experiment
Ten male Wistar rats (Charles River, Hollister, CA), each weighing between 225 and 250 g at the beginning of the study, served as subjects. Rats were housed in groups of two in cages with a reversed 12:12-h light/dark cycle with lights on at 8:00 p.m. Food and water were available ad libitum throughout the study. All experimental sessions were conducted during the dark (active) cycle. Rats were implanted with stainless steel bipolar electrodes (Plastics One® Inc., Roanoke, VA) into the lateral hypothalamus (AP, −0.5 mm; L, ±1.7 mm; DV, 8.3 mm from dura with the incisor bar set at 5 mm above interaural line). Half of the rats were implanted with electrodes in the right side of the brain, and the others in the left side of the brain.
ICSS sessions were held in operant chambers that were placed in light- and sound-attenuating cubicles (23×31× 24 cm; Med Associates Inc., St. Albans, VT). The chamber had a metal wheel manipulandum in one wall and a house light on the opposite wall. Rats were connected to the stimulation circuit via a bipolar lead and a gold contact swivel commutator. Brain stimulation was administered in 60-Hz sinusoidal waves with 200 ms of a train duration when a rat rotates a wheel at least a quarter turn.
ICSS was performed according to a modified procedure of the Kornetsky and Esposito discrete-trial current-threshold procedure (Kornetsky and Esposito 1979). At the start of each trial, a noncontingent electrical stimulus was administered to a rat, and the rat had 7.5 s to rotate the wheel at least a quarter turn to receive a contingent stimulus identical to the non-contingent stimulus. Each correct response was followed by an intertrial interval ranging 7.5 to 12.5 s. If no response occurred within 7.5 s after the noncontingent stimulus, the intertrial interval followed and that trial ended. Any responding during the intertrial interval resulted in a 10-s delay before the start of the next trial.
Each ICSS session was composed of four columns with a total of 50 trials. Three trials were presented at each current intensity. In the first column, current intensity decreased by 5 μA until a rat did not respond for at least two of three trials in two consecutive current intensities, and the column switched to the second where current intensity increased until the rat started to respond for more than two of three trials in two consecutive current intensities. This series repeated one more time, resulting in two ascending and two descending columns in each session. An ICSS threshold was calculated in each column as the midpoint between two consecutive current intensities that yielded “positive scores” (a rat responded for at least two of three trials) and two consecutive current intensities with “negative scores” (a rat did not respond for at least two of three trials). The overall ICSS threshold for a given session was calculated as the mean of the ICSS thresholds across four columns. Each session lasted approximately 30 min, and rats received two sessions per day, one in the morning and the other in the afternoon.
After the rats demonstrated reliable performance under a discrete-trial current-threshold procedure, the rats were implanted with indwelling intravenous catheters (0.3 mm ID×0.64 mm OD; Dow Corning Co. Midland, MI) into the right external jugular vein as described in a previous study (Wee et al. 2007a), and subsequently were trained to self-administer 0.05 mg/kg/infusion of methamphetamine in daily 1-h sessions under a fixed ratio (FR) 1 schedule of reinforcement, where one lever press resulted in a drug delivery.
Methamphetamine self-administration sessions were held in an operant chamber that was placed in a light- and sound-attenuating cubicle (28×26×20 cm; Med Associates Inc., St. Albans, VT). The chamber had two retractable response levers mounted on a sidewall. A stimulus light was mounted above each lever. At the start of a session, two response levers were presented into the chamber, and one response on the right lever resulted in the delivery of 0.1 ml of a drug solution over 4 s (fixed ratio 1 schedule). The stimulus light above the active lever was illuminated at the onset of each infusion and remained illuminated throughout the time-out period (20 s) during which responses were recorded without consequence. The offset of the cue light signaled the availability of the next infusion. This has been our standard intravenous self-administration procedure (Roberts et al. 1980). Pressing the left (inactive) lever was counted but had no other programmed consequences. The session ended by the withdrawal of the levers from the chamber.
The experimental design was within-subjects, with rats first tested with 1-h access (ShA) to methamphetamine and subsequently with 6-h access (LgA) to methamphetamine (see Fig. 1). After four initial 1-h methamphetamine self-administration sessions, the rats entered a test phase where they received an ICSS session 1 h before (22 h of methamphetamine withdrawal) and 3 h after each methamphetamine self-administration session for a total of 11 days. After the final determination of ICSS thresholds in rats with 1-h access to methamphetamine, the session duration for methamphetamine self-administration was extended to 6 h. ICSS thresholds in this phase were measured daily 1 h before (17 h of methamphetamine withdrawal) and 3 h after each 6-h methamphetamine self-administration session, again for a total of 11 days. After the final determination of ICSS thresholds in this 6-h access phase, rats were withdrawn from drug self-administration for 37 days, with ICSS thresholds measured once daily during this forced abstinence phase. Finally, after 37 days of abstinence the rats were re-exposed to 6-h access to methamphetamine self-administration for an additional 11 days, with ICSS thresholds measured 1 h before onset of drug self-administration on each day.
Fig. 1.
