Abstract
Given the importance of memory cytotoxic T lymphocytes (CTLs) in eliminating altered self-cells, including virus-infected and tumor cells, devising effective vaccination strategies for generating memory CTLs is a priority in the field of immunology. Herein, we elaborate upon a novel boosting approach that utilizes synthetic peptides and Toll-like receptor (TLR) agonists as adjuvants to generate sufficient numbers of memory CTLs to protect against infection in mice. Peptide boosting with lipopolysaccharide (LPS), a TLR4-ligand, has been shown to progressively enhance memory CTLs. Whether this result is strictly dependent on activation of TLR4 or can be similarly achieved by signaling through other TLRs is of practical interest in vaccine development but is yet unknown. In this report, we present evidence that intravenous peptide boosting together with TLR3 and TLR9 agonists (Poly IC and CpG, respectively) is highly effective and induces large quantities of memory CTLs of effector memory phenotype after three boosts. Compared to LPS, CpG and Poly IC generate more robust immune responses after the first and second boosts, indicating that a protective level of CTLs might be achieved with fewer boosts when CpG or Poly IC is used. Lastly, the resultant memory CTLs from boosting with different TLR agonists as adjuvant are equally protective against pathogen challenge and are not immune senescent. Therefore, TLR agonists are effective adjuvants in intravenous peptide boosting for the generation of functional memory CTLs.
Keywords: T cells, cytotoxicity, peptide, memory, adjuvant, boosting, TLR agonist
Introduction
Immunological memory is the defining feature of adaptive immunity and the basis for vaccination. While we have been successful at containing or even eradicating numerous infectious diseases through vaccination, other diseases, such as HIV and malaria, have proven more difficult to manage. Effectors of cellular immunity, CD8 T cells, or cytotoxic T lymphocytes (CTLs), are able to kill infected cells through direct lysis and the secretion of antiviral cytokines (e.g. IFN-γ and TNF-α) [1,2], and they play a critical role in the control of bacteria- and virus-infected host cells and cancer cells [3,4]. The memory CTL population, derived from naïve CD8 T cells after full activation, is long-lived and rapidly initiates a robust response upon reencountering the same or a similar pathogen [5]. Protective memory can last for many years after initial exposure to antigen, and in some cases it can last a lifetime [6,7].
Given the importance of memory CTLs in eliminating altered self-cells, including virus-infected and tumor cells, devising effective vaccination strategies for generating memory CTLs is a priority in the field of immunology. Prime-boosting with different vectors has proven effective [8], but the limited availability of appropriate vectors constrains the practical applications of this strategy [9,10]. Peptide-vaccines are a promising alternative to vector-based vaccines. However, peptides are notorious for being poorly immunogenic. One approach to increasing the efficacy of peptide immunization is co-administration of adjuvant. Adjuvants typically work by prolonging antigen persistence, enhancing costimulatory signals, increasing local inflammation, and/or triggering the nonspecific proliferation of lymphocytes [1,11]. Selection of an adjuvant depends on the species to be vaccinated, the likelihood of side effects, and the ability to promote the desired immune response (e.g. cell-mediated or humoral immunity) [12,13].
The most widely used adjuvants in humans are alum-based [12,13]. Alum salts induce the formation of an antigen depot at the inoculation site, enabling the slow release of antigen. Unfortunately, alum-based adjuvants seldom induce cellular immune responses [12,13]. Another class of adjuvants, oil-based adjuvant emulsions, are commonly used in veterinary vaccines and two formulations have been approved in Europe for use in adjuvanated human influenza vaccines [13]. Similar to alum-based adjuvants, adjuvant emulsions work through formation of an antigen depot and typically do not promote strong cellular immunity [12]. A relatively new class of vaccine adjuvant – ligands of Toll-like receptors (TLRs) - shows promise for its ability to increase the crosstalk between the innate and adaptive immune systems and enhance the specific immune response against co-inoculated antigens [14]. Many successful live-attenuated or killed vaccines are naturally immunogenic, as they possess motifs that trigger TLR pathways [13]. TLR ligands likely contribute to CD8 T cell memory by stimulating antigen presenting cells (APCs) - promoting up-regulation of MHC molecules, costimulatory molecules, and inflammatory cytokines which can program CTLs to memory [5,15,16].
