Abstract
OBJECTIVE
We sought to evaluate the effect of tumor size, location, and clinical nodal status on outcomes after thoracoscopic lobectomy for lung cancer.
METHODS
All patients who underwent attempted thoracoscopic lobectomy for lung cancer between June 1999 and October 2010 at a single institution were reviewed. A model for morbidity including published risk factors as well as tumor size, location, and clinical N status was developed by multivariable logistic regression.
RESULTS
During the study period, 916 thoracoscopic lobectomies met study criteria: 329 for peripheral, clinical N0 tumors ≤3cm and 504 for tumors that were central, clinical node positive, or >3cm; tumor location could not be documented for 83 patients. Conversions to thoracotomy occurred in 36 patients (4%); patients with clinically node positive disease had higher conversion rates [11 conversions in 153 clinical N1-N3 patients (7.2%) vs 25 in 763 clinical N0 patients (3.3%), p=0.03]. Overall operative mortality was 1.6% (14 patients) and morbidity was 32% (296 patients). Although patients with larger tumors (p=0.006) and central tumors (p=0.01) had increased complications by univariate analysis, tumor size >3cm (p=0.17) and central location (p=0.5) did not significantly predict overall morbidity in multivariate analysis. Clinical node status did not predict increased complications by univariate or multivariate analysis. Significant predictors of morbidity in multivariable analysis were increasing age, decreasing FEV1, prior chemotherapy, and congestive heart failure.
CONCLUSIONS
Thoracoscopic lobectomy for lung cancers that are central, clinically node positive, or larger than 3 cm does not confer increased morbidity compared to peripheral, clinical N0 cancers that are smaller than 3 cm.
Keywords: Lobectomy, Thoracoscopy, Lung Cancer, Outcomes
Introduction
A thoracoscopic approach to lobectomy has less morbidity compared to thoracotomy and has been advocated to be the gold standard resection approach for early-stage non-small cell lung cancer (NSCLC) [1-6]. The feasibility of performing a thoracoscopic lobectomy for lung nodules smaller than 3 cm suspected to be NSCLC was demonstrated by a prospective, multi-institution trial [7]. Although thoracoscopic techniques for more advanced tumors and resections have been reported, there is a lack of literature on the impact of attempting a thoracoscopic lobectomy for larger, central, and higher stage tumors [8-12]. This study was performed to evaluate the effect of tumor size, location, and clinical nodal status on outcomes after thoracoscopic lobectomy for NSCLC. The purpose of the study was to test the hypothesis that performing a thoracoscopic lobectomy for tumors that are larger than 3 cm, have a central location, or have clinically positive lymph nodes does not have increased perioperative morbidity compared to thoracoscopic lobectomy for peripheral tumors that are smaller than 3 cm and have clinically negative nodes.
Methods
After local Institutional Review Board approval was granted, the Duke University Medical Center Data Center was queried for Current Procedural Terminology codes linked with pulmonary resection by a thoracoscopic approach between June 1999 and October 2010. Careful attention was paid to individual operative notes and surgical pathology reports to identify all patients who underwent anatomic lobectomy via thoracoscopy for primary lung cancer. Patients who had attempted lobectomy via thoracoscopy but had conversions to thoracotomy for any reason were included in the thoracoscopic group for the analysis. Patients who had resection of more than one lobe or who underwent concomitant chest wall resection were excluded from the study.
Retrospective review of an institutional, prospective database maintained on all thoracic surgery patients was performed. Data collected included demographics, preoperative functional status, the use of induction therapy, smoking history, significant comorbidities, the histology and stage of disease, intraoperative details, and postoperative course. Chart review was utilized as necessary to complete data collection. Any postoperative event prolonging or otherwise altering the postoperative course was recorded along with all operative deaths, which were defined as deaths that occurred within 30 days after operation or those that occurred later but during the same hospitalization. Deaths were captured both by chart review and use of the Social Security Death Index Database. The definitions of postoperative events were based on version 2.08 of the Society of Thoracic Surgeons General Thoracic Surgery Database [13]. Morbidity was defined as the occurrence of at least one of these postoperative events. This composite endpoint definition was chosen because all events can cause patient discomfort, require additional testing and treatment, lead to longer hospital stays, and increase overall costs, and thus are all clinically significant
The recorded tumor size was that of the largest nodule in cases in which more than one nodule was present. Tumor location definition was based on location of tumor relative to branching lobar bronchi and blood vessels. A tumor was classified as central if removal would require division of lobar structures that would render any remaining lobar lung tissue non-functional. Tumors that can be removed via wedge resection while leaving remaining functional lobar lung tissue were classified as peripheral. Therefore, tumors for which a wedge resection could be performed to establish diagnosis prior to completion lobectomy were classified as peripheral. Tumors for which a diagnosis had not been established prior to resection but were considered not to be amenable to wedge at the time of resection by the operating surgeon were classified as central. Pre-resection imaging for all other patients was reviewed by an attending thoracic surgeon (MFB), and classified as central/peripheral based on the above criteria.