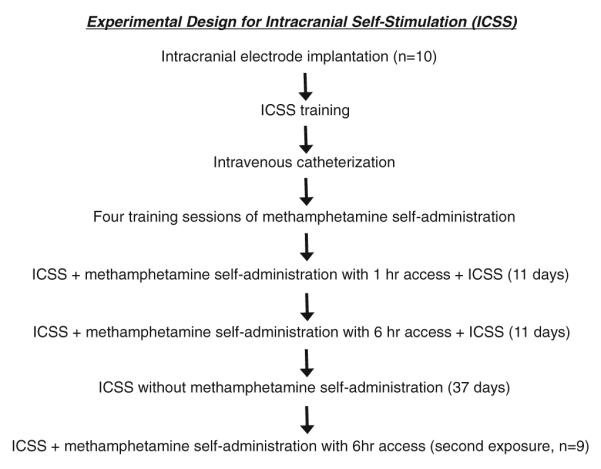
Experimental design for ICSS. Ten male Wistar rats were allowed to receive ICSS and to self-administer methamphetamine (0.05 mg/kg/injection) with 1-h access and then 6-h access and were withdrawn from methamphetamine for 37 days during which the rats were only allowed to receive ICSS. During the second exposure to methamphetamine self-administration with extended access, one rat was excluded because of a catheter failure
Novelty-suppressed feeding and open-field experiments
This novelty suppressed feeding test was shown to be sensitive to anxiolytic drugs in rats (Britton and Britton 1981). Additionally, this test was sensitive to chronic (but not acute) treatment with antidepressants (Bodnoff et al. 1988) and there has been reported a significant correlation in measurements between depression tests, such as forced swim and sucrose consumption, and novelty-suppressed feeding (Bessa et al. 2009). Accordingly, we hypothesized that the novelty-suppressed feeding test might reflect depressive-like states in rats, perhaps in addition to novelty-induced anxiety, during methamphetamine withdrawal.
A separate cohort of rats (ShA, n=11; LgA, n=11) was used for these experiments. The rats (n=22) were implanted with indwelling intravenous catheters and were trained to self-administer 0.05 mg/kg/infusion of methamphetamine under 1-h access for 10–13 sessions. After establishing stable self-administration, the rats were divided into two groups balanced by total infusions of methamphetamine in the final training session. One group of rats (n=11) was allowed to self-administer methamphetamine with 6-h access (LgA) whereas the other group (n=11) was maintained with daily 1-h access (ShA). The rats self-administered methamphetamine under either 1- or 6-h access for 57 days, during which they were tested with capsazepine in a Latin square design (1, 2.5, and 5 mg/kg), a TRPV1 receptor antagonist on methamphetamine self-administration (unpublished). Also, capsazepine (10 mg/kg) did not alter open-field behaviors in rats and had no effect on depressive-like behaviors in the forced swim test as well as in the tail suspension test in rats (Panlilio et al. 2009; Hayase 2011). Therefore, all rats had equivalent drug history upon being entered into the current study, and it does not appear that the previous exposure of the rats to acute capsazepine could have interfered with the present outcome (Hayase 2011).
For purposes of the present study, these rats were tested after 1 or 14 days withdrawal from methamphetamine self-administration. The rats were food deprived and tested for anxiety-like behaviors in open fields and for the novelty-suppressed feeding as previously described (Dulawa and Hen 2005). In detail, food was removed from the cage at each withdrawal time point, and 23 h later, the rat was placed in the center of an open field (80×80×50 cm) with a dim illumination for 5 min. The floor of the apparatus had a grid drawn on it so that line crossings and time spent in each of the 24 squares could be determined. On the following day of the open-field test, a small piece of familiar chow was placed in the center of a novel arena. At the start of the experiment, each animal was placed in the corner of the novel arena, and the time to approach and start to feed was determined as latency to feed. Cutoff time was 6 min. Immediately after the animal began to eat, the subject was transferred to a clean housing cage where preweighed chow was placed and remained there for 5 min. At the end of the 5-min period, the amount of food consumed was measured, and the animal was returned to its home cage.
Defensive burying experiment
A new cohort of male Wistar rats (n=24, Charles River) served as subjects. All the conditions for housing is as described above in the ICSS experiment. Rats were implanted with indwelling intravenous catheters into the right external jugular vein as described in a previous study (Wee et al. 2007a) and were trained to self-administer 0.05 mg/kg/infusion of methamphetamine in daily 1-h sessions under a fixed ratio 1 schedule of reinforcement. After 11 sessions, they were divided into two groups balanced by the mean number of methamphetamine injections in the last two sessions. One group of the rats (n=12) was allowed to self-administer methamphetamine with 6-h access per day while the other group (n=12) was kept at 1-h access. After 18 sessions of methamphetamine self-administration with extended access, the animals were subjected to a defensive burying test at 1-day withdrawal time point. Testing was performed in a clear Plexiglas housing cage (43×20×20 cm) which was evenly covered with sawdust (5 cm height). A Teflon probe (6.5× 1 cm diameter), wrapped with two wires, was inserted through a hole at 2 cm above the even surface of the sawdust, in the center of the narrow side of the cage. Electric shock (0.5 mA) was generated by a shock generator scrambler (Staco Energy Products Dayton, Ohio, USA) and delivered through the wires of the probe whenever the animal touched with a forepaw or the snout to both wires simultaneously. Each session lasted 15 min. Between animals, the probe was cleaned with alcohol, and the sawdust was changed. Five LgA rats were excluded from the study before being tested in the defensive burying due to compromised health issues.