We previously determined that intravenous peptide boosting generates a large number of functional memory CTLs when applied with lipopolysaccharide (LPS) (Smyth et al. Accepted by BBRC). LPS acts through TLR4, utilizing the adaptor molecule MyD88 to initiate a signaling cascade through an IL-1R-associated kinase (IRAK) ultimately activating NF-κB [17]. NF-κB activation stimulates the production of type I interferons (IFN-α and IFN-β) and promotes IL-12 production which enhances the cellular immune response [18]. This MyD88-dependent pathway is utilized by all TLRs except for TLR3, which signals through the adaptor molecule TRIF [14,17,18]. In the absence of MyD88, TLR4 can also employ the TRIF-dependent pathway. This pathway activates interferon regulatory factor 3 (IRF3) and culminates in the production of type I interferons [18,19]. The ability to signal through both MyD88- and TRIF-dependent pathways is exclusive to TLR4 [14,17,18]. Whether peptide boosting with other TLR ligand adjuvants, which operate solely through the MyD88-dependent pathway or the TRIF-dependent pathway, such as agonists of TLR9 and TLR3, respectively, can similarly enhance functional memory CTLs has yet to be examined. Thus, the goal of this study was to determine if a TLR3 ligand, Poly IC (polyriboinosinic:polyribocytidylic acid), and a TLR9 ligand, CpG, induce memory CTLs of similar phenotype and function to LPS when administered intravenously with peptide.
Materials and Methods
Mice
OT-I mice were gifts from MF Mescher, University of Minnesota, MN. OT-I mice possess T cells with transgenic TCRs specific for H-2Kb and OVA257–264 [15,20]. C57BL/6 mice were purchased from the National Cancer Institute. Mice were maintained under specific pathogen-free conditions at the University of Maryland. These studies have been reviewed and approved by the Institutional Animal Care and Use Committee (protocol ID R-12-23).
Reagents and immunizations
All conjugated fluorescent antibodies were purchased from eBioscience (San Diego, CA), BD Biosciences (San Diego, CA), or Biolegend (San Diego, CA). Tetramer was a gift from SC Jameson, University of Minnesota. LPS, CpG OD1826, and Poly IC were purchased from Invivogen (San Diego, CA). SIINFEKL peptide was purchased from New England peptide (Gardner, MA). Boosting was performed through i.v. tail injection, and each boost consisted of 50 ug LPS, CpG OD1826, or Poly IC, and 50 ug of SIINFEKL in a total volume of 300 ul DPBS.
Viruses and bacteria
Recombinant VV-GFP-JAW-OVA (VV-OVA) expressing the OVA257–264 epitope and recombinant LM expressing full-length secreted OVA (LM-OVA) were gifts from SC Jameson, University of Minnesota, as previously described [15,21]. Mice were infected i.p. with VV-OVA at 5×106 PFUs and i.v. with LM-OVA at 104 CFU for primary infection and 5×105 CFU for test of protection.
Naive T cell purification
Lymph nodes (LN) (inguinal, axillary, brachial, cervical, and mesenteric) were harvested from wild-type (WT) OT-I mice. LNs were pooled and disrupted to obtain a single-cell suspension. Enrichment of CD8+CD44low cells was acheived by negative selection using MACS magnetic cell sorting (Miltenyi Biotec, Auburn CA), as we described before [15,21].
Adoptive transfer and flow cytometric analysis
C57BL/6NCr mice recieved purified naive OT-I cells through adoptive transfer by i.v. tail injection at the numbers indicated for each experiment. Mice were infected one day after transfer. Single-cell suspensions were obtained from tissues, viable cell counts were done using trypan blue, and the percentage of OT-I cells in the sample was determined by flow cytometry using a FACSCalibur flow cytometer and CellQuest software (BD Biosciences), as previously described [15,21]. As is common practice in immunology[22], we used thirty days as a cutoff point for examination of memory CTLs as we reported previously[15,23,24].
Intracellular cytokine staining after in vitro rechallenge
Isolated cells from mice having received OT-I adoptive transfer were incubated at 2×106 cells/ml in RP-10 with 0.2 µM OVA257–264 peptide and 1 µl of GolgiPlug (BD Biosciences) for 3.5 h at 37°C. Following incubation, cells were fixed for 15 min in Cytofix buffer (BD Biosciences) at 4°C, permeabilized in saponin-containing Perm/Wash buffer (BD Biosciences) for 15 min at 4°C, and stained with conjugated Abs for 30 min at 4°C. Cells were then washed once with Perm/Wash buffer and once with PBS containing 2% FBS and analyzed by flow cytometry.
