Abstract
Objectives
To describe neurodevelopmental outcomes in infants with single ventricle (SV) physiology and determine factors associated with worse outcomes.
Study design
Neurodevelopmental outcomes for infants with SV enrolled in a multicenter drug trial were assessed at 14 months of age using the Bayley Scales of Infant Development-II. Multivariable regression analysis was used to identify factors associated with worse outcomes.
Results
Neurodevelopmental testing was performed at 14±1 months in 170/185 subjects in the trial. Hypoplastic left heart syndrome was present in 59% and 75% had undergone the Norwood operation. Mean psychomotor (PDI) and mental developmental indices (MDI) were 80±18 and 96±14 respectively (normal 100±15, P<0.001 for each). Group-based trajectory analysis provided a two-group model (high” and “low”) for height z-score trajectory and brain type natriuretic peptide (BNP) trajectory. The predicted PDI scores were 15 points higher in the “high” height z-score trajectory compared with the “low” cluster (P<.001). A higher number of serious adverse events during the trial was associated with lower PDI scores (P=.02). The predicted MDI scores were 13–17 points lower in “low height trajectory- high BNP trajectory” group compared with the other three groups (P<.001). MDI scores were also lower in subjects who required extracorporeal membrane oxygenation during the neonatal hospitalization (P=.01) or supplemental oxygen at discharge (P=.01).
Conclusions
Neurodevelopmental outcome at 14 months of age is impaired in infants with SV physiology. Low height trajectory and high BNP trajectory were associated with worse neurodevelopmental outcomes. Efforts to improve nutritional status alone may not improve neurodevelopmental outcomes.
Academic difficulties, behavioral problems, motor impairment, speech delays, inattention and hyperactivity are common in survivors of cardiac surgery in infancy, with the greatest risk in children with single ventricle (SV) physiology [1]. The etiology of neurodevelopmental disability is likely multifactorial with the precise role of specific medical, surgical, and socio-demographic factors not fully delineated [2–3]. Previous investigators have focused on peri-operative events as important etiologic factors [4–6]. Growth failure during early infancy has been implicated in impaired development of executive function and worse school performance in children without congenital heart defects (CHD) [7–9]. In other “at risk” populations such as extreme prematurity, catch-up growth in weight and head circumference from birth to hospital discharge has been associated with better neurocognitive outcome at 5 years (10). Poor growth occurs frequently in infants with SV physiology particularly prior to performance of the superior cavopulmonary connection (SCPC) [11–16]. Despite this, the impact of poor growth on neurodevelopmental outcome in infants with SV has not been studied.
As part of the Infant Single Ventricle (ISV) trial, survivors underwent neurodevelopmental testing at 14 months and growth was measured longitudinally from enrollment. Using group-based trajectory modeling to characterize growth patterns during infancy, we sought to evaluate the impact of growth pattern on neurodevelopmental outcome [18]. Our hypothesis was that lower trajectory of weight, height, and head circumference z-scores would be associated with worse neurodevelopmental outcome. We also explored the association between neurodevelopmental outcomes and patient characteristics, peri-operative course during neonatal and second stage surgeries, ventricular function by echocardiography, and measures of heart failure including serum brain type natriuretic peptide (BNP) concentration and Ross classification.
Methods
The ISV trial conducted by the Pediatric Heart Network (PHN) was a randomized, double-blind, placebo-controlled trial designed to determine whether enalapril improved growth and ventricular function in infants with SV physiology [14]. 230 subjects were enrolled in 10 centers in North America between August 2003 and May 2007. The study protocol was approved by the Institutional Review Board or Institutional Ethics Board at each participating center, and written informed consent was obtained from parents prior to trial enrollment. This clinical trial is registered at www.clinicaltrials.gov (NCT00113087).
Infants between one week and 45 days of age with SV physiology with stable systemic and pulmonary blood flow in whom a SCPC was planned were eligible for participation in the trial. Exclusion criteria included prematurity (gestational age less than 35 weeks), small for gestational age (birth weight less than 10th percentile for gestational age), and presence of chromosomal or phenotypic syndromes known to be associated with growth failure [19].
Neurodevelopmental testing with the BSID-II was performed at 14±1 months at the PHN sites by a designated PHN site psychologist certified by the Data Coordinating Center’s neuropsychological testing consultant (DCB). The BSID-II was administered in English or Spanish depending on the dominant language spoken at home. The BSID-II provides two scores: the Psychomotor Developmental Index (PDI), which assesses gross motor and fine motor skills, and the Mental Developmental Index (MDI), which measures cognitive functioning through assessment of memory, problem solving, number concepts, vocalization, language and social interaction skills. The mean PDI and MDI score for the normal population is 100, with a standard deviation (SD) of 15, and a minimal score of 50. Subjects who were too impaired to complete neurodevelopmental testing were assigned a score of 50.