Forced swim test
A new cohort of male Wistar rats (n=12, Charles River) were implanted with indwelling intravenous catheters and were trained to self-administer 0.05 mg/kg/infusion of methamphetamine in daily 1-h sessions under a fixed ratio 1 schedule of reinforcement. After 15 sessions, they were divided into two groups balanced by the mean number of methamphetamine injections in the last two sessions. One group of the rats (n=6) was allowed to self-administer methamphetamine with 6-h access per day while the other group (n=6) was kept at 1-h access. On the following day after 13 sessions of methamphetamine self-administration with extended access, the animals were subjected to a forced swim test. A modified 2-day procedure of Porsolt forced swim test was used. On the first day, rats were placed in a clear Plexiglas cylinder (25 cm diameter×61 cm height) that was filled with water (22–24 °C, 45 cm depth) for 15 min. On the secondday, the rats were placed in the same condition for 5 min. After each session, rats were towel dried and returned to their home cage. Swimming behaviors were videotaped throughout the session. Two LgA rats were excluded from the study before being tested in the defensive burying due to health issues.
Data analysis
Prism 5 (GraphPad Software Inc.) was used for all data analysis.
Effect of methamphetamine self-administration on ICSS thresholds
ICSS thresholds, measured before and after each methamphetamine self-administration session, were expressed as percentage of the baseline ICSS threshold that was determined 1 day before the first methamphetamine self-administration session of each access condition. After log transformation of the data, changes in ICSS thresholds during methamphet-amine self-administration were examined using repeated measures two-way ANOVA (access condition×daily session) with Bonferroni post hoc tests as dictated by the outcome of the overall ANOVA. Additionally, methamphetamine intake data also were log transformed and examined using repeated measures two-way ANOVA (access×daily session) with Bonferroni post hoc test after data were transformed to log values. The percentage of the baseline ICSS threshold and methamphetamine intake were log transformed only for statistical analysis to homogenize error variance between ShA and LgA groups. Correlations between methamphetamine self-administration and ICSS thresholds were determined using Pearson’s product moment correlation.
Effect of prolonged methamphetamine withdrawal on ICSS thresholds
ICSS data on all methamphetamine withdrawal days were expressed as percent of the ICSS threshold determined 1 h before the first methamphetamine self-administration session with 6-h access. Changes in ICSS thresholds during prolonged withdrawal were examined in comparison to the ICSS thresholds 1 h before the first methamphetamine self-administration with 6-h access using repeated measures oneway ANOVA with Dunnett’s post hoc test after the data were transformed to log values. With respect to methamphetamine self-administration and ICSS thresholds during re-exposure to extended access methamphetamine after protracted withdrawal, increases in methamphetamine intake and in ICSS thresholds over daily sessions were examined using repeated measures one-way ANOVA with Dunnett’s multiple comparison post hoc test. The rates of increase in ICSS thresholds between the first and second exposures to methamphetamine self-administration with 6-h access were compared using nonlinear regression analysis by comparing ED50 sessions, the number of sessions that took to reach half of the maximum increase in ICSS thresholds.
Effect of methamphetamine withdrawal on novelty-induced suppression of feeding and open-field test
In novelty-induced suppression of feeding, data were expressed as latency to onset of feeding and total amount of food consumed. In open-field test, data were expressed as percent of the time spent in center, and center crossing counts. Significant difference between the ShA and LgA groups was analyzed by Student’s t test. Additionally, the effect of withdrawal time point on the dependent variables was examined using two-way ANOVA (access×withdrawal time point).
Defensive burying experiment
Methamphetamine self-administration in ShA and LgA rats is expressed as the number of injections/session as well as milligrams per kilogram per session. The latency to bury was defined as the period between the time of receiving an electric shock and the time of exhibiting the first burying behavior. Duration of burying was determined by adding the time that an animal spent in burying during an entire session. After the session ended, the highest height of the bedding over the electric probe was measured. Latency to touch the probe as soon as an animal was placed in a chamber was also measured. Additionally, we measured the duration of being immobile after rats received am electric shock until they started burying the probe. Specifically, the immobility was defined as being still in a position for the minimum of 2 s without lying, sniffing, or exploring. Self-administration data were analyzed using repeated measures two-way ANOVA (access×daily session) with Bonferroni post hoc tests. Defensive burying behaviors were analyzed by Student’s t test. The data were transformed when variances differed between the groups.
Forced swim test
Methamphetamine self-administration in ShA and LgA rats is expressed as the number of injections/session as well as milligrams per kilogram per session and were analyzed using repeated measures two-way ANOVA (access×daily session) with Bonferroni post hoc tests. Forced swimming behaviors on the second test session were subjected to analysis. Specifically, latency to be immobile and the duration of immobility were scored twice by a trained person under the condition of being blind to an experimental treatment, and the mean values were used for a comparison between ShA and LgA rats using Student’s t test. Immobility was noted and was measured when an animal floated with the minimum movements of the hind limbs to maintain floating at least over 2 s. The data were transformed when variances differed between the groups.
