Abstract
As systemically used therapeutics for treatmenting acute or chronic pains or inflammations, non-steroidal anti-inflammatory drugs (NSAID) also associate with the adverse gastrointestinal and renal effects and cardiovascular risks. Thus, it is beneficial to develop topical gels that selectively inhibit cyclooxygenase-2 (COX-2) for the management of local inflammation. In this work, we demonstrate that the covalent conjugation of D-amino acids to naproxen (i.e., an NSAID) not only affords supramolecular hydrogelators for the topical gels, but also unexpectedly and significantly elevates the selectivity towards COX-2 about 20 times at little expense of the activity of naproxen. This work illustrates a previously unexplored approach that employs D-amino acids for the development of functional molecules that have dual or multiple roles and exceptional biostability, which offers a new class of molecular hydrogels of therapeutic agents.
This communication reports the design, synthesis, and characterization of hydrogelators made of D-amino acids and a non-steroidal anti-inflammatory drug (NSAID) for the development of multifunctional supramolecular hydrogelators that have excellent selectivity for inhibiting COX-2. As a widely, systemically used drugs for the treatment of acute or chronic pains or inflammations, NSAIDs usually are administered in high dosage, which causes the adverse gastrointestinal and renal effects1 when they inhibit COX-1,2 and associate with cardiovascular risks with the inhibition of COX-2.2,3 Such paradoxical effects demand the selectivity of NSAID to be modulated according to the therapeutic objectives, as well as minimize the systemic use of NSAIDs for localized acute or chronic pains.4 Therefore, it is clinically beneficial to explore gels of NSAIDs as a topical agent for treating inflammation and relieving pain, as demonstrated by the use of diclofenac lotion for managing moderate osteoarthritis.5 The encouraging results of diclofenac lotion indicate that it is worthwhile to develop gels of other NSAIDs for more effective localized treatment of severe chronic pains. Thus, we choose to develop supramolecular hydrogels of NSAIDs because, despite their promising potential, they are less explored,6 particularly in term of how to enhance biostability and maintain desired activity—two essential requirements of topical gels for sustained release.
Supramolecular hydrogels, as a type of hydrogels resulted from the self-assembly of small molecules (usually termed as hydrogelators) in water,7 have become an attractive choice of soft nanomaterials8 for a variety of applications, such as scaffolds for tissue engineering,9,10 carriers for drug delivery,11,12 ultrathin membranes,13 and new matrices for enzyme assay,14 antibacterial cell culture,10 gel electrophoresis,15 and protein pull-down assay.16 Since the hydrogelators only associate with each other through noncovalent interactions, supramolecular hydrogels are inherently biocompatible and biodegradable, which also makes them attractive candidates for self-delivery therapeutics, that is, the drug molecules themselves are hydrogelators.12 Comparing to the use of biodegradable polymers to encapsulate therapeutic agents for controlled release of drugs by adjusting the pore sizes and functionality of the polymer networks,17 self-delivery hydrogels based on supramolecular hydrogelators minimize several inherent shortcomings, such as inflammations,18 limited loading of drug molecules,19 and the difficulties of functionalizing the polymers with drug molecules, that limit the application of polymeric hydrogels.
Since the formation of supramolecular hydrogels relies on the small molecules (i.e., hydrogelators) that self-assemble in water via non-covalent interactions and the sustained drug-release depends on the biostability of the hydrogels, the essential requirements for the hydrogelators made of NSAIDs are (i) enabling the self-assembly of NSAIDs without compromising the activity of the NSAIDs; (ii) resisting the premature degradation due to proteolytic hydrolysis. To satisfy these two requirements and to demonstrate the concept of the design, we use one of the prescription NSAIDs, naproxen (denoted as Npx in this report), to generate new hydrogelators that only compose of naproxen and small peptides made of D-amino acids. Based on the ability of diphenylalanine (Phe-Phe)20 motif to enable functional molecules to self-assembly in water,21 we conjugate D-Phe-D-Phe with Npx to afford NSAID containing hydrogelators. The use of D-amino acids for the conjugates not only confers proteolytic resistance to the hydrogelators, but also, unexpectedly and significantly, enhances the selectivity of the hydrogelators as the Npx derivatives for inhibiting COX-2. As the first example of the D-amino acids to improve the selectivity and to maintain the desired activity of a therapeutic agent, this work demonstrated a new approach for improving the selectivity of clinically used therapeutics and for developing multifunctional small molecules that self-assemble in water to result in new supramolecular hydrogels of therapeutic agents that are biostable, target specific, and potent.