Patient characteristics, medical and surgical data were prospectively collected during the neonatal and SCPC hospitalizations, and patients were followed until the age of 14 months. Data collected included patient demographics, socioeconomic status (SES), details of anatomic diagnosis, type of neonatal surgery, intra-operative support times during neonatal surgery, post-operative duration of mechanical ventilation, hospital length of stay (LOS), type and mode of feeding at hospital discharge, and use of supplemental oxygen at hospital discharge, and adverse events from enrollment until the age of 14 months such as neurologic events and use of extracorporeal membrane oxygenation (ECMO) (Appendix 2; available at www.jpeds.com). Measurement of serum BNP concentration, Ross heart failure classification (class 1–4), and assessment of ventricular size and function by echocardiography was performed before the SCPC and at the 14 month visit [18]. BNP concentration was measured at a central laboratory using the Shinogi-32 human assay. A single core laboratory reader interpreted all echocardiograms.
Detailed anthropometric measurements (weight, height and head circumference) were made at seven time points: study enrollment, 4 days after study enrollment, 2 weeks after enrollment, pre-SCPC, 7 days after restarting study drug following SCPC, age 10 months, and age 14 months. Study coordinators performing anthropometric measurements underwent standardized training to ensure accurate and reliable measurements of weight and height using training modules from the Health Resources and Service Administration Maternal and Child Health Bureau (http://dept.washington.edu/growth/). Quality assurance measures included the use of dedicated, appropriately calibrated scales, duplicate measurements, and third measurements if the first two were not in agreement (within 0.1 kg for weight, within1.0 cm for height). Measurements used in this analysis were limited to those performed at PHN sites by trained study coordinators. All growth data were converted into age-adjusted z-scores based on World Health Organization standards [14].
Statistical Analyses
Data are described as frequencies, medians with 25th and 75th percentile values, and means with SD as appropriate. The study cohort used for analysis was restricted to those patients with non-missing PDI and MDI scores. The data of subjects with missing MDI and PDI scores (n=15, see details below) were compared with those with non-missing scores (n=170). The variables listed in Appendix 2 were tested in a univariate manner for association with PDI and MDI scores. Backward stepwise regression along with bootstrapping to assess reliability of parameter estimates was used for selection of a final multivariable model. The significance level for entry into the final multivariable model was set at 0.05.
In order to use longitudinal somatic measurements, such as weight, height, and head circumference as predictors in regression analyses, we divided subjects into groups based on individual trajectories. We used a semi-parametric, group-based approach to characterize trajectories of weight, height, and head circumference z-scores. This approach, known as group-based trajectory modeling (and based on the maximum-likelihood method) allows for an easy identification of population heterogeneity both in terms of an attribute (e.g. height z-score) at a given age and how this attribute changes over time [18, 20–22]. This methodology also has the advantage of handling missing data and allows the retention of subjects with incomplete data during the course of the trial. Clustering of subjects into groups based on individual trajectories allowed 1) simplification of regression modeling by replacing seven correlated continuous predictors (e.g. height z-score from baseline until the 14-month visit) with one categorical cluster indicator and 2) assessment of clinically relevant associations between growth trajectory profiles and neurodevelopmental outcomes. Determining the appropriate number of clusters is a combination of science (the Bayesian information criterion or BIC), and art (“whether the final model adequately addresses the research question under investigation”). To determine the appropriate number of clusters, we used the BIC as well as the variance in outcome (PDI and MDI scores) explained by the cluster indicator. Although the association between a categorical cluster indicator (a predictor) and PDI or MDI score (a one-dimensional dependent variable) was being explored, a higher number of clusters did not necessarily improve the model. Some variables such as serum BNP concentration and Ross heart failure classification were measured at two study visits, pre-SCPC and at 14-months. The distribution of serum BNP concentration was highly skewed and was normalized by logarithmic transformation (log BNP). For multivariable modeling, log BNP was transformed into a categorical cluster indicator using the group-based trajectory modeling approach described above. Dichotomous categorical variables measured at 2 time points (pre-SCPC and at 14-months) were transformed into categorical variables with 4 levels (Appendix 2).
Results
From August 2003 to May 2007, 230 infants with SV physiology were randomized, and 185 subjects were followed from study enrollment until the final study visit at the age of 14±1 months. Of these, 174 returned for BSID-II testing, however three test results were deemed invalid following expert review (DCB), and one subject was excluded due to a missing MDI score. Thus 170 subjects had valid PDI and MDI scores and constituted the study population. Baseline characteristics of subjects with and without valid PDI and MDI scores did not differ with the exception of the type of palliative surgery (P=.02) (Table I; available at www.jpeds.com). For the 170 subjects, the median gestational age was 38 weeks, 9% of patients had gestational age between 35–37 weeks, and 71% were males. Hypoplastic left heart syndrome (HLHS) was the most common diagnosis (59%). The initial palliative surgery was a Norwood procedure in 75%, a systemic-to-pulmonary artery shunt in 20%, and a pulmonary artery band in 5%.
Table 1.