Results
Effect of methamphetamine self-administration on ICSS thresholds
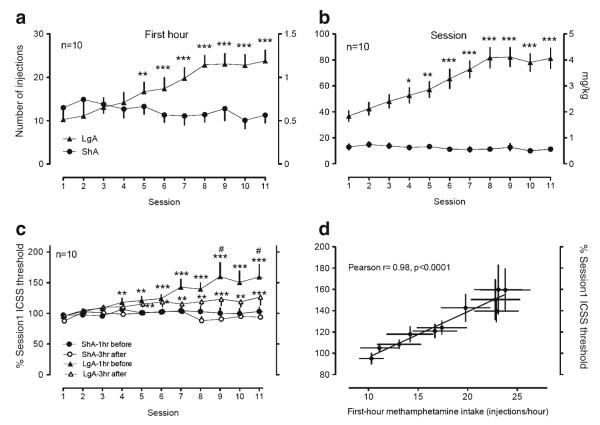
Methamphetamine self-administration significantly increased over sessions with 6-h access (LgA) compared with 1-h access (ShA) [Fig. 2a, b; session data: access×session interaction F(10, 180)=6.75, p<0.001; main effect access F(1, 180)=109, p<0.001; main effect session F(10, 180)= 2.67, p<0.01; first-hour data: access×session interaction F(10, 180)=9.61, p<0.001; main effect access F(1, 180)= 4.96, p<0.05; main effect session F(10, 180)=4.22, p< 0.001]. Similarly, ICSS thresholds significantly increased in LgA compared to ShA conditions [Fig. 2c, 1 h before (22 h withdrawal): access×session interaction F(10, 180)=4.36, p< 0.001; main effect access F(1, 180)=11.5, p<0.01; main effect session F(10, 180)=5.54, p<0.001; 3 h after: access×session interaction F(10, 180)=2.84, p<0.01, access F(1, 180)=7.70, p<0.05, session F(10, 180)=2.66, p<0.01]. Additionally, ICSS thresholds measured 1 h before a self-administration session were significantly higher than those measured 3 h after the session in LgA rats in days 9 and 11 [time×session interaction F(10, 99)=1.9, p=0.059; main effect time F(1, 99)=21, p<0.001; main effect session F(10, 99)=3.0, p<0.01]. Methamphetamine intake with 6-h access was strongly correlated with ICSS threshold measured at 1 h before and 3 h after each self-administration session (Fig. 2d, correlation between ICSS thresholds 1 h before and first-hour intake: Pearson r= 0.980, p<0.0001; session intake: Pearson r=0.982, p<0.0001; correlation between ICSS thresholds 3 h after and first-hour intake, data not shown: Pearson r=0.939, p<0.0001; session intake: Pearson r=0.941, p<0.0001).
Fig. 2.
Methamphetamine self-administration and ICSS by rats. Rats were daily allowed to receive ICSS at the lateral hypothalamus 1 h before and 3 h after intravenous methamphetamine self-administration with either 1- or 6-h access. The upper panels represent the data of methamphetamine self-administration during the first hour of each session (a) or during a whole session (b). c The data of ICSS measured 1 h before and 3 h after methamphetamine self-administration. d The correlation between the first-hour methamphetamine intake and the percent change of ICSS thresholds in rats with 6-h access. When error bars do not appear, they are within the symbols. *p<0.05, **p<0.01, ***p<0.001 compared with session 1. #p<0.05 compared with LgA 3 h after
Effect of prolonged methamphetamine withdrawal on ICSS thresholds
The increased ICSS thresholds in LgA conditions remained significantly elevated for at least 7 days after the cessation of methamphetamine self-administration [Fig. 3, F(10, 109)=5.18, p<0.001]. However, with protracted withdrawal, ICSS thresholds in rats returned toward the baseline ICSS thresholds before the reexposure to 6-h access to methamphetamine.
Fig. 3.