In cases where clinical N stage was not explicitly recorded prior to resection, pre-treatment imaging was reviewed to establish the clinical N stage. Nodes were considered clinically positive if an official PET report described the presence of nodal activity suspicious for metastatic disease. The actual values for the standardized update ratio were not routinely provided in PET scans at our institution. For patients that had CT scans but not PET scans, patients were considered clinically N positive if the official radiology report described abnormally enlarged lymph nodes greater than 1 cm in short axis. When patients had both CT and PET scans, the PET result was used to determine clinical stage.
Thoracoscopic lobectomy was performed without any rib spreading with the thoracoscope placed in the 8th intercostal space in the midaxillary line and a 4-5cm anterior utility incision in the 5th intercostal space [14]. An epidural catheter for postoperative pain relief was routinely offered to all patients regardless of planned operative approach. Chest tubes were routinely placed on water seal immediately postoperatively and removed when no air leak was present and drainage over 24 hours was less than 200 cc.
Univariate analyses were performed relating operative morbidity to the following patient characteristics: age, tumor size (≤3cmversus >3cm), tumor location (central versus peripheral), clinical N stage (N0 versus N1-N3), the presence of diabetes, the presence of renal insufficiency, history of prior chemotherapy, history of prior radiation, a history of smoking, history of congestive heart failure, history of coronary artery disease, previous thoracic surgery, and preoperative pulmonary function tests (Forced Expiratory Volume in one second (FEV1) and Diffusion Capacity of the Lung to Carbon Monoxide (DLCO)) . Tumor size was analyzed as classified as above and not according to T stage to account for tumors in which T stage was upstaged due to the presence of additional nodules in the lobe or pleural involvement. The variables that were significant at p < .20 were entered into a multivariable logistic regression with morbidity as the dependent variable and significance set at the 0.05 level.
Propensity score analysis that created subclasses of patients with central and peripheral tumors with similar covariate distributions was used as an alternative method of assessing the impact of tumor location on overall morbidity. Multivariable analysis was performed with tumor location as the outcome and age, percent predicted FEV1, percent predicted DLCO, tumor size (≤3cm versus >3cm), clinical N stage (N0 versus N1-N3), a history of smoking, history of coronary artery disease, previous thoracic surgery, and history of prior chemotherapy as potential predictors. Propensity scores were calculated using a logistic regression model that was created using stepwise variable selection with a p-value of 0.2 for entry into the model and a p-value of 0.05 to stay in the model. Only patients with propensity scores between 0.40 and 0.60 were then included in subsequent analysis where baseline characteristics and outcomes were compared based on tumor location.
Unpaired student’s t tests were used to compare continuous data, Fisher’s exact tests for dichotomous data, and χ2 for categorical variables. A two-tailed p value of less than 0.05 was considered significant. Data are presented as mean ± standard error of the mean unless otherwise noted. The SAS 9.0 statistical package (SAS Institute, Cary, North Carolina) was used for statistical analyses.
Results
Of 1195 thoracoscopic lobectomies performed during the study period, 916 met the study criteria. Overall, 620(68%) lobectomies were for tumors less than or equal 3 cm in size (median 2 cm) and 296(32%) lobectomies were for tumors greater than 3 cm in size (median 4.3 cm). Most patients (n=763,83%) were clinical stage N0, while 153 (17%) were clinical stage N1-N3. Of the patients with tumors larger than 3 cm, 213 patients had tumors between 3.1 cm and 5cm, 60 between 5.1cm and 7cm, and 23 larger than 7cm. Tumor location could not be documented for 83 patients. Of 833 patients with documented tumor location, 487(58%) tumors were peripheral and 346(42%) were central.