Baseline characteristic comparing 170 subjects with valid PDI and MDI scores and those without valid scores
| Characteristic | Valid PDI and MDI score | No valid PDI and MDI score | p-value |
|---|---|---|---|
| N | 170 | 15 | |
| Mean Age at Enrollment, Days | 21.0 ± 9.3 | 22.5 ± 10.4 | 0.56 |
| Median Age at Enrollment, Days | 19 (14, 27) | 22 (13, 35) | 0.59 |
| Male | 71% | 73% | 1.00 |
| Race | 0.07 | ||
| White | 81% | 80% | |
| Black or African American | 15% | 7% | |
| Asian | 1% | 13% | |
| Other | 4% | 0% | |
| Hispanic | 14% | 20% | 0.47 |
| Median Gestational Age, Weeks (imputed)* | 38 (37, 39) | 39 (37, 40) | 0.53 |
| Gestational age < 37 weeks | 9% | 7% | 1.00 |
| Mean Weight, kg | 3.38 ± 0.55 | 3.22 ± 0.49 | 0.27 |
| Weight-for-Age Z-score-WHO | −1.28±1.31 | −1.71±1.26 (15) | 0.22 |
| Mean height, cm | 51.1 ± 2.4 | 50.6 ± 2.1 | 0.40 |
| Height-for-Age Z-score-WHO | −1.06±1.31 | −1.45±1.10 (15) | 0.27 |
| Mean Head Circumference, cm | 34.3±1.6 | 34.1±1.3 (15) | 0.56 |
| Head Circumference Z-score-WHO (n=169) | −1.72±1.44 | −2.02±1.17 (15) | 0.43 |
| Single Ventricle Anatomic Diagnosis | 0.68 | ||
| Single Ventricle | 32% | 20% | |
| Hypoplastic Left Heart Syndrome | 59% | 73% | |
| Other Functional Single Ventricle | 9% | 7% | |
| Unclassified | 1% | 0% | |
| Type of Surgery | 0.02 | ||
| Norwood | 72% | 73% | |
| Systemic-to-Pulmonary Shunt | 20% | 0% | |
| PA Band | 5% | 13% | |
| Damus-Kaye-Stansel | 2% | 13% | |
| Overall AV valve regurgitation-dichotomous | 0.35 | ||
| None/Mild | 78% | 67% | |
| Moderate/Severe | 23% | 33% | |
| Systemic Ventricular Dysfunction | 0.59 | ||
| None | 83% | 79% | |
| Mild | 12% | 14% | |
| Moderate | 5% | 7% | |
| Severe | 0% | 0% | |
| Treatment Arm (ITT) | 0.10 | ||
| Enalapril | 51% | 27% | |
| Placebo | 49% | 73% |
Gestational age imputed as 40 weeks for 2 full-term patients, WHO-World Health Organization, PA-pulmonary artery, AV-atrioventricular, ITT-intention to treat.
Mean PDI and MDI scores for all 170 subjects were 80.3±18.1 and 95.6±14.5 respectively, both below the population mean of 100±15 (p<0.001 for both PDI and MDI). A PDI score < 85, which is 1 SD below the population mean, was seen in 58% of subjects, and 28% had a PDI score < 70 (i.e. 2 SD below the population mean). A MDI score of < 85 was seen in 21% of subjects and 10% had a MDI score < 70.
Group-based trajectory analyses provided a two-group model (“high” and “low”) for height z-score trajectory, and a three-group model (low, medium, and high) for weight and head circumference z-score trajectories. The process is illustrated in the Figure, where height z-score trajectories for all subjects (panel A) were divided into “high” and “low” groups (panels B and C) with distinct mean values (panel D). With this method, 109 or 63% of subjects were assigned to the “high” height z-score cluster, and 61 or 37% of subjects were assigned to the “low” height z-score cluster. Of note, a majority of subjects in the “high” cluster had higher height z-score at trial enrollment compared with those in the “low” cluster (Figure, B and C). Overall subjects in the “high” group maintained higher height z-scores throughout the study period compared with those in the “low” group, though there was some overlap between the two groups.
Figure.
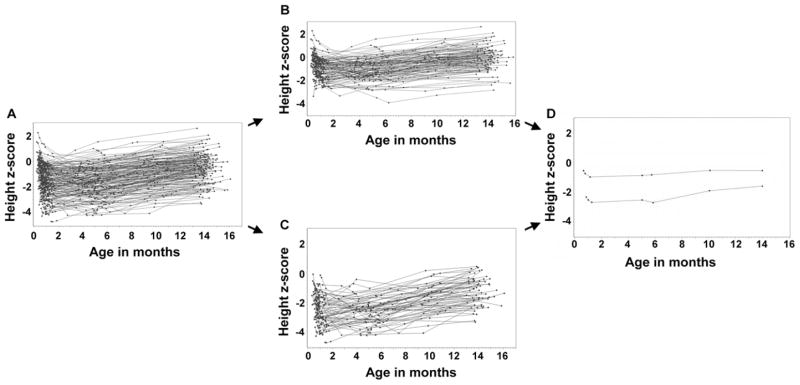
Trajectories of height z-scores over time for 170 subjects with PDI and MDI scores (7 visits): Individual trajectories for A, all subjects, B, 109 subjects in the “high” cluster, and C, 61 subjects in the ‘low’ cluster. D, Mean values by visit for each cluster.
Similarly, group-based trajectory modeling provided a two-group model (“high” and “low”) for log BNP trajectory. Using this method, 118 subjects were assigned to the “low” log BNP cluster and 52 were assigned to the “high” log BNP cluster.