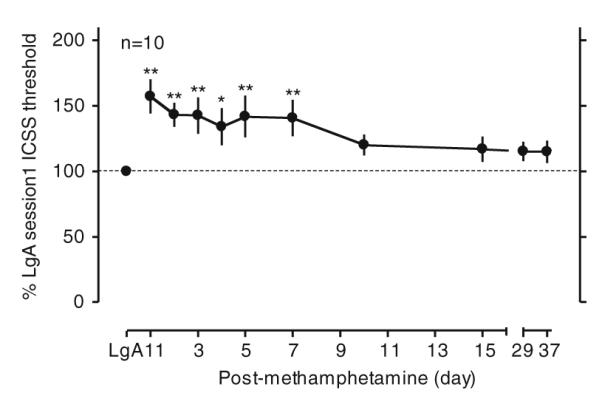
ICSS in rats during protracted withdrawal from methamphetamine self-administration with 6-h access. The data are expressed as the percentage of the ICSS threshold measured 1 h before the first exposure to 6-h access of methamphetamine self-administration (LgA session1 ICSS threshold). After 11 days of methamphetamine self-administration with 6-h access, rats were withdrawn from methamphetamine self-administration and allowed to receive ICSS once a day. *p<0.05, **p<0.01 compared with LgA session1 ICSS threshold
Effect of re-exposure to extended access to methamphetamine self-administration on ICSS thresholds
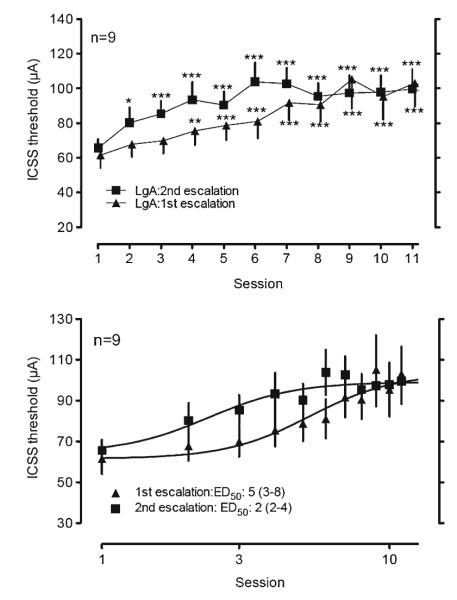
When re-exposed to 6-h access to methamphetamine self-administration, ICSS thresholds that had returned to the baseline with protracted withdrawal again increased in the rats [Fig. 4 top, repeated measure one-way ANOVA, F(10, 98)=15.5, p<0.001]. When compared between the first and the second exposure to 6-h access to methamphetamine, the rate of increase in ICSS thresholds was significantly faster during the second exposure to extended access to methamphetamine than during the first one (Fig. 4 bottom, nonlinear fit Log ED50 comparison, p<0.05).
Fig. 4.
Re-escalation of ICSS thresholds in rats upon re-exposure to methamphetamine self-administration with 6-h access. After 37 days of drug abstinence, rats were again allowed to self-administer methamphetamine with 6-h access for 11 days. ICSS thresholds were measured 1 h before daily methamphetamine self-administration. The lower panel represents the data of the ED50 sessions that it took to reach a half of the maximum increase of ICSS thresholds in rats with 6-h access to methamphetamine using nonlinear regression analysis. The numbers in parenthesis indicate 95 % confidence intervals. *p<0.05, **p<0.01, ***p<0.001 compared with session 1
Open-field and novelty-suppressed feeding tests
When measured 1 day after the last methamphetamine self-administration, LgA rats tended to cross the center of an open field less frequently than ShA rats (Table 1). However, there was no statistically significant difference between ShA and LgA groups in any measurement. At 14 days of withdrawal, both ShA and LgA rats showed no difference in the open-field behaviors. However, both groups spent significantly less time in the center on the 14th day of withdrawal than on the 1st day of withdrawal (two-way ANOVA, the main effect of withdrawal time point, F(1, 40)=18, p<0.001).
Table 1.
Open-field behaviors during early withdrawal from methamphetamine self- administration in rats with short or extended access
| Withdrawal | Parameters | ShA (n=11) | LgA (n=11) | |
|---|---|---|---|---|
| 1 day | Center crossing (% of total crossings) | 14.5±3.3 | 8.7±4.8 | p=0.06 |
| Time spent in center (%) | 8.7±1.7 | 7.1±1.7 | ns | |
| Total crossing (counts) | 107.3±15.5 | 124.1±9.8 | ns | |
| 14 days | Center crossing (% of total crossings) | 9.1±2.0 | 6.5±1.5 | ns |
| Time spent in center (%) | 3.0±0.8 | 2.2±0.6 | ns | |
| Total crossing (counts) | 90.8±14.5 | 75.6±9.3 | ns |
ns not significant
At 2-day withdrawal, LgA rats showed a greater latency to approach a food pellet than ShA rats (Table 2). However, the amount of food consumed in a cage after the test session did not differ between the two groups. At 15 days withdrawal, there was no difference in latency to approach a food pellet or the amount of food consumption between the two groups. Additionally, there was no difference in the amount of food consumption between the 2- and 15-day withdrawal time points within each group.
Table 2.
Novelty-suppressed feeding during withdrawal from methamphetamine self- administration in rats with short or extended access
| Withdrawal | Parameters | ShA (n=11) | LgA (n=8) | |
|---|---|---|---|---|
| 2 days | Latency to feed (s) | 245.8±16.1 | 315.5±21.8 | p<0.05 |
| Food consumption (mg/kg) | 3.6±0.3 | 3.2±0.5 | ns | |
| 15 days | Latency to feed (s) | 287.3±30.8 | 270.5±37.3 | ns |
| Food consumption (mg/kg) | 3.6±0.2 | 3.2±0.4 | ns |
ns not significant
Defensive burying experiment
LgA rats in this experiment also showed an increase in methamphetamine self-administration over sessions compared with ShA rats [access×session interaction F(17, 289)=3.2, p<0.001; main effect of access F(1, 289)=134, p<0.001; main effect of session F(17, 289)=3.0, p<0.001, data not shown]. When exposed to an electric shock at 1-day withdrawal, LgA rats were immobile for a longer time than ShA rats after they received an electric shock (Table 3). However, ShA rats spent a significantly more time in burying a probe and produced a higher mount of bedding over the probe than the LgA rats.