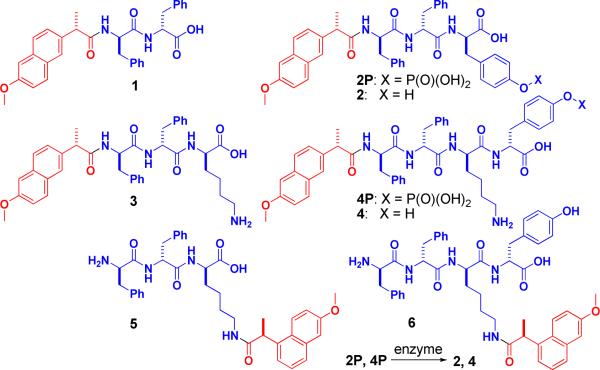
We design the hydrogelators based on the crystal structure of COX-2,22 which suggests that the conjugation of amino acids to Npx hardly disrupts the binding of Npx to COX-2. Figure 1 shows an example of the design. According to the binding of Npx (red) with COX-2 enzyme (gray) (Figure 1A), the carboxylate end of Npx is available for modification after Npx binds to COX-2 due to the large open space in the structure of COX-2. Figure 1B shows the predicted binding model of hydrogelator Npx-D-Phe-D-Phe (1, Npx-ff, blue spheres represent D-Phe-D-Phe) and COX-2: the connection of a rather bulky D-Phe-D-Phe dipeptide to Npx still allows the Npx to bind to the active site of COX-2. In order to generate the hydrogels in situ (or in vivo) via enzymatic reactions,23 we introduce D-tyrosine phosphate to 1. Additionally, we connect Npx to the side chain of small peptides of D-amino acids for evaluating the correlation between the structure and the activity of the hydrogelators of NSAIDs. Scheme 1 shows the molecular structures of the designed derivatives of Npx in this work. The connection of D-Phe-D-Phe, D-Phe-D-Phe-D-Tyr, D-Phe-D-Phe-D-Lys or D-Phe-D-Phe-D-Lys-D-Tyr to Npx results in molecules Npx-ff (1), Npx-ffy (2), Npx-ffk (3), or Npx-ffky (4), respectively, that contains Npx at the backbone of the small peptide. The conjugation of Npx to the side chain of D-Phe-D-Phe-D-Lys or D-Phe-D-Phe-D-Lys-D-Tyr via the ε-amino group of the D-Lys residue produces molecules ffk(Npx) (5) and ffk(Npx)y (6). The addition of a phosphate group on the tyrosine residue of 2 and 4 affords the precursors (2P and 4P) that would convert to molecules 2 and 4 followed by the dephosphorylation catalyzed by phosphatases.23,24
Figure 1.
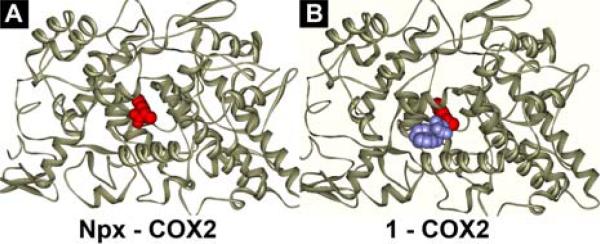
The binding of (A) Npx and (B) 1 with COX-2 enzyme (the ligands as CPK model and the COX-2 as ribbons).
Scheme 1.
The structures of the hydrogelators consisting of D-amino acids and naproxen (Npx).