For PDI score, statistically significant relationships by univariate analysis were seen for the following variables: HLHS diagnosis, hospital LOS and ECMO during neonatal hospitalization, number of other cardiac procedures during SCPC hospitalization, Ross heart failure class at 14 months, trajectories of height z-score, weight z-score, and head circumference z-score, log BNP trajectory, and number of serious adverse events (SAE) during the trial period (Table II).
Table II.
Variables significantly associated with PDI and MDI scores by univariate analysis
| Variable | N* | Value | Parameter Estimate** | p-value** (site-adjusted) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| PDI | MDI | PDI | MDI | |||
| Patient factors | ||||||
| Hispanic origin | 167 | 24 | −3.9 | −7.0 | 0.32 | 0.05 |
| HLHS | 170 | 100 | −3.5 | −2.8 | 0.03 | 0.17 |
| Neonatal hospitalization | ||||||
| Feeding Mechanism at discharge | 170 | 0.74 | 0.04 | |||
| Tube | 83 | 0.0 | 0.0 | |||
| Bottle | 81 | 3.4 | 5.0 | |||
| Breast | 6 | −1.2 | −6.1 | |||
| Supplemental oxygen at discharge | 170 | 13 | 4.8 | 3.0 | 0.25 | 0.01 |
| Length of stay (days) | 170 | 32.2±25.8 | −0.2 | −0.1 | 0.002 | 0.01 |
| Pre-SCPC visit | ||||||
| No other procedures at SCPC | 169 | 150 | 11.6 | 6.7 | 0.006 | 0.01 |
| 14 month visit | ||||||
| AVVR ┼ (None/Mild) | 166 | 142 | 2.2 | 5.5 | 0.37 | 0.02 |
| Growth trajectories cluster indicator | ||||||
| Height trajectories | 170 | <0.001 | <0.001 | |||
| high | 109 | 15.8 | 8.9 | |||
| low | 61 | 0.0 | 0.0 | |||
| Weight trajectories | 170 | 0.02 | 0.09 | |||
| high | 38 | 4.7 | 0.6 | |||
| medium | 80 | 0.0 | 0.0 | |||
| low | 52 | −9.6 | −4.9 | |||
| Head Circumference trajectories | 170 | 0.009 | 0.12 | |||
| high | 57 | 11.9 | 4.9 | |||
| medium | 44 | 0.0 | 0.0 | |||
| low | 69 | 1.9 | 0.9 | |||
| Log BNP trajectories | 170 | 0.01 | 0.004 | |||
| high | 52 | −9.4 | −6.7 | |||
| low | 118 | 0.0 | 0.0 | |||
| Height and Log BNP trajectories cluster indicator interaction | 170 | N/A | 0.006 | |||
| High-High | 27 | N/A | 3.96 | |||
| High-Low | 82 | N/A | 2.68 | |||
| Low-High | 25 | NA | −14.4 | |||
| Low-Low | 36 | NA | 0.0 | |||
| ECMO (neonatal surgery): | 170 | 6 | −19.0 | −15.0 | 0.04 | 0.01 |
| Total number of serious adverse events | 170 | 1.8±2.2 | −1.9 | −2.2 | <.001 | <.001 |
| Total number of serious adverse events | 170 | 0.001 | 0.011 | |||
| <2 | 97 | 14.8 | 14.8 | |||
| 2–4 | 61 | 6.9 | 10.1 | |||
| >4 | 12 | 0.0 | 0.0 | |||
| Clinical site | 170 | N/A | - | - | 0.07 | 0.05 |
total number of subjects with a measurement
From linear regression modeling
parameter estimate represents change in the dependent variable associated with a one integer unit change in the explanatory variable, HLHS-hypoplastic left heart syndrome, min-minutes, BNP-brain type natriuretic peptide, AVVR-atrioventricular valve regurgitation, SCPC-superior cavopulmonary connection, ECMO-extracorporeal membrane oxygenation, NA-not applicable.
In the final multivariable model, height z-score trajectory was positively correlated with PDI score with subjects in the “high” cluster scoring 15 points higher than those in the “low” cluster (P<.001) (Table III). The total number of SAEs over the entire trial negatively impacted PDI score; subjects experiencing fewer than two SAEs scored 10.6 points higher than those with more than four SAEs (P=.02). Clinical site was also associated with PDI scores (P=.02). This multivariable model explained 31% of the variance in PDI scores.
Table 3.