Table 3.
Defensive burying behaviors during early withdrawal from methamphetamine self-administration in rats with short or extended access
| Parameters | ShA (n=12) | LgA (n=7) | |
|---|---|---|---|
| Latency to touch the probe (s) |
25.5±8.2 | 52.3±18.5 | ns |
| Immobility after the shock (s) | 6.4±2.7 | 19.3±9.0 | p<0.05 |
| Latency to bury after the shock (s) |
60.3±14.2 | 104.6±30.9 | ns |
| Duration of burying (s) | 149.4±22.3 | 95.1±13.9 | p<0.05 |
| Height of the bedding (cm) | 11.7±0.6 | 9.3±0.7 | p<0.01 |
ns not significant
Forced swim test
LgA rats in this experiment also showed an increase of methamphetamine intake over 13 sessions compared with ShA rats [access×session interaction F(12, 96)=2.0, p< 0.05; main effect of access F(1, 96)=36.8, p<0.001; main effect of session F(12, 96)=1.7, p>0.05, data not shown]. Additionally, LgA rats showed longer immobility times in water than ShA rats (Table 4).
Table 4.
Forced swimming behaviors during early withdrawal from methamphetamine self-administration in rats with short or extended access
| Parameters | ShA (n=6) | LgA (n=4) | |
|---|---|---|---|
| Duration of immobility (s) | 149.6±33.6 | 260.0±29.6 | p<0.05 |
| Latency to immobility (s) | 87.9±39.4 | 52.0±5.9 | ns |
ns not significant
Discussion
In the present study, extended access (LgA) to methamphetamine produced an increase of methamphetamine intake in rats, consistent with previous findings (Kitamura et al. 2006; Wee et al. 2007b). Although the dose–response function of methamphetamine was not measured here, it was previously shown that increased methamphetamine intake with extended access under an FR schedule was accompanied by an upward-shifted dose–response function of methamphetamine under a progressive ratio schedule in rats (Wee et al. 2007b), which suggested increased motivation to self-administer the drug. Therefore, this rodent model of methamphetamine self-administration with extended access may have face validity for compulsive drug intake and seeking in humans with methamphetamine addiction.
Similar to the finding with cocaine (Ahmed et al. 2002), the increase of methamphetamine intake was correlated with an increase in ICSS thresholds. Electrical stimulation of brain regions such as the lateral hypothalamus or ventral tegmental area consistently supports responding for ICSS in rats (Esposito et al. 1978; Olds and Milner 1954), implying activation of reward systems at those regions. Moreover, acute administration of drugs of abuse decreased current thresholds for ICSS in rats, suggesting a relationship between increased sensitivity to brain stimulation and the rewarding effects of the drugs (Kornetsky and Esposito 1979). In contrast, chronic mild stress that induced a depressive-like state in animals (Willner 1997) and acute administration of the stress peptide CRF both increased ICSS thresholds in rats (Macey et al. 2000; Moreau et al. 1995), suggesting a relationship between decreased rewarding efficacy of brain stimulation and a dysphoric-like states in rats. Accordingly, the present data suggest that extended access to methamphetamine induced a dysphoric-like state in rats, which was correlated with the increase in methamphetamine intake. Indeed, prolonged exposure to cocaine, ethanol, nicotine, and morphine have also been shown to produce an increase in ICSS thresholds in rats during withdrawal, suggesting that dysphoric-like symptoms are common to withdrawal from multiple classes of abused drugs (Epping-Jordan et al. 1998; Kenny et al. 2006; Markou and Koob 1991; Schulteis et al. 1995). In the present study, ICSS thresholds measured 1 h before a self-administration session were significantly higher than those measured 3 h after the session in LgA rats in days 9 and 11. This suggests that methamphetamine still produced the acutely decreasing effect of ICSS thresholds in LgA rats. Nevertheless, the drug was not able to bring ICSS thresholds back to the previous baseline level. This observation suggests that repeated methamphetamine use in humans at this point in binge would not produce a positive hedonic effect possibly reflecting tolerance, but rather would contribute to allostatic-like increases in reward threshold hypothesized to occur in addiction (Koob and Le Moal 2001).
Neither ICSS thresholds nor methamphetamine intake was altered in the rats over 11 days of 1-h access to methamphetamine. In this rodent model of cocaine self-administration with extended access, it was hypothesized that a constant rate of drug intake with short access might represent a nondependent, more “recreational” pattern of drug use, in contrast to compulsive drug use in addiction that is commonly modeled in rodents with extended drug access. The present findings appear to support the hypothesis that compulsive methamphetamine intake in addiction is associated with a negative emotional state during drug withdrawal, which is not observed in animals with 1-h access.