Statistics
Unpaired two-tailed Student’s t-tests were used to determine significant differences between treatments using Prism (GraphPad Software).
Results
Repetitive peptide boosting with TLR-L adjuvants induces large numbers of antigen-specific CTLs
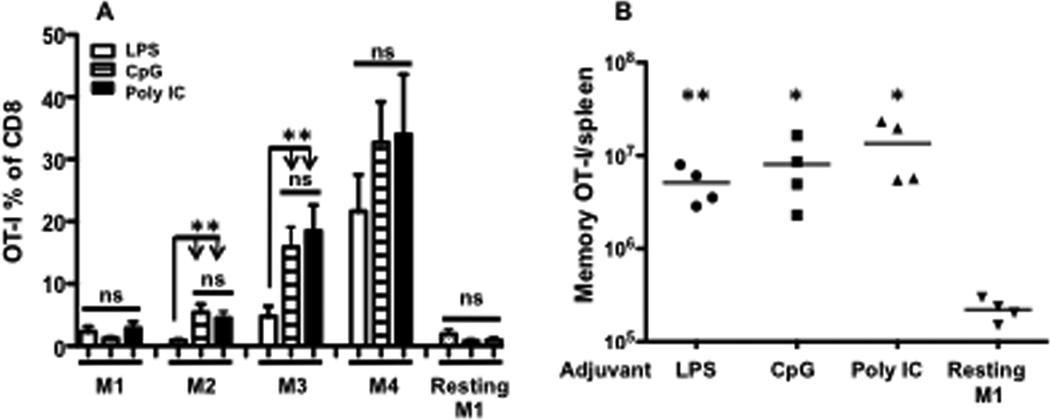
Although LPS is a well-characterized TLR4 ligand and commonly used adjuvant in animal experiments, it is not approved for use in human trials due to its toxic effects. Several other molecular adjuvants targeting different TLRs, especially CpG and Poly IC, have proven effective in vaccination [17,25,26,27]. To test if these adjuvants have similar efficacy, purified naïve OT-I cells were transferred into B6 mice, and the mice were primed with VV-OVA the next day to induce low but detectable background memory. Thirty days later, circulating memory OT-I cells in the blood were examined to confirm the presence of memory (designated as M1). Boosting with peptide (SIINFEKL), peptide plus LPS, peptide plus CpG, or peptide plus Poly IC was repeated every 32 days. Memory OT-I cells in the blood were examined 30 days after each boost. Analysis of these memory OT-Is in the blood showed that all three adjuvants (plus peptide) progressively enhanced memory CTLs (Fig. 1A). However, both CpG and Poly IC were more potent in increasing memory CTLs after the first (M2) and second (M3) boosts, indicating that they may be superior to LPS as adjuvants. The third boost, on the other hand, led to an increase in memory CTLs in all three adjuvant groups (Fig. 1A). Lack of a significant difference between treatments could be explained by variation among individual animals, as a few samples reached OT1 numbers above 60% of total CD8 in the CpG and Poly IC groups (data not shown). At these high levels, we promptly stopped further boosting in the same animals due to the potentially lethal effects of further antigen stimulation. In agreement with our previous report (Smyth et al. Accepted by BBRC), the resting memory OT-Is (resting M1) from VV-OVA priming did not change over time (Fig. 1A).
Figure 1. Repetitive peptide boosting with different TLR-L adjuvants generates large numbers of antigen-specific memory CTLs.
Naive OT-I CD8 T cells were transferred into B6 mice 105/mouse, which were primed with VV-OVA the next day. Boosts were performed three times with different adjuvants at 32-day intervals, and blood samples were drawn every 30 days after each priming or boosting. Thirty days after the third boost, memory mice were sacrificed to harvest the major memory CTL reservoir spleen. (A) Memory OT-I percentage of total CD8 in blood. The bar indicates mean of 10–15 mice per group, plus SEM. Comparison was performed between LPS group and CpG or Poly IC groups in each memory stage. (B) Total memory OT-I cells from spleens of resting M1 mice and mice having received three boosts with LPS, CPG, or Poly IC. Each bar represents the mean plus SEM. Asterisks indicate statistical significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns: not significant. These will be followed in the rest of the paper.