Final multivariable models for PDI and MDI scores at 14 months
| PDI | MDI | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Estimate | Bootstrapping Reliability*, % | P | Estimate | Bootstrapping Reliability**, % | P |
| Height trajectories cluster indicator, High vs. Low | 14.99 | 100 | <0.001 | |||
| Total Number of SAEs | 56.2 | 0.02 | ||||
| <2 | 10.61 | |||||
| 2–4 | 3.81 | |||||
| >4 | reference | |||||
| Site | 85.5 | 0.02 | - | 94.7 | 0.001 | |
| ECMO | −13.30 | 48.2 | 0.01 | |||
| Supplemental Oxygen (after neonatal surgery) | −11.00 | 56.6 | 0.01 | |||
| Height and Log BNP trajectories cluster indicator interaction | 75.3 | <0.001 | ||||
| High§-High | 4.45 | |||||
| High-Low§ | 4.58 | |||||
| Low--High | −12.79 | |||||
| Low-Low | reference | |||||
| Hispanic | −5.58 | 57.7 | 0.05 | |||
PDI: N=170, R2 = 0.31, R2 adjusted = 0.30, MDI: N=167, R2 = 0.36, R2 adjusted = 0.33
on a subset of 6 key variables with bootstrapping reliability above 30% and univariate p-values under 0.2
On a subset of 9 key variables with bootstrapping reliability above 30% and univariate p-values under 0.2, SAEs-serious adverse events, ECMO-extracorporeal membrane oxygenation, BNP-brain type natriuretic peptide
High and low are labels for two levels of the trajectories cluster indicator
For MDI score, statistically significant relationships by univariate analysis were seen for the following variables: Hispanic ethnicity, neonatal hospitalization outcomes including ECMO, mode of feeding at discharge, use of supplemental oxygen at discharge, and LOS, presence of moderate-to-severe atrioventricular valve regurgitation at 14 months, height z-score trajectory, log BNP trajectory, total number of SAEs during the trial, and clinical site (Table II).
We also tested for an interaction between BNP and height by analyzing the relationship of log BNP trajectory with MDI score in the “high” and “low” height z-score trajectories. In the final multivariable model, subjects in the “low height trajectory- high log BNP trajectory” scored on average 13–17 points lower than those in the other three groups (low height-low BNP, high height-low BNP, high height-high BNP, P<.001; Table III). Mean MDI score was 14 points lower in subjects requiring ECMO during the neonatal hospitalization (P<.01), and 11 points lower in those discharged home on supplemental oxygen after neonatal palliative surgery (p=0.01). Clinical site was also associated with MDI scores (p<0.001). This multivariable model explained 36% of the variance in MDI scores.
Discussion
Severe stunting (height-for-age ≤ 2SD below normal) is an indicator of chronic malnutrition, and is a major public health problem around the world. Children from developing countries who are stunted in early childhood have poorer cognition, school achievement, and psychosocial function in later childhood [23–24]. This study in the CHD population demonstrates that lower height z-score trajectory in early infancy is associated with neurodevelopmental disability.
Poor growth is a significant problem in infants with CHD, especially in those with SV physiology prior to performance of the SCPC [14–16]. Infants with SV may experience feeding difficulties, gastro-esophageal reflux, uncharacterized genetic influences, alteration in serum growth factors and growth hormone, and inadequate nutrition, which can impact growth [25, 26]. In a retrospective study of 50 infants with HLHS, weight-forage z-score at discharge was lower in children with longer hospital LOS, longer intensive care unit LOS, shorter duration of parenteral nutrition and higher diuretic dosage at discharge [27]. Similarly, the ISV trial demonstrated that infants with SV physiology undergoing staged reconstruction are “stunted” with both mean weight and height being below normal [14].
Protein-energy malnutrition in fetal and early neonatal animal models results in a reduction in brain size due to changes in structural proteins, growth factor concentrations, and neurotransmitter production [28]. The rapid rate of brain growth along with the developmental changes that occur during the third trimester of gestation and in the early postnatal stage make it vulnerable to an inadequate diet. Smaller average head circumferences and microcephaly have been reported in infants with HLHS [29]. This suggests a disruption of normal fetal brain growth, which may increase the vulnerability of the brain to poor nutrition in infants with HLHS.
It is interesting to note that weight z-score trajectory did not have an impact on neurodevelopment in multivariable analysis, even though it was associated with PDI score in univariate analysis. This may be explained by the unexpected finding of greater impairment of height rather than weight during the first 14 months in infants with SV physiology (14). In a secondary analysis of data from the ISV study to determine factors that affect growth, the mean change in weight z-score was −0.37±1.15 from enrollment to SCPC, and +1.12±0.89 from SCPC to 14 months. Similarly height z-score decreased by −0.26 ± 1.16 from enrollment to SCPC, however there was a less dramatic improvement in height z-score by +0.34±0.97 from the time of SCPC to 14 months [16]. Thus clinicians should be attentive to poor linear growth in the first year of life as a marker for potential neurodevelopmental abnormalities. Similarly, head circumference z-score trajectory was not independently associated with neurodevelopmental outcome. With the SCPC, elevation of the intra-thoracic central venous pressure is transmitted to the venous drainage of the brain, altering cerebrospinal fluid re-absorption and increasing intracranial pressure at a time when the skull is still quite malleable. This may represent pseudo-normalization of head circumference measurements and alter the relationship between head circumference and brain volume so that changes in head circumference do not necessarily reflect brain growth.