When rats were withdrawn from methamphetamine self-administration with extended access, increased ICSS thresholds remained elevated in the rats for a week, but gradually returned toward the level prior to the exposure to extended access to methamphetamine. The gradual reversal suggests that the neuroadaptations that underlie the development of dysphoric-like states in rats with extended access to methamphetamine are reversible with protracted withdrawal. Upon re-exposure to methamphetamine with extended access, however, ICSS thresholds re-escalated rapidly, with significant increases seen by the second day of re-exposure (Fig. 4), and the rate of increase in ICSS thresholds was significantly faster during the second exposure to methamphetamine than during the first exposure. The rapid reescalation in ICSS threshold suggests that although neuroadaptations underlying the increase of ICSS thresholds were reversed with protracted withdrawal, there were residual changes that contributed to a faster increase in ICSS thresholds during the second exposure to extended access to methamphetamine in rats.
Additionally, we examined depressive-like behaviors of rats in novelty-induced suppression of feeding and in a forced swim test. As noted earlier, novelty-induced suppression of feeding has been shown to be sensitive to the effects of anxiolytics on chow consumption in an open field by food-deprived rats (Britton and Britton 1981), suggesting the measurement of anxiety-like states in animals. However, later studies (Bessa et al. 2009; Bodnoff et al. 1988) suggested that this test has also predictive validity for antidepressants. In the present study, LgA rats showed a longer latency to approach a food pellet in a novel environment than ShA rats at an early withdrawal time point. In contrast, there was no significant difference in total food consumed in a cage measured right after the test. This suggests that the LgA rats developed depressive-like states compared with ShA rats during early withdrawal, without changes in food consummatory behavior, consistent with the findings in ICSS. At 15 days of withdrawal, however, the ShA and LgA groups of rats did not differ significantly in latency to approach a food pellet or in food consumption. This result suggests that depressive-like states manifest during early withdrawal rather than during protracted withdrawal from methamphetamine, and is consistent with the finding that ICSS thresholds had returned to baseline prior to 15 days of abstinence. The data in the forced swim test further support the development of depression-like states in LgA rats during early withdrawal from methamphetamine.
One concern with the present study is the interaction of the well-documented anorexigenic effect of methamphetamine with novelty-suppressed feeding in LgA rats during early withdrawal. The rats in the present study were food deprived for a day before being tested on the second day of withdrawal from methamphetamine, which should have allowed enough time for clearance of methamphetamine from the system and enhanced motivation for food. In novelty-suppressed feeding, animals are tested for their food consumption during a 5-min period upon returning to their home cage to see whether the difference in latency was due to differences in hunger (Snyder et al. 2011). In the present study, there was no difference in the amount of food consumption between the 2- and 15-day withdrawal points within each group or between ShA and LgA groups at each withdrawal time. Therefore, the results do not appear to have been compromised by the anorexic effect of methamphetamine.
In an open-field test, LgA rats showed a strong tendency of decreased frequency to cross the center compared with short-access rats during early withdrawal, which appears to be consistent with the longer latency to approach chow in the center of a novel environment by LgA rats in the novelty-suppressed feeding test. However, there was no statistically significant difference in any open-field measurement between the groups. It was reported that rats with extended access to cocaine self-administration showed sensitized anxiety-like responses in defensive burying during withdrawal, compared with drug-naïve and short-access groups, which lasted for 42 days (Aujla et al. 2008). In a defensive burying paradigm, an animal is exposed to a stressor, an electric shock. Therefore, we hypothesized that rats with extended access to methamphetamine would show anxiety-like behaviors only as measured by increased sensitivity to a stressor. The present results, however, suggest that ShA rats, compared with LgA rats, developed anxietylike states in the presence of a stressor. One possible explanation for this finding might be that the LgA rats became too passive from a depressive-like state to actively cope with the presence of a stressor, see forced swim results.
The focus of the present study was on finding evidence of negative emotional-like states during drug withdrawal in rats with extended access to methamphetamine. Therefore, we did not explore the involvement of any specific neural system in the observed behaviors. We previously reported that chronic daily methamphetamine self-administration (49 days) decreased the basal level of plasma corticosterone in rats with both short and extended access compared with drug-naïve rats (Mandyam et al. 2008). This finding is consistent with that of rats with extended access to cocaine (Mantsch et al. 2003, 2007) and that of alcohol-dependent rats with a low level of plasma corticosterone level compared with nondependent rats (Richardson et al. 2008). In contrast, we have found the increased basal level of CRF peptides in the basolateral amygdala and the dorsal raphe nucleus, but not in the central nucleus of amygdala, of LgA cocaine rats compared with ShA cocaine rats (Zorrilla et al. 2011), which are brain regions implicated in the mediation of different aspects of emotional responses (Bruchas et al. 2009, 2011; Land et al. 2009). These observations suggest that, with prolonged exposure to drugs, the HPA activity of animals is blunted while the central CRF system becomes sensitized, which we hypothesis to mediate some of the negative emotional responses during drug withdrawal. Future studies investigating the role of increased CRF in the basolateral amygdala and in the dorsal raphe nucleus on depression-like behaviors in rats with extended access during methamphetamine withdrawal are warranted.