To compare the resultant memory CTLs in tissue from different adjuvants, we harvested spleen, the major reservoir for memory CTLs (Smyth et al. Accepted by BBRC), 30 days after the third boost. For each adjuvant group, memory OT-Is reached numbers of approximately 10 million per animal, which were all significantly higher than resting M1 controls (Fig.1B). Although the mean of memory OT-Is in the LPS group was lower than CpG and Poly IC groups, the difference was not significant. The endogenous peptide-specific CTLs were undetectable in all of the memory mice (data not shown).
Repetitive peptide boosting with different TLR agonists generates CTLs of similar phenotype
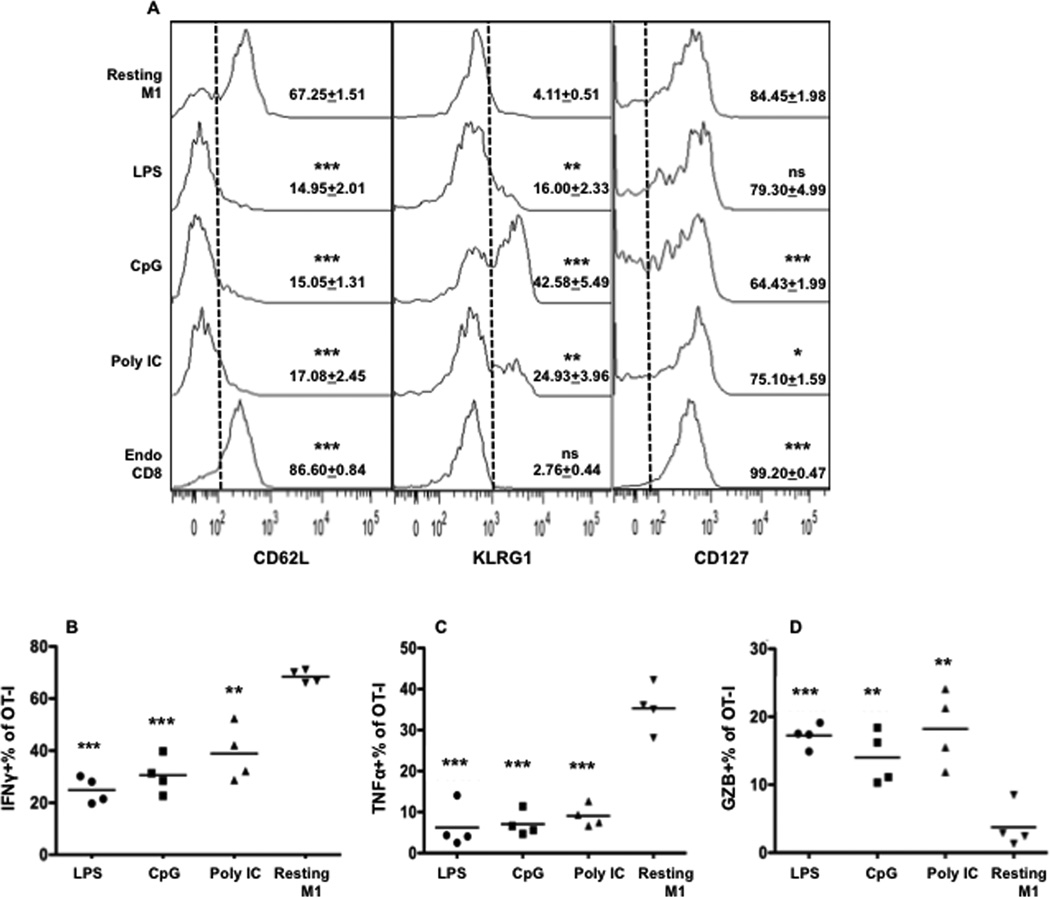
Peptide boosting in the presence of LPS, CpG, or Poly IC resulted in CTLs of a similar effector memory phenotype (CD62LloCD127hi)[28,29] (Fig. 2A). However, the CpG group had higher KLRG1 expression and lower CD127 expression (Fig. 2A). KLRG1 is an inhibitory receptor expressed on activated CD8 T cells [30,31]. High expression of KLRG1 has been related to short-lived terminal effector CD8 T cells[32]. There was a decreased number of IFNγ- and TNFα-producing memory CTLs from all three adjuvant groups, but an increased number of GZB-producing cells compared to the CTLs after priming (control, resting M1) (Fig. 2B–D). This indicates that the development of memory CTLs with reduced IFNγ production but enhanced GZB production is a general trend that results from repetitive peptide boosting and is independent of the adjuvant applied.