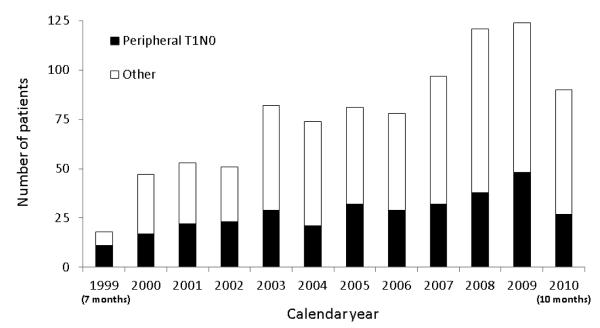
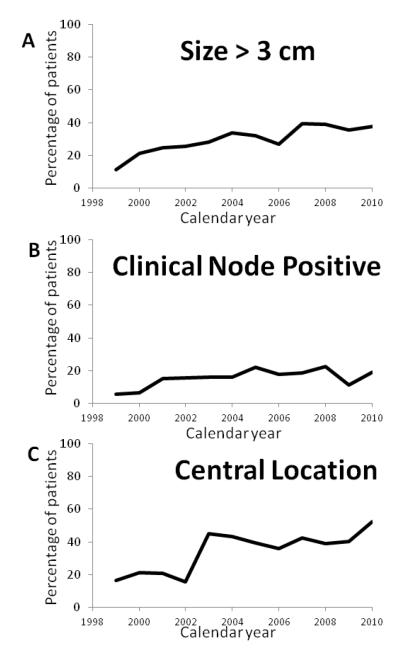
The number of thoracoscopic lobectomies per year, both overall and stratified by peripheral T1AN0 versus other, is depicted in Figure 1. Overall, 329 (39%) patients had peripheral, clinical N0 tumors ≤3cm and 504 (61%) patients had tumors that were larger than 3 cm, central, or clinical node positive. The percentage of procedures performed for larger tumors, central tumors, and clinically positive nodal disease all trended upwards through the course of the study period (Figure 2). Over the same timeframe, 541 lobectomies via thoracotomy (89 sleeve resections) were performed. The pathologic stage distribution of the thoracotomy lobectomies was: 73 patients stage IA (13%); 109 patients stage IB (20%); 119 patients stage IIA (22%); 76 patients stage IIB (14%); 140 patients stage IIIA (26%); 11 patients stage IIIB (2%); and 11 patients stage IV (2%).
Figure 1.
Distribution of thoracoscopic lobectomy for smaller and larger tumors per study year (Note: 1999 only included 7 months for analysis and 2010 only included 10 months for analysis).
Figure 2.
Percentage of thoracoscopic lobectomy performed in each year of the study for (a) tumors larger than 3 cm, (b) tumors with clinically positive nodal disease, and (c) tumors in a central location.
Demographic, baseline characteristics, and comorbid conditions are shown in Table 1. Compared to patients with tumors that were peripheral, smaller than 3 cm, and had clinically negative nodes, patients with central tumors, tumors larger than 3 cm, or clinically positive nodes had worse pulmonary function and were more likely to have had coronary artery disease, previous thoracic surgery, and induction chemotherapy. The ages of these two groups were not significantly different, and the incidences of smoking history, diabetes, prior radiation, congestive heart failure, and renal insufficiency were also not significantly different.
Table 1.
Demographics and perioperative outcomes for all patients and stratified by tumor size, location, and clinical N status.