We synthesize the molecules in Scheme 1 according to the synthetic procedures that combine solid phase synthesis and N-hydroxysuccinimide (NHS) assisted coupling reaction.25 After the synthesis of the designed hydrogelators, the gelation test indicates that all the hydrogelators in Scheme 1 are able to form stable hydrogels at the concentration of 0.8 wt% (Figure 2), but the hydrogels exhibit a slightly different appearance. For example, the aid of sonication and heating affords the aqueous solution of 1 at pH 9.0, which turns into an opaque hydrogel upon the adjustment of the pH to 4.0 at room temperature. Unlike the case of 1, the addition of 0.2 U/mL of alkaline phosphatase into the solution of 2P results in a transparent hydrogel of 2 at pH 7.6. By changing the pH and temperature, we obtain the hydrogels of 3, 5, and 6, respectively.25 By adding 0.2 U/mL of alkaline phosphatase into the solution of 4P, we obtain the hydrogel of 4.
Figure 2.
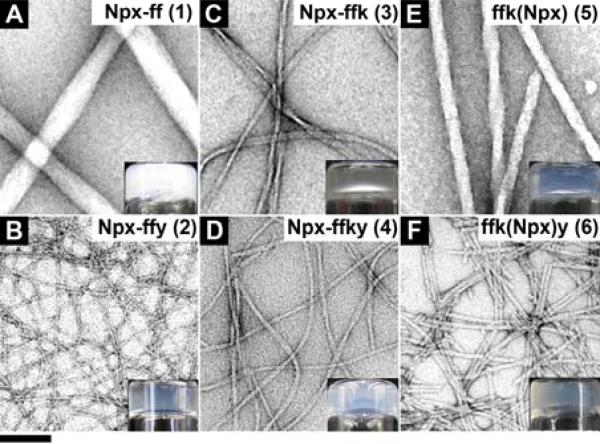
The TEM images of the hydrogels of (A) 1 (pH 4.0); (B) 2 (pH 7.6); (C) 3 (pH 7.6); (D) 4 (pH 7.6); (E) 5 (pH 7.0); (F) 6 (pH 7.0) (inset: optical images). Hydrogels of 2 and 4 made by adding 0.2 U/mL alkaline phosphatase to the solutions of 2P and 4P. All hydrogels are at 0.8 wt% (f = D-Phe, k = D-Lys, y = D-Try). The scale bar is 100 nm.
We use transmission electron microscopy (TEM) to examine the Npx containing hydrogels for evaluating the characteristics of the molecular assemblies. As shown in Figure 2A and 2B, hydrogelator 1 self-assembles to afford large and rigid nanofibers with average width of 54±7 nm, while hydrogelator 2 gives long, thin, and flexible nanofibers with average width of 7±2 nm (Figure 2B). Figure 2C shows the nanofibers with helical structure formed in hydrogel of 3, of which average width is 16±3 nm. The enzymatically formed hydrogel of 4 comprises of long and flexible nanofibers with average width of 10±2 nm (Figure 2D). Hydrogel of 5 exhibits helical, rigid, and long nanofibers with average widths of 26±3 nm (Figure 2E), meanwhile, hydrogelator 6 self-assembles to give rigid but short nanofibers with average widths of 7±2 nm, which tend to form bundles (Figure 2F). As shown in the bottom row of the images in Figure 2, the hydrogels containing D-Tyr (i.e., hydrogels of 2, 4, and 6) exhibit smaller diameter nanofibers that entangle to form network with higher density than their corresponding hydrogels (i.e., hydrogels of 1, 3, and 5) without D-tyrosine. The incorporation of D-Lys in hydrogelator 3 also makes it to form more flexible and narrower nanofibers than the nanofibers of hydrogelator 1. Hydrogel 4 contains the nanofibers that have similar morphologies to those in hydrogel 2. The hydrogels of 5 and 6, which have Npx connected at the side chain, contains nanofibers that are rigid and straight, which differ from those flexible and long nanofibers in the hydrogels of 3 and 4. These differences in the morphologies of these hydrogels indicate the position of Npx and the presence of tyrosine at the C-terminal of the hydrogelators likely play a role in their self-assembly in water.