Figure 2. Repetitive peptide boosting with different TLR agonists generates CTLs of similar phenotype.
(A) Spleen samples were from Fig. 1B. Representative histograms of expression of CD62L, KLRG1 and CD127, with statistics as mean of gated areas +SEM. Asterisks indicate statistical significance compared to resting M1. Every group had 4 animals. (B–D) Comparison of IFNγ, TNFα and GZB expression in memory OT-I cells in the spleens from Fig.1B. Each group was compared to resting M1.
Memory CTLs from peptide boosts with TLR-L adjuvants protect against LM-OVA challenge
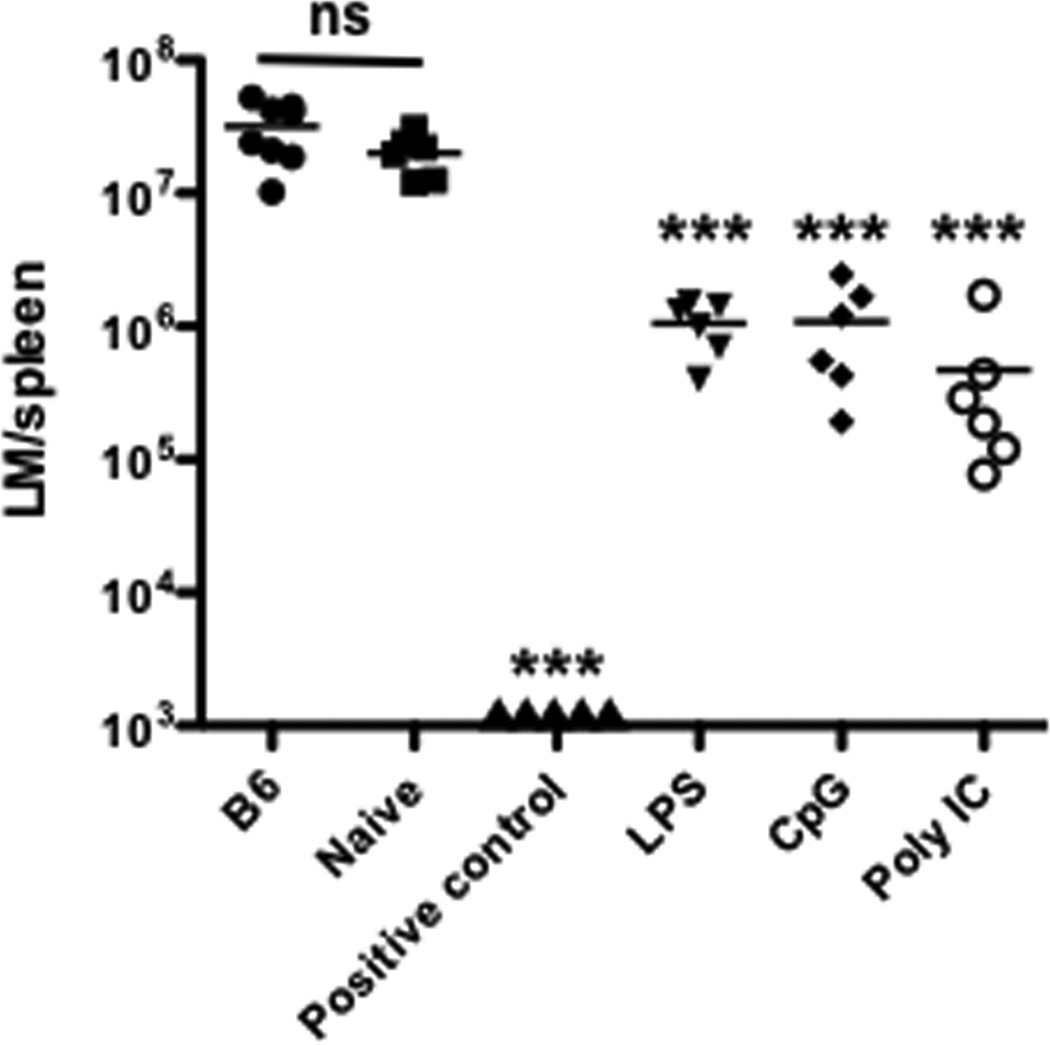
KLRG1 is a marker for short-lived effectors [30] and may be indicative of impaired function. The increase in KLRG1 expression in CTLs resulting from boosting with peptide and CpG prompted us to assess the protective abilities of CTLs generated by each treatment directly on a per-cell basis. We transferred an equal number of memory CTLs from each adjuvant group. As a positive control, we directly challenged M1 mice (resting M1 after priming). As expected, the bacterial burden was lowest in the positive control. In this case, the reduced bacterial load is due to the combined action of far more than 105 memory CTLs in M1. Compared to the naïve group, the three adjuvant groups displayed similar protection against LM-OVA challenge (Fig. 3).