| All Patients (n=916) |
Peripheral , ≤ 3cm, and Clinical N0 (n=329) |
Central, > 3 cm, or Clinical N1- N3 (n=504) |
P* | |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Median Age (Range) | 67 (21-93) | 67 (38-88) | 68 (21-93) | 0.2 |
| % Predicted Forced Expiratory Volume in One | 74±20 | 78±21 | 72±20 | <0.0001 |
| Second (%) % Predicted Diffusion Capacity to Carbon Monoxide | 77±21 | 82±21 | 75±21 | <0.0001 |
| Smoking History | 792 (86%) | 274 (83%) | 444 (88%) | 0.051 |
| Coronary Artery Disease | 196 (21%) | 61 (18%) | 125 (25%) | 0.04 |
| Diabetes | 150 (16%) | 60 (18%) | 80 (16%) | 0.4 |
| Previous Thoracic Surgery | 144 (16%) | 39 (12%) | 92 (18%) | 0.01 |
| Prior Chemotherapy | 60 (6%) | 9 (3%) | 48 (10%) | 0.0001 |
| Prior Radiation | 48 (5%) | 14 (4%) | 31 (6%) | 0.3 |
| Congestive Heart Failure | 43 (5%) | 18 (5%) | 22 (4%) | 0.4 |
| Renal Insufficiency | 41 (4%) | 13 (4%) | 26 (5%) | 0.5 |
| Outcomes | ||||
| Perioperative Mortality | 14 (1.6%) | 4 (1.2%) | 8 (1.6%) | 0.8 |
| Conversion | 36 (4%) | 11 (3%) | 23 (5%) | 0.5 |
| Hospital Stay (Days) | 4.9±4.3 | 4.4±3.7 | 5.3±4.8 | 0.005 |
| Hospital Stay > 14 days | 25 (3%) | 6 (2%) | 19 (4%) | 0.15 |
| Overall Morbidity | 296 (32%) | 85 (26%) | 188 (37%) | 0.0007 |
| Pathologic Stage | <0.0001 | |||
| Stage I | 634 (69%) | 280 (85%) | 296 (59%) | |
| Stage II-IV | 282 (31%) | 49 (15%) | 208 (41%) | |
| Atrial fibrillation | 128 (14%) | 41 (12%) | 76 (15%) | 0.3 |
| Prolonged Air Leak Greater than 5 Days | 107 (12%) | 28 (9%) | 70 (14%) | 0.02 |
| Need for post-operative Bronchoscopy | 54 (6%) | 9 (3%) | 44 (9%) | 0.0004 |
| Post-operative Transfusion | 47 (5%) | 12 (4%) | 34 (7%) | 0.06 |
| Pneumonia | 30 (3%) | 8 (2%) | 21 (4.2%) | 0.2 |
| Need for New Chest Tube | 22 (2%) | 5 (2%) | 16 (3%) | 0.2 |
| Confusion/Delirium | 16 (2%) | 4 (1%) | 11 (2%) | 0.4 |
| Re-Intubation | 15 (2%) | 1 (0.3%) | 14 (3%) | 0.007 |
| Renal Failure | 13 (1%) | 5 (2%) | 8 (2%) | 1 |
| Myocardial Infarction | 4 (0.4%) | 0 | 3 (0.6%) | 0.3 |
| Tracheostomy | 4 (0.4%) | 1 (0.3%) | 3 (0.6%) | 1 |
| Reoperation for Bleeding | 3 (0.3%) | 0 | 3 (0.6%) | 0.3 |
| Empyema | 3 (0.3%) | 1 (0.3%) | 2 (0.4%) | 1 |
comparison between Peripheral, ≤ 3cm, and Clinical N0 versus Central, > 3 cm, or Clinical N1-N3
Outcomes are summarized in table 1. Overall operative mortality was 1.6% (14 patients) and morbidity was 32% (296 patients). The median chest tube duration for all patients was 3 days, and the median hospitalization was 4 days. The most common postoperative events were atrial arrhythmia (n=128, 14%), air leak greater than 5 days (n=107, 12%), post-operative bronchoscopy (n=54, 6%), and post-operative transfusion (n=47, 5%). Table 1 also shows the final pathologic stage for the patients in the study. As expected, patients with smaller, peripheral, clinically node negative tumors were much more likely to be pathologic stage I than patients with larger, central, or clinically node positive tumors. Of the 763 patients who were clinically node negative, 98 (13%) patients were upstaged to pathologic N1 (10%, n=77) or pathologic N2 (3%, n=21).
In univariate analysis, patients with central tumors, tumors larger than 3 cm, or clinically positive nodes had increased overall morbidity, as well as increased rates of prolonged air leaks, postoperative bronchoscopy, postoperative transfusion, and re-intubation, compared to the patients with tumors that were peripheral, smaller than 3 cm, and had clinically negative nodes. However, multivariate analysis did not demonstrate tumor size, location, or nodal status to be significant risk factors for overall morbidity (Table 2). Although patients with larger tumors (p=0.006) and central tumors (p=0.01) had increased complications by univariate analysis, tumor size larger than 3 cm (p=0.17) and central location (p=0.5) did not significantly predict overall morbidity in multivariate analysis. Clinical node status did not predict increased complications by univariate or multivariate analysis. The significant predictors of morbidity by multivariable analysis were age, FEV1 prior chemotherapy, and congestive heart failure.