Collectively, it appears that extended access to methamphetamine produced more depressive-like and dysphoriclike withdrawal states in rats during early withdrawal whereas short access to methamphetamine enhanced anxiety-like stress responses. In support of our findings, while chronic methamphetamine abusers reported both depression and anxiety-like states during 4–7 days of withdrawal (London et al. 2004), methamphetamine-dependent patients showed depression-like symptoms including anhedonia and inactivity with less severe anxiety-like symptoms during acute withdrawal in a time course study (McGregor et al. 2005). These results suggest a continuum of development of negative emotional responses during psychostimulant withdrawal following compulsive drug taking, with withdrawal from short access to methamphetamine enhancing anxiety-like responses, and with extended access to methamphetamine producing dysphoric-like withdrawal responses. These motivational effects of drug withdrawal may contribute to compulsive drug intake and the rapid reinstatement of compulsive drug seeking in humans with methamphetamine addiction.
Acknowledgments
We gratefully acknowledge the technical assistance of Jovy Quiocho, Anjali Samant, Nicole Ng, and Tali Nadav who were undergraduate students of the University of California in San Diego in the self-administration and self-stimulation. We also thank Clay Archer, Robert Lintz, and Mike Arends for the technical and editorial assistance. This is publication number 21070 from The Scripps Research Institute. This study was supported by National Institute on Drug Abuse grant DA010072 (G.F.K), National Research Foundation of Korea grant NRF-2009-013-E00056 (C.G.J), Brain Research Center of the 21st Century Frontier Research Program grant 2010K000812 (C.G.J) funded by the MEST, Republic of Korea, The Pearson Center for Alcoholism and Addiction Research, and a Veterans Health Administration Research Career Scientist Award (GS).
Footnotes
Conflict of interest All the authors declare that there is no relationship with the organization supporting this research and no conflict of interest.
Contributor Information
Choon-Gon Jang, Department of Pharmacology, School of Pharmacy, Sungkyunkwan University, Suwon, South Korea.
Timothy Whitfield, Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, San Diego, CA, USA.
Gery Schulteis, Research Service, VA San Diego Healthcare System, and Department of Anesthesiology, School of Medicine, University of California, San Diego, CA, USA.
George F. Koob, Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, San Diego, CA, USA
Sunmee Wee, Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, San Diego, CA, USA; Department of Molecular Therapeutics, Scripps Florida, 130 Scripps Way, Jupiter, FL 33458, USA.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22(4):413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, et al. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5(7):625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33(8):1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Bessa JM, et al. A trans-dimensional approach to the behavioral aspects of depression. Front Behav Neurosci. 2009;3:1. doi: 10.3389/neuro.08.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff SR, et al. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95(3):298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Britton DR, Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav. 1981;15(4):577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, et al. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4(12):e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71(3):498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29(4–5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, et al. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Epstein DH, et al. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito RU, Motola AH, Kornetsky C. Cocaine: acute effects on reinforcement thresholds for self-stimulation behavior to the medial forebrain bundle. Pharmacol Biochem Behav. 1978;8(4):437–439. doi: 10.1016/0091-3057(78)90082-5. [DOI] [PubMed] [Google Scholar]
- Hayase T. Differential effects of TRPV1 receptor ligands against nicotine-induced depression-like behaviors. BMC Pharmacol. 2011;11:6. doi: 10.1186/1471-2210-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, et al. Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann N Y Acad Sci. 2001;939:366–380. doi: 10.1111/j.1749-6632.2001.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, et al. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26(22):5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, et al. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186(1):48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38(11):2473–2476. [PubMed] [Google Scholar]
- Land BB, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA. 2009;106(45):19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866(1–2):82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, et al. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64(11):958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, et al. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28(7):836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, et al. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4(1):17–26. [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. Guilford Press; New York: 1985. [Google Scholar]
- McGregor C, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100(9):1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- Moreau JL, et al. Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behav Pharmacol. 1995;6(7):682–687. [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278(5335):66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47(6):419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, et al. Anandamide-induced behavioral disruption through a vanilloid-dependent mechanism in rats. Psychopharmacology (Berl) 2009;203(3):529–538. doi: 10.1007/s00213-008-1399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali L, et al. Role of autophagy during methamphetamine neurotoxicity. Ann N Y Acad Sci. 2008;1139:191–196. doi: 10.1196/annals.1432.016. [DOI] [PubMed] [Google Scholar]
- Richardson HN, et al. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28(8):1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, et al. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12(5):781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Schulteis G, et al. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92(13):5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS, et al. Neurotoxicity in dopamine and 5-hydroxytryptamine terminal fields: a regional analysis in nigrostriatal and mesolimbic projections. Ann N Y Acad Sci. 1988;537:161–172. doi: 10.1111/j.1749-6632.1988.tb42104.x. [DOI] [PubMed] [Google Scholar]
- Snyder JS, et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio SE, et al. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196(3):473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. (NSDUH Series H-41). HHS Publication No. (SMA) 11-4658. [Google Scholar]
- Thompson PM, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007a;320(3):1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Wee S, et al. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007b;32(10):2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, et al. Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addict Biol. 2011;17(2):300–308. doi: 10.1111/j.1369-1600.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]