We use oscillatory rheology to examine the viscoelastic properties of the hydrogels. All the Npx containing hydrogels exhibit viscoelastic properties of a solid-like material because of the storage moduli (G’) being significantly higher than the loss moduli (G”) and the frequency independence of the storage moduli of the hydrogels (Figure S14). Table 1 summarizes the critical strains and the moduli of hydrogels measured from strain sweep and dynamic frequency sweep. The relatively large critical strains of 3 and 4 suggest that the ε-amino group from the lysine residue makes the networks of the hydrogels to be resilient. The low critical strains of hydrogels 1, 5, and 6 apparently agree with the rigidity of the nanofibers in those hydrogels, which also confers relatively high storage moduli (G’). These results provide insights on the correlation between molecular structures of the hydrogelators and the viscoelasticity of the supramolecular hydrogels. Moreover, the critical strains of the hydrogels are comparable or higher than that of commercial topical gels (e.g., Gelrite® with the critical strain of 1%26), suggesting that the hydrogels are rheologically suitable for topical use.
Table 1.
The rheological properties and TEM characteristics of the hydrogels of the conjugates of d-amino acids and naproxen.
| Dynamic strain sweep | Dynamic frequency sweep | TEM images | |
|---|---|---|---|
| Critical strain (%) | G’, G”a (Pa) | Fiber width (nm) | |
| 1 | 1.0 | 5.3 × 104, 2.3 × 103 | 54±7 |
| 2 | 1.6 | 6.2 × 102, 6.7 × 10 | 7±2 |
| 3 | 5.2 | 3.9 × 102, 4.9 × 10 | 16±3 |
| 4 | 5.5 | 1.5 × 102, 3.0 × 10 | 10±2 |
| 5 | 0.41 | 3.8 × 103, 4.2 × 102 | 26±3 |
| 6 | 0.40 | 1.4 × 103, 4.4 × 102 | 7±2 |
The value is taken at frequency equals 6.28 rad/s.
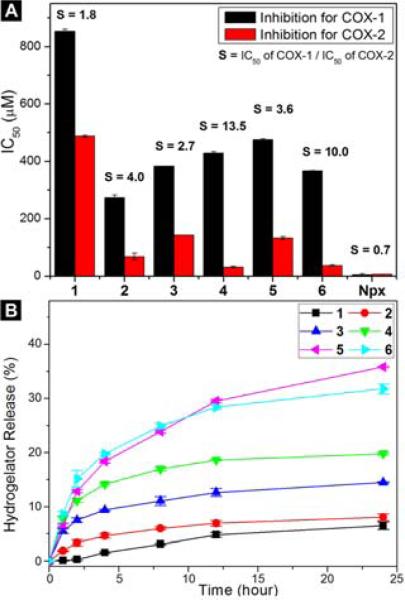
We perform in vitro inhibition assays27 for both COX-1 and COX-2 enzymes to evaluate the efficacies of the NSAID containing hydrogelators. As shown in Figure 3A, the IC50 values of COX-1 enzyme of hydrogelators 1, 2, 3, 4, 5, and 6 are 853.8, 273.7, 383.5, 428.9, 476.3, and 367.3 μM, respectively. All these value are almost two orders of magnitude higher than the reported IC50 values of naproxen (0.6-4.8 μM28) in the literature. Undoubtedly, the attachment of the small D-peptides to naproxen greatly reduces its binding to COX-1, which should reduce the associated adverse gastrointestinal and renal effects. For COX-2 enzyme, an inducible enzyme at the sites of inflammation, the IC50 values of hydrogelators 1, 2, 3, 4, 5, and 6 are 487.7, 68.8, 143.2, 31.7, 132.2, and 36.7 μM, respectively. Since the reported IC50 of naproxen to COX-2 is 2.0-28.4 μM,28 hydrogelators 4 and 6, obviously, afford reasonable IC50 values for the inhibition of COX-2. Thus, hydrogelators 4 and 6 exhibit excellent selectivity, S = 13.5 and S = 10.0, respectively, towards COX-2. These results not only validate 4 and 6 as potential candidates for topical NSAID gels, but also suggest that the presence of D-tyrosine on the D-peptides is beneficial for the activity and selectivity regardless the position of Npx on either the side chain or the main chain of the D-peptide. In the control compound, the use of L-amino acids (L-Phe, L-Lys, and L-Tyr) to replace the D-amino acid residues in hydrogelators 1, 2, 3, and 4 results in the hydrogelators that exhibit higher IC50 values and poor selectivity towards COX-2 (Figure S12). For example, L-4 (Npx-FFKY) exhibits IC50 values of 38.0 and 114.8 μM for COX-2 and COX-1, respectively, which affords the selectivity for COX-2 inhibition to be about 3. These results indicate the advantages of using D-peptide for generating the hydrogelators containing Npx to achieve high selectivity.