Another unique finding of this study is the association of serum BNP concentration with Neurodevelopmental outcome and the interaction between height trajectory and BNP trajectory for MDI scores. Serum BNP is a surrogate marker of heart failure, and elevated serum BNP concentration has been correlated with increased right and left sided intra-cardiac pressures [30–31]. Serum BNP concentration is expected to decline following the volume unloading that occurs after the SCPC [13]. Persistent elevation of serum BNP concentration at 14 months may be due to the presence of ventricular dysfunction and/or atrioventricular valve regurgitation, each of which can cause heart failure. Subjects in the “low height trajectory-high log BNP trajectory” had the lowest mean MDI scores. This interaction suggests that subjects with persistent heart failure and lower height z-scores over the first 14 months of life have worse neurodevelopmental outcome. This finding implies that interventions to improve neurodevelopmental outcomes in infants with SV physiology will need to include measures beyond improving nutritional status.
Consistent with previous studies, complications such as ECMO and a greater number of SAEs during the trial were associated with worse BSID-II scores. This suggests that hemodynamic instability and other morbidities both in the post-operative period, and after hospital discharge affect neurodevelopmental outcomes. Lower systemic venous oxygen saturation suggestive of low cardiac output has been shown to negatively impact neurodevelopment at 4.5 years [31]. Similarly longer hospital LOS, which is often a surrogate for a complicated post-operative course, has also been associated with worse outcome [32].
Clinical site was also associated with BSID-II scores at 14 months. This could represent a true difference between subjects due to unmeasured variation in patient characteristics or management strategies across sites. Alternatively, differences in ascertainment of neurodevelopmental status may be due to variation in testing by the site psychologist. Although psychologists at all sites were certified by a PHN expert, the difference may also be related to subjective differences in scoring by the designated site psychologists. It is interesting to note that, contrary to other studies, factors such as socioeconomic status, diagnosis of HLHS, or the Norwood procedure as neonatal palliative surgery were not associated with neurodevelopmental outcomes in our cohort (34, 35).
This study has some important limitations. Only subjects less than 45 days of age who were well with stable systemic and pulmonary blood flow were approached for enrollment. Sicker subjects who may be at the highest risk for worse neurodevelopmental outcome were not included in this study. Subjects with recognizable genetic or phenotypic syndromes were excluded from the ISV trial. However most subjects did not undergo formal genetic evaluation and unrecognized genetic abnormalities may have been present in our study cohort. Another limitation of this study is the lack of information about nutritional intake. The study protocol did not include assessment of nutritional intake at discharge from neonatal hospitalization. Although the protocol included collection of data on caloric intake during the seven study visits, this information was incomplete and not obtained consistently. The study was also limited by lack of formal neurologic evaluation or neuroimaging. The predictive value of neurodevelopmental testing at the age of14 months is limited. The BSID-II provides a good assessment of developmental strengths and weaknesses. However, it is not an intelligence test and assessment of one-year mental and motor development may correlate only modestly with performance later in life [36]. Our study design allowed us to identify factors associated with worse neurodevelopmental outcome, but not to determine causality.
Clinical efforts should be directed at improving nutritional status and decreasing heart failure in infants with single ventricle physiology.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (NHLBI; HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, and HL085057) and theFood and Drug Administration’s Office of Orphan Products Development. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or National Institutes of Health.
Pediatric Heart Network Investigators include:
National Heart, Lung, and Blood Institute: Gail Pearson, Victoria Pemberton, Mario Stylianou, Marsha Mathis
Network Chair: Lynn Mahony, University of Texas Southwestern Medical Center
Data Coordinating Center: New England Research Institutes, Lynn Sleeper (PI), Steven Colan, Lisa Virzi, Lisa Wruck*, Victor Zak, David F. Teitel
Core Clinical Site Investigators: Children’s Hospital Boston, Jane W. Newburger (PI), Roger Breitbart, Jami Levine, Ellen McGrath, Carolyn Dunbar-Masterson; Children’s Hospital of New York, Daphne Hsu* (Study Chair), William Hellenbrand (PI), Ashwin Prakash*, Seema Mital*, Darlene Servedio*; Children’s Hospital of Philadelphia, Victoria L. Vetter (PI), Chitra Ravishankar, Sarah Tabbutt*, Meryl Cohen, Katherine Lee, Marisa Nolan, Stephanie Piacentino, Michelle Toms; Cincinnati Children’s Medical Center, D. Woodrow Benson (PI), Catherine Dent Krawczeski, Lois Bogenschutz, Teresa Barnard, Steven Schwartz*, David Nelson; North Carolina Consortium: Duke University, East Carolina University, Wake Forest University, Page A. W. Anderson (PI) – deceased, Jennifer Li (PI), Wesley Covitz, Kari Crawford, Michael Hines, James Jaggers, Theodore Koutlas, Charlie Sang, Jr, Lori Jo Sutton, Mingfen Xu; Medical University of South Carolina, J. Philip Saul (PI), Andrew Atz, Girish Shirali, Eric M. Graham, Teresa Atz; Primary Children’s Medical Center and the University of Utah, Salt Lake City, Utah, L. LuAnn Minich (PI), John A. Hawkins, Richard V. Williams, Linda M. Lambert, Marian E. Shearrow; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Elizabeth Radojewski, Nancy Slater, Svetlana Khaikin, Susan McIntyre.