Table 2.
Multivariate risk model for complications.
| Univariate | Multivariate Analysis | |||
|---|---|---|---|---|
| p-value | Odds Ratio |
95% Confidence Interval |
p-value | |
| Age (per 1 year increase) | <0.0001 | 1.06 | 1.04-1.08 | <0.0001 |
| % predicted Forced Expiratory Volume in 1 Second (per 1% increase) |
<0.0001 | 0.98 | 0.97-0.99 | <0.0001 |
| Prior Chemotherapy | 0.02 | 2.40 | 1.14-5.08 | 0.02 |
| Congestive Heart Failure | 0.003 | 2.16 | 1.03-4.54 | 0.04 |
| % predicted Diffusion Capacity to Carbon Monoxide (per 1% decrease) |
<0.0001 | 0.1 | ||
| Tumor Size (>3cm vs ≤3 cm) | 0.006 | 0.17 | ||
| Tumor Location (Central vs Peripheral) | 0.01 | 0.5 | ||
| Previous Thoracic Surgery | 0.009 | 0.5 | ||
| Smoking History | 0.04 | 0.6 | ||
| Coronary Artery Disease | 0.004 | 0.8 | ||
| Prior Radiation Therapy | 0.16 | 1 | ||
| Diabetes | 0.4 | |||
| Clinical N stage (N1-N3 vs N0) | 0.5 | |||
| Renal Insufficiency | 0.6 | |||
Propensity score analysis identified 135 patients with central tumors and 122 patients with peripheral tumors with similar baseline characteristics (Table 3). There were no statistically significant differences in perioperative mortality, overall morbidity, or hospital stay between the patients with central tumors and the patients with peripheral tumors. These results are consistent with the above multivariate analysis results that show central location does not independently predict increased overall morbidity.
Table 3.
Baseline characteristics and outcomes for centrally and peripheral located tumors after propensity scoring used to create subclasses of patients with similar baseline characteristic distributions.
| Central Tumors (n=135) | Peripheral Tumors (n=122) | p-value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age | 67.5±8.6 | 67.5±9.3 | 1 |
| % predicted FEV1 | 68±21 | 70±22 | 0.5 |
| % predicted DLCO | 72±22 | 72±18 | 0.9 |
| History of Smoking | 124 (92%) | 107 (88%) | 0.3 |
| Coronary Artery Disease | 40 (30%) | 23 (19%) | 0.06 |
| Previous Thoracic Surgery | 17 (13%) | 19 (16%) | 0.6 |
| Prior Chemotherapy | 11 (8%) | 6 (5%) | 0.4 |
| Tumor Size | 0.2 | ||
| ≤3cm | 40 (30%) | 46 (38%) | |
| >3cm | 95 (70%) | 76 (62%) | |
| Clinical Nodal Status | 0.3 | ||
| Clinical N0 | 102 (76%) | 99 (81%) | |
| Clinical N1-N3 | 33 (24%) | 23 (19%) | |
| Outcome Variables Perioperative Mortality | 3 (2.2%) | 2 (1.6%) | 1 |
| Overall Morbidity | 56 (41%) | 44 (36%) | 0.4 |
| Hospital Stay | 5.3±3.6 | 5.8±6.7 | 0.4 |
Other stratifications of tumor size were also evaluated to assess the impact of tumor size on outcomes. The difference between morbidity of patients with tumors greater than 5 cm (40%, 33 of 83 patients) and patients with tumors smaller than 5 cm (32%, 263 of 833) was not statistically significant (p=0.1). Morbidity stratified by more discrete size subgroups were as follows: 29% (182 of 620 patients) for tumors smaller than 3 cm; 38% (81 of 213 patients) for tumors between 3 cm and 5 cm; 43% (26 of 60 patients) for tumors between 5cm and 7cm; and 30% (7 of 23 patients) for tumors larger than 7 cm.