Figure 3.
(A) The IC50 values of the Npx based hydrogelators for inhibiting COX enzyme (the selectivity, defined as the IC50 ratio of COX-1 and COX-2, is labeled on the top of the bars). (B) The release profiles of the Npx based hydrogelators from hydrogels of 1, 2, 3, 4, 5, and 6.
After studying their drug efficacies, we evaluate the sustained release of Npx containing hydrogelators from 0.8 wt% of hydrogels. We incubate 100 μL of hydrogels at 37 °C for 24 hours with 100 μL of PBS buffer solution (pH 7.4), which is refreshed and monitored at 1h, 2h, 4h, 8h, 12h, and 24h. Figure 3B shows the release profile of these hydrogelators. After 24 hours, hydrogel 1 slowly and steadily releases 6.5% of gelators. With increased solubility contributed from hydrophilic amino acid residues (i.e., Tyr and Lys), hydrogels 2, 3, and 4 release gelators of 8.0%, 14.5%, and 19.8%, respectively. Hydrogels 5 and 6 release 35.8% and 31.7% of gelators after 24h, likely due to their good solubility of the hydrogelators and the brittleness of the nanofiber networks (as indicated by low critical strains of the hydrogels 5 and 6). Dynamic light scattering of the solutions of the released hydrogelators (1 or 6) exhibits little difference with that of the PBS buffer (Figure S15), suggesting that the hydrogelators unlikely exist as self-assembled nanofibers after being released from the hydrogels. These results suggest that these Npx containing hydrogels may serve as topical gels for sustained drug delivery.
We also examined the biocompatibility of the Npx containing hydrogelators by incubating them with HeLa cells for 72 hours at 37 °C. As shown in Figure S13, all of these hydrogelators have the IC50 values higher than 500 μM, except 1 with IC50 value of 357 μM. The high IC50 values of the hydrogels indicate that they are cell compatible. Although the absorption of formazan in the MTT assay indicates the promotions of the growth of the cells when the cells are incubated with hydrogelators 2, 5, or 6, we find no promotion of the cell proliferation based on the change of the numbers of the HeLa cells. Though the exact mechanism remains to be determined, these increase absorptions likely originate from the increase of metabolic activity in those HeLa cells treated by 2, 5, or 6, without increasing viability of cells. 29
In conclusion, we have developed multifunctional supramolecular hydrogelators made of D-amino acids and NSAID, which is a new approach for delivering therapeutic agents by biostable, target specific, and potent hydrogels. In addition, the incorporation of D-amino acids makes Npx a highly selective NSAID for inhibiting COX-2 and minimizing its adverse effect. This approach may provide useful strategy for reducing the adverse drug reactions (ADR) of other therapeutic agents or candidates, a subject being investigated further. Although the phosphate trigger in 2P or 4P appears less relevant to topical gel application of NSAID as a pain reliever, the enzyme triggered gelation may find new applications in other setting when the change of physiological conditions accompanied by the presence of phosphatases.