Auxiliary Sites: Children’s Hospital of Wisconsin, Nancy Ghanayem, Kathy Mussatto, Michele Frommelt, Lisa Young-Borkowski; University of Michigan, Albert Rocchini, Laurie Rodgers-Augustyniak
Echocardiography Core Laboratory: Children’s Hospital Boston: Steven Colan, Renee Margossian
Genetics Core Laboratory: Children’s Hospital of New York: Wendy Chung, Liyong Deng, Patricia Lanzano
Protocol Review Committee: Michael Artman, Chair; Judith Massicot-Fisher, Executive Secretary; Timothy Feltes, Julie Johnson, Thomas Klitzner, Jeffrey Krischer, G. Paul Matherne
Data and Safety Monitoring Board: John Kugler, Chair; Rae-Ellen Kavey, Executive Secretary; David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Holly Taylor, Catherine L. Webb
*no longer at the institution listed
Appendix 2
Patient factors (8 variables)
Gestational age (weeks)
Race
Sex
Ethnicity
Anatomic Diagnosis (Hypoplastic left heart syndrome/HLHS – yes/no)
Household income (annual, Socioeconomic status/SES)
Highest grade of school completed by parent/guardian filling the questionnaire (SES)
Ventricular type (Left vs. Right/Mixed)
Feeding regimen (1 variable)
Feeding mechanism* (Tube/Bottle/Breast) – at the time of discharge after neonatal surgery
Factors during neonatal hospitalization (9 variables)
Age at palliative surgery (days)
Type of palliative surgery (Norwood –yes/no) (‘no’ includes 5 patients without neonatal surgery)
Total bypass time (minutes)
Aortic cross clamp time (minutes)
Circulatory arrest (yes/no)
Concurrent procedures (number of) (transformed to dichotomous: 0 or > 0)
Length of hospital stay (for neonatal surgery, days) (transformed to quartiles)
Total time on ventilator following neonatal surgery (days, transformed to quartiles)
Supplemental oxygen at discharge (yes/no)
Factors during SCPC hospitalization (3 variables)
Age at SCPC surgery (days)
Concurrent cardiac surgical procedures (number of) (transformed to dichotomous: 0 or > 0)
Length of hospital stay (days) (transformed to quartiles)
ECHO (4 variables)
Overall Atrioventricular/AV valve regurgitation (None/Mild vs. Moderate/Severe)* at pre-SCPC and 14-month visits
Systemic Ventricular Dysfunction (None vs. Mild/Moderate/Severe) at baseline
BNP (5 variables)
Logarithm of BNP at pre-SCPC and 14-month visits
Dichotomous variable BNP <100 pg/ml (Yes/No)* at pre-SCPC and 14-month visits
ROSS (3 variables)
Ross heart classification (class 1 vs. classes 2–4)* at pre-SCPC and 14-month visits
*In addition to dichotomous (Yes/No) variables at pre-SCPC and 14-month visits we created a variable with 4 levels (YY, YN, NY, NN) accounting for both visits and used this variable in multivariate modeling.
RAAS gene polymorphism (1 variable)
High risk (Yes/No) (high risk genotype (homozygous) present in at least 2 genes out of five vs. in only one gene, or absent)
GROWTH (22 continuous variables, converted into 3 categorical)
Growth z-scores (weight, height and head circumference) at randomization, 4 days, two weeks, pre-Glenn, 7days after restarting study drug, 10 month and14 month visits. Birth weight z-score
ADVERSE EVENTS (AE) (over the duration of the trial, 3 variables)
Total number of serious AE (varies from 0 to17: a categorical form will be used: <2, 2–4, >4)
Neurological SAE (yes/no) (only 10 subjects have neurological SAE, each has 1)
ECMO (yes/no) (only 6 subjects have ECMO, one of them twice)
SITE (1 variable)
Footnotes
The authors declare no conflicts of interest. Registered at www.clinicaltrials.gov: NCT00113087
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16:92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 2.Gaynor JW, Gerdes M, Zackai EH, Bernbaum J, Wernovsky G, Clancy RR, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–1745. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Bove EL, Schork MA, et al. Neurodevelopmental outcome of patients after the Fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000;137:646–652. doi: 10.1067/mpd.2000.108952. [DOI] [PubMed] [Google Scholar]
- 5.Visconti KJ, Rimmer D, Gauvreau K, del Nido P, Mayer JE, Jr, Hagino I, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one-year neurodevelopmental outcome. Ann Thorac Surg. 2006;82:2207–2211. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 6.Tabbutt S, Nord AS, Jarvik GP, Bernbaum J, Wernovsky G, Gerdes M, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic heart syndrome. Pediatrics. 2008;121:476–483. doi: 10.1542/peds.2007-1282. [DOI] [PubMed] [Google Scholar]
- 7.Black MM, Dubowitz H, Krishnakumar A, Starr RH., Jr Early intervention and recovery among children with failure to thrive: follow-up at age 8. Pediatrics. 2007;120:59–69. doi: 10.1542/peds.2006-1657. [DOI] [PubMed] [Google Scholar]
- 8.Dykman RA, Casey PH, Ackerman PT, McPherson WB. Behavioral and cognitive status in school-aged children with a history of failure to thrive during early childhood. Clin Pediatr. 2001;40:63–70. doi: 10.1177/000992280104000201. [DOI] [PubMed] [Google Scholar]