Conversions to thoracotomy occurred in 36 patients (4%). Conversion was achieved by enlarging the anterior utility incision in 19 patients and performing a posteriolateral thoracotomy in 15 patients; conversion method wasn’t specified in two patients. Conversion indication was bleeding in 21 patients (16-pulmonary artery branch, 2-unspecified location, one-pulmonary vein, one-bronchial vessel, and one-lung parenchyma) and difficult dissection/hilar adenopathy in 9 patients. In addition, conversions were made in one patient for each of the following reasons: inability to locate nodule; pulmonary artery invasion; need to perform pulmonary arterioplasty after lobectomy had resulted in stenosis; inability to maneuver the lung; failure to progress; and failure to obtain adequate lung isolation. None of the conversions resulted in intra-operative death, and the difference in perioperative mortality between patients that required conversion and patients without conversion was not statistically significant [5.6% (2 of 36 patients) versus 1.4% (12 of 880 patients, p=0.1]. Patients that required conversion did have significantly higher overall morbidity compared to patients without conversion [61% (22 of 36 patients) versus 31% (274 of 880 patients), p=0.0004]. The individual complication that was significantly higher in the converted patients was postoperative transfusion [25% (9 of 36 patients) versus 4% (38 of 880 patients), p<0.0001]. The conversion rate was not higher for tumors larger than 3cm [12 conversions in 296 patients (4%) with tumors larger than 3 cm versus 24 conversions in 620 patients (3.9%) with tumors smaller than 3 cm, p=0.7] or for central tumors [17 conversions in 346 patients (4.9%) with central tumors versus 17 conversions in 487 patients (3.5%) with peripheral tumors, p=0.3]. The conversion rate was increased for patients with clinically node positive disease [11 conversions in 153 clinical N1-N3 patients (7.2%) versus 25 conversions in 763 clinical N0 patients (3.3%), p=0.03].
Discussion
Thoracoscopic lobectomy was first reported in the 1990s and since then several studies have shown advantages to this technique over thoracotomy. These advantages include less pain, better pulmonary function, shorter hospitalization, decreased overall costs, and fewer overall complications [1-5,14-21]. Despite these demonstrated advantages, a thoracoscopic approach was used in less than 45 percent of lobectomies as recently as 2010 [22].
The relatively limited application of thoracoscopic lobectomy is likely multifactorial, including surgeon training, experience, preferences, and biases. Concerns regarding safety, completeness of oncologic resection, and technical difficulties may also have prevented more widespread use of the thoracoscopic technique. Indeed, increased T and N stages, hilar adenopathy, previous thoracic radiation, and induction therapy were all considered contraindications to thoracoscopic lobectomy even in the mid-2000s [23].The prospective multi-center trial that was performed to evaluate the feasibility of this technique limited inclusion to patients with small, peripheral, clinical stage 1A tumors [7]. However, thoracoscopic lobectomy has been associated with improved compliance to adjuvant chemotherapy compared to thoracotomy [24,25]. Considering the importance of multimodality therapy to survival of advanced-stage lung cancer, investigation into the use of thoracoscopic lobectomy in these cases is warranted.
The purpose of the current study was to analyze the impact of tumor size, location, and clinical N stage on outcomes after thoracoscopic lobectomy for lung cancer. In this study, overall operative mortality and morbidity was comparable to reported outcomes for thoracoscopic lobectomy in the STS database [2]. A high proportion (55%) of patients had tumors that were either >3cm in size, central or clinical node positive. None of these characteristics was associated with increased postoperative complications by multivariate analysis. The factors that were significant predictors of morbidity in multivariable analysis were increasing age, decreasing FEV1, prior chemotherapy, and congestive heart failure. Our results demonstrate that thoracoscopic lobectomy is safe and feasible for lung cancers that are central, clinically node positive, or larger than 3cm in size.
Other recent studies have also shown the safety of thoracoscopic lobectomy in locally advanced lung cancer, including a retrospective review of 114 patients who underwent lobectomy for advanced clinical stage NSCLC between January 1, 2002 and July 31, 2007 [8]. Advanced clinical stage NSCLC was defined as tumors ≥4cm, T3 or T4 (based on the American Joint Committee on Cancer, 6th edition), and/or tumors that received neoadjuvant chemotherapy. Thoracoscopic lobectomy was attempted in 95 patients and completed in 73 patients. Conversion to thoracotomy was necessary in 22 patients (23%). Muscle-sparing thoracotomy was the procedure of choice in 19 patients. Length of stay and major postoperative complications were similar between the thoracoscopy group and the open group. A significantly higher percentage of patients was able to receive adjuvant therapy in the thoracoscopy group compared to the open group (37.2% for thoracoscopy VS 5.3% for open, P= 0.006). The thoracoscopic and open groups did not differ significantly in overall survival or disease-free survival. The authors concluded that thoracoscopic lobectomy for advanced lung cancer can be performed safely with an acceptable mortality rate, although with a higher conversion rate than thoracoscopic lobectomy for early-stage NSCLC.