Supplementary Material
ACKNOWLEDGMENT
This work is partially supported by Human Frontier Program (HFSP, RGP 0056/2008), start-up grant from Brandeis University, and NIH (R01CA142746), and assisted by the Brandeis University EM facility (supported by NIH P01 GMGM-62580).
Footnotes
Supporting Information
Synthesis of hydrogelators, HPLC traces and LC-MS data of the reduction of hydrogelators, and rheological data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agarwal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. JAMA-J. Am. Med. Assoc. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 2.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FitzGerald GA. N. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 3.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA, Investigators APT. N. Engl. J. Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 4.Banning M. Expert Opin. Pharmacotherapy. 2008;9:2921–2929. doi: 10.1517/14656566.9.16.2921. [DOI] [PubMed] [Google Scholar]
- 5.Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. BMC Musculoskelet. Disord. 2004;5:28. doi: 10.1186/1471-2474-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]; Grace D, Rogers J, Skeith K, Anderson K. J. Rheumatology. 1999;26:2659–2663. [PubMed] [Google Scholar]; Cevc G, Blume G. Biochim. Biophys. Acta-Biomembranes. 2001;1514:191–205. doi: 10.1016/s0005-2736(01)00369-8. [DOI] [PubMed] [Google Scholar]
- 6.Bhuniya S, Seo YJ, Kim BH. Tetrahedron Lett. 2006;47:7153–7156. [Google Scholar]
- 7.Terech P, Weiss RG. Chem. Rev. 1997;97:3133–3159. doi: 10.1021/cr9700282. [DOI] [PubMed] [Google Scholar]; Sangeetha NM, Maitra U. Chem. Soc. Rev. 2005;34:821–836. doi: 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]; Estroff LA, Hamilton AD. Chem. Rev. 2004;104:1201–1217. doi: 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]; Yang Z, Liang G, Xu B. Acc. Chem. Res. 2008;41:315–326. doi: 10.1021/ar7001914. [DOI] [PubMed] [Google Scholar]; Yan CQ, Pochan DJ. Chem Soc Rev. 2010;39:3528–3540. doi: 10.1039/b919449p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu B. Langmuir. 2009;25:8375–8377. doi: 10.1021/la900987r. [DOI] [PubMed] [Google Scholar]
- 9.Cui HG, Pashuck ET, Velichko YS, Weigand SJ, Cheetham AG, Newcomb CJ, Stupp SI. Science. 2010;327:555–559. doi: 10.1126/science.1182340. [DOI] [PMC free article] [PubMed] [Google Scholar]; Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]; Matson JB, Stupp SI. Chem Commun. 2012;48:26–33. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jayawarna V, Ali M, Jowitt TA, Miller AE, Saiani A, Gough JE, Ulijn RV. Adv. Mater. 2006;18:611–614. [Google Scholar]; Zhou M, Smith AM, Das AK, Hodson NW, Collins RF, Ulijn RV, Gough JE. Biomaterials. 2009;30:2523–2530. doi: 10.1016/j.biomaterials.2009.01.010. [DOI] [PubMed] [Google Scholar]; Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Science. 2008;319:1812–1816. doi: 10.1126/science.1154586. [DOI] [PubMed] [Google Scholar]
- 10.Salick DA, Kretsinger JK, Pochan DJ, Schneider JP. J. Am. Chem. Soc. 2007;129:14793–14799. doi: 10.1021/ja076300z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Kuang Y, Guo ZF, Guo ZH, Krauss IJ, Xu B. J. Am. Chem. Soc. 2009;131:13576–13577. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]