- 9.Bhoomika K, Shobini R, Chandramouli B. Cognitive development in children with chronic protein energy malnutrition. Behavioral and Brain Functions. BioMed Central. 2008:1–12. doi: 10.1186/1744-9081-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5. 4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;120:e101–e109. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 11.Varan B, Tokel K, Yilmaz G. Malnutrition and growth failure in cyanotic and acyanotic congenital heart disease with and without pulmonary hypertension. Arch Dis Child. 1999;81:49–52. doi: 10.1136/adc.81.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JB, Beekman RH, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, et al. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Burnham N, Ittenbach RF, Stallings VA, Gerdes M, Zackai E, Bernbaum J, et al. Genetic factors are important determinants of impaired growth after infant cardiac surgery. J Thorac Cardiovasc Surg. 2010;140:144–149. doi: 10.1016/j.jtcvs.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medoff-Cooper B, Irving SY, Marino BS, García-España JF, Ravishankar C, Bird GL, et al. Weight change in infants with a functionally univentricular heart: from surgical intervention to hospital discharge. Cardiol Young. 2010;12:1–9. doi: 10.1017/S104795111000154X. [DOI] [PubMed] [Google Scholar]
- 16.Williams RV, Zak V, Ravishankar C, Altmann K, Anderson J, Atz AM, et al. Factors affecting growth in infants with single ventricle physiology: a report from the Pediatric Heart Network Infant Single Ventricle trial. J Pediatr. 2011;159:1017–1022. doi: 10.1016/j.jpeds.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley Scales of Infant Development, Second Edition. 2. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 18.Nagin DS, Odgers CL. Group-based modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 19.Hsu DT, Mital S, Ravishankar C, Margossian R, Li JS, Sleeper LA, et al. Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle. Am Heart J. 2009;157:37–45. doi: 10.1016/j.ahj.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, et al. Trajectories of Adherence to Airway Clearance Therapy for Patients with Cystic Fibrosis. Journal of Pediatric Psychology. 2010;35:1028–1037. doi: 10.1093/jpepsy/jsq015. [DOI] [PubMed] [Google Scholar]
- 21.Shaw DS, Lacourse E, Nagin DS. Developmental trajectories of conduct problems and hyperactivity from ages 2 to 10. Journal of Child Psychology and Psychiatry. 2005;46:931–942. doi: 10.1111/j.1469-7610.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Social Methods Res. 2001;29:374–93. [Google Scholar]
- 23.Chang SM, Walker SP, Grantham-McGregor S, Powell SP. Early childhood stunting and later fine motor abilities. Dev Med Child Neurol. 2010;52:831–836. doi: 10.1111/j.1469-8749.2010.03640.x. [DOI] [PubMed] [Google Scholar]
- 24.Grantham-McGregor S, Cheung YB, Cueto S, Cueto S, Glewwe P, Richter L, et al. the International Child Development Steering Group. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens JL, Ndidiamaka M. Nutritional support with neonatal cardiac surgery. Nutr Clin Pract. 2009;24:242. doi: 10.1177/0884533609332086. [DOI] [PubMed] [Google Scholar]
- 26.Dinleyici EC, Kilic Z, Buyukkaragoz B, Ucar B, Alatas O, Aydogdu SD, et al. Serum IGF-1, IGFBP-3 and growth hormone levels in children with congenital heart disease: relationship with nutritional status, cyanosis and left ventricular functions. Neuro Endocrinol Lett. 2007;28:279–83. [PubMed] [Google Scholar]
- 27.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage I Norwood procedure. Nutrition. 2006;22:237–244. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Georgieff MK. Am J Clin Nutr. 2007;85:614S–20S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 29.Shillingford AJ, Ittenbach RF, Marino BS, Rychik J, Clancy RR, Spray TL, et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young. 2007;17:189–195. doi: 10.1017/S1047951107000248. [DOI] [PubMed] [Google Scholar]
- 30.Mukoyama M, Nakao K, Hosada K, Suga S, Saito Y, Ogawa Y, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hystad ME, Geiran OR, Attramadal H, Spurkland A, Vege A, Simonsen S, et al. Regional cardiac expression and concentration of natriuretic peptides in patients with severe chronic heart failure. Acta Physiol Scand. 2001;171:395–403. doi: 10.1046/j.1365-201X.2001.00805.x. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman GM, Mussatto KA, Brosig CL, Ghanayem NS, Musa N, Fedderly RT, et al. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2005;130:1094–1100. doi: 10.1016/j.jtcvs.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Limperopoulos C, Majnemer A, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C, et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. 2002;141:51–58. doi: 10.1067/mpd.2002.125227. [DOI] [PubMed] [Google Scholar]
- 34.Bellinger DC, Wypij D, duPlessis AJ, Rappaport RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston circulatory arrest trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 35.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early Developmental Outcome in Children With Hypoplastic Left Heart Syndrome and Related Anomalies: The Single Ventricle Reconstruction Trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114:e572–6. doi: 10.1542/peds.2003-0983-L. [DOI] [PubMed] [Google Scholar]