Kim and colleagues [9] analyzed the outcomes of unexpected pathologic N1 and N2 disease after thoracoscopic lobectomy for clinical stage I NSCLC. The medical records of 89 patients were retrospectively reviewed to assess clinical characteristics, early postoperative complications, recurrence patterns, and survival. Complications occurred in 16 (18%) patients. For patients with N1 disease, overall survival was 98% at 1 year and 98% at 3 years. For patients with N2 disease, overall survival was 98% at 1 year and 89% at 3 years. The authors concluded that survival after thoracoscopic lobectomy for patients with pathologic N1 or N2 disease was comparable with survival after lobectomy through a thoracotomy. However, clinicians must continue to collect and evaluate long-term follow-up data in larger numbers of patients to ensure that a thoracoscopic approach does not compromise oncological efficacy.
The conversion rate to thoracotomy in the present study was 4%, which is relatively low in comparison to other series [1,7-9, 14]. The conversion rate was not higher for tumors larger than 3cm or for central tumors. Similarly, Hennon and colleagues [8] did not find either an association between larger tumors and need for conversion. The conversion rate was increased for patients with clinically node positive disease, which is likely due to the fact that involvement of hilar nodes can increase the difficulty of dissection around the pulmonary hilar structures. In this study, conversion to thoracotomy did not increase mortality but did result in increased morbidity, primarily due to an increased rate or postoperative transfusion. When planning a thoracoscopic approach in the setting of clinically positive nodes, surgeons should consider discussing the potentially higher chance of conversion with the patient preoperatively. In addition, surgeons may also wish to have a lower threshold to conversion in this setting, especially when they are early in their thoracoscopic lobectomy experience.
As the experience with thoracoscopic lobectomy has grown, thoracoscopy is now used for potentially challenging technical situations such as previous thoracic surgery and following induction therapy. In addition, the thoracoscopic approach is currently applied to more complex resections including chest wall resection, sleeve lobectomy, and pneumonectomy [10-12]. It appears that in experienced centers, these procedures are performed with acceptable morbidity and mortality. As the application of thoracoscopy expands to more complex situations, it is important to determine risk factors for potential complications to maximize patient safety. A major strength of this study is that it includes a large number of patients, allowing the creation of a model for morbidity that incorporates multiple clinical variables to elucidate those factors.
The main limitation of this study is the retrospective nature and the selection bias in which patients were chosen for a thoracoscopic approach, particularly early in experience. However, approximately 20% of thoracoscopic approach patients in recent years have had clinically positive nodes. This shift in distribution reflects a general institutional practice of performing thoracoscopic exploration in most patients, even when a thoracotomy is considered likely based on expected difficulty with hilar exposure and dissection due to tumor size or location. Thoracoscopic exploration may identify unexpected metastatic disease or another indicator of unresectability and spare the patient a thoracotomy, which likely will allow the patient to begin other needed systemic therapy more quickly.
The positive effects of approach on outcome in this study also cannot necessarily be immediately generalized to all thoracic surgeons, considering the extensive experience of surgeons at our institution with thoracoscopic lobectomy. In particular, the majority of tumors in this study were smaller than 3 cm and only 9% (n=83) were larger than 5 cm. Surgeons may not want to attempt a thoracoscopic approach for tumors larger than 3 cm until they feel completely comfortable with a thoracoscopic approach for smaller tumors. The decision of if and when to convert to thoracotomy should also be based on the surgeon’s overall experience with a thoracoscopic approach, and less experienced thoracoscopic surgeons should likely have a lower threshold for conversion when dissection is difficult. However, surgeons that become comfortable with thoracoscopic lobectomy can likely achieve similar results to those shown in this series and should consider this approach preferentially for lung cancer in all patients, even for larger tumors and in the presence of adhesions from previous surgery or higher cancer stages.