- 12.Zhao F, Ma ML, Xu B. Chem. Soc. Rev. 2009;38:883–891. doi: 10.1039/b806410p. [DOI] [PubMed] [Google Scholar]; Caron P, Beckers A, Cullen DR, Goth MI, Gutt B, Laurberg P, Pico AM, Valimaki M, Zgliczynski W. J. Clin. Endocrinol. Metab. 2002;87:99–104. doi: 10.1210/jcem.87.1.8153. [DOI] [PubMed] [Google Scholar]
- 13.Cameron PJ, Johnson EK, Adams DJ. J. Am. Chem. Soc. 2010;132:5130–5136. doi: 10.1021/ja909579p. [DOI] [PubMed] [Google Scholar]
- 14.Kiyonaka S, Sada K, Yoshimura I, Shinkai S, Kato N, Hamachi I. Nat Mater. 2004;3:58–64. doi: 10.1038/nmat1034. [DOI] [PubMed] [Google Scholar]
- 15.Yamamichi S, Jinno Y, Haraya N, Oyoshi T, Tomitori H, Kashiwagi K, Yamanaka M. Chem Commun. 2011;47:10344–10346. doi: 10.1039/c1cc13826j. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Long MJC, Shi JF, Hedstrom L, Xu B. Chem Commun. 2012;48:8404–8406. doi: 10.1039/c2cc33631f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]; Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 18.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Adv. Mater. 2006;18:1345–1360. [Google Scholar]; Langer R. Adv. Mater. 2009;21:3235–3236. doi: 10.1002/adma.200902589. [DOI] [PubMed] [Google Scholar]
- 19.Torchilin VP. J. Control. Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]; Brazel CS, Peppas NA. Polymer. 1999;40:3383–3398. [Google Scholar]
- 20.Reches M, Gazit E. Science. 2003;300:625–627. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]; Gorbitz CH. Chem.-Eur. J. 2001;7:5153–5159. doi: 10.1002/1521-3765(20011203)7:23<5153::aid-chem5153>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Kuang Y, Gao YA, Xu B. Langmuir. 2011;27:529–537. doi: 10.1021/la1020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duggan KC, Walters MJ, Musee J, Harp JM, Kiefer JR, Oates JA, Marnett LJ. J. Biol. Chem. 2010;285:34950–34959. doi: 10.1074/jbc.M110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang ZM, Liang GL, Wang L, Xu B. J. Am. Chem. Soc. 2006;128:3038–3043. doi: 10.1021/ja057412y. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Shi JF, Yuan D, Xu B. Nat. Commun. 2012;3:1033. doi: 10.1038/ncomms2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.supporting information.
- 26.Carlfors J, Edsman K, Petersson R, Jornving K. Eur. J. Pharm. Sci. 1998;6:113–119. doi: 10.1016/s0928-0987(97)00074-2. [DOI] [PubMed] [Google Scholar]
- 27.Blobaum AL, Marnett LJ. J. Med. Chem. 2007;50:1425–1441. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]; Kargman S, Wong E, Greig GM, Falgueyret JP, Cromlish W, Ethier D, Yergey JA, Riendeau D, Evans JF, Kennedy B, Tagari P, Francis DA, Oneill GP. Biochem. Pharmacol. 1996;52:1113–1125. doi: 10.1016/0006-2952(96)00462-5. [DOI] [PubMed] [Google Scholar]; Cheong E, Ivory K, Doleman J, Parker ML, Rhodes M, Johnson IT. Carcinogenesis. 2004;25:1945–1952. doi: 10.1093/carcin/bgh184. [DOI] [PubMed] [Google Scholar]; Cordero JA, Camacho M, Obach R, Domenech J, Vila L. Eur. J. Pharm. Biopharm. 2001;51:135–142. doi: 10.1016/s0939-6411(00)00149-1. [DOI] [PubMed] [Google Scholar]
- 28.Barnett J, Chow J, Ives D, Chiou M, Mackenzie R, Osen E, Nguyen B, Tsing S, Bach C, Freire J, Chan H, Sigal E, Ramesha C. Biochim. Biophys. Acta-Protein Struct. Molec. Enzym. 1994;1209:130–139. doi: 10.1016/0167-4838(94)90148-1. [DOI] [PubMed] [Google Scholar]; Laneuville O, Breuer DK, Dewitt DL, Hla T, Funk CD, Smith WL. J. Pharmacol. Exp. Ther. 1994;271:927–934. [PubMed] [Google Scholar]
- 29.Wang P, Henning SM, Heber D. PLoS ONE. 2010;5:e10202. doi: 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.