Acknowledgements
This work was in part supported by the NIH funded Cardiothoracic Surgery Trials Network (M.F.B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Western Thoracic Surgical Association’s 38th Annual Meeting, June, 2012
References
- 1.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–16. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Villamizar NR, Darrabie MD, Burfeind WR, Petersen RP, Onaitis MW, Toloza E, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg. 2009;138:419–25. doi: 10.1016/j.jtcvs.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–78. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Berry MF, Hanna J, Tong BC, Burfeind WR, Jr, Harpole DH, D’Amico TA, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88:1093–9. doi: 10.1016/j.athoracsur.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo SM, Park BJ, Wilton AS, Seshan VE, Bains MS, Downey RJ, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–5. doi: 10.1016/j.athoracsur.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig MG, D’Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg. 2010;89:S2098–101. doi: 10.1016/j.athoracsur.2010.02.102. [DOI] [PubMed] [Google Scholar]
- 7.Swanson SJ, Herndon JE, 2nd, D’Amico TA, Demmy TL, McKenna RJ, Jr, Green MR, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–7. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 8.Hennon M, Sahai RK, Yendamuri S, Tan W, Demmy TL, Nwogu C. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol. 2011;18:3732–6. doi: 10.1245/s10434-011-1834-9. [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Choi YS, Kim J, Shim YM, Kim K. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:1288–93. doi: 10.1016/j.jtcvs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Berry MF, Onaitis MW, Tong BC, Balderson SS, Harpole DH, D’Amico TA. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg. 2012;41:888–92. doi: 10.1093/ejcts/ezr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahtabifard A, Fuller CB, McKenna RJ., Jr Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg. 2008;85:S729–32. doi: 10.1016/j.athoracsur.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg. 2010;89:S2102–6. doi: 10.1016/j.athoracsur.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Society of Thoracic Surgeons. http://www.sts.org/sites/default/files/documents/pdf/trainingmanuals/adult2.61/Section_P_COMPLICATIONS.pdf.
- 14.Onaitis MW, Petersen PR, Balderson SS, Toloza E, Burfeind WR, Harpole DH, Jr, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg. 2006;244:420–5. doi: 10.1097/01.sla.0000234892.79056.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case control study. Ann Thorac Surg. 1999;68:194–200. doi: 10.1016/s0003-4975(99)00467-1. [DOI] [PubMed] [Google Scholar]
- 16.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–5. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 17.Tschernko E, Hofer S, Beiglmayer C, Wisser W, Haider W. Video-assisted wedge resection/lobectomy versus conventional axillary thoracotomy. Chest. 1996;109:1636–42. doi: 10.1378/chest.109.6.1636. [DOI] [PubMed] [Google Scholar]
- 18.Kaseda S, Aoki T, Hangai N, Shimizu K. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg. 2000;70:1644–6. doi: 10.1016/s0003-4975(00)01909-3. [DOI] [PubMed] [Google Scholar]
- 19.McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–6. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 20.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited anterior thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72:879–84. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 21.Burfeind WR, Jr, Jaik NP, Villamizar N, Toloza EM, Harpole DH, Jr, D’Amico TA. A cost-minimisation analysis of lobectomy: thoracoscopic versus posterolateral thoracotomy. Eur J Cardiothorac Surg. 2010;37:827–32. doi: 10.1016/j.ejcts.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Ceppa DP, Kosinski AS, Berry MF, Tong BC, Harpole DH, Mitchell JD, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: A Society of Thoracic Surgeons database analysis. Ann Surg. 2012 doi: 10.1097/SLA.0b013e318265819c. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onaitis M, D’Amico TA. Lung Cancer: Minimally Invasive Approaches. In: Selke FW, de Nido PJ, Swanson SJ, editors. Sabiston & Spencer Surgery of the Chest. 7th ed Volume 1. Elsevier Saunders; Philadelphia: 2005. pp. 277–284. [Google Scholar]
- 24.Petersen RP, Pham D, Burfeind WR, Hanish SI, Toloza EM, Harpole DH, Jr, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007;83:1245–9. doi: 10.1016/j.athoracsur.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 25.Nicastri DG, Wisnivesky JP, Litle VR, Yun J, Chin C, Dembitzer FR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008;135:642–7. doi: 10.1016/j.jtcvs.2007.09.014. [DOI] [PubMed] [Google Scholar]