Figure 3. Memory CTLs from peptide boosts with TLR-L adjuvants are protective against challenge with LM-OVA.
Splenocytes containing 105 memory (M4) or naïve OT-I cells were transferred into naïve B6 mice, which were challenged with 5×105 LM-OVA i.v. the next day. Spleens were harvested for LM-OVA counting three days after LM-OVA challenge. VV-OVA-primed memory mice (with OT-I transferred) were used as positive controls. Each group was compared to the naïve OT-I transferred group.
Memory CTLs induced by repetitive peptide boosts with different adjuvants are proliferative
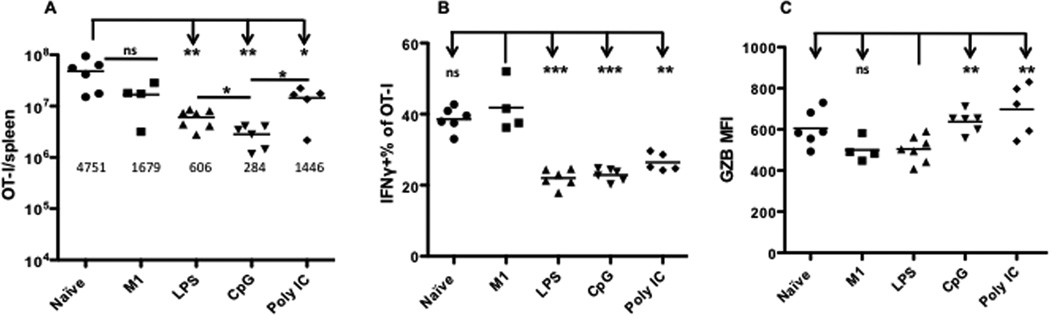
Effector memory CTLs can quickly kill infected cells and thus control infection without going through dramatic expansion upon pathogen challenge[22,33,34]. Although the resultant effector memory CTLs were protective, it was still possible that they were senescent due to experiencing multiple rounds of expansion. Ceased proliferation in response to antigen stimulation is the major feature of this phenomenon [29,35,36,37,38,39,40,41]. To examine the possibility of immune senescence, we compared the proliferative response of memory CTLs from Fig.1A that had experienced three rounds of peptide boosting with different adjuvants. 105 memory OT-Is from each group were transferred into new B6 recipients, and naïve OT-I and resting M1 were included as controls. Recipients were challenged with LM-OVA the day after transfer. Spleens were harvested six days after challenge. Repetitively boosted memory CTLs achieved substantial expansion (Fig. 4A), although the number was significantly higher in the naïve OT-I group compared to all except the immune group (Fig. 4A). The slightly superior expansion ability of naïve CTLs to memory CTLs is also observed in other reports[29,42]. Among adjuvant groups, expansion was the highest in the Poly IC-experienced memory CTLs, and lowest in CpG-experienced memory CTLs (Fig. 4A). Furthermore, these reactivated memory OT-I cells produced IFNγ, but at a level significantly lower than in resting M1 (Fig. 4B), consistent with data in Fig.2B. Interestingly, production of Granzyme B, a key functional molecule, was highest in the Poly IC group and lowest in the LPS group (Fig. 4C). These results suggest that memory CTLs from multiple rounds of peptide stimulation with adjuvant have normal proliferative responsiveness, and are able to produce certain level of functional molecules.
Figure 4. Memory CTLs from repetitive boosting are effective in responding to pathogen challenge.
Three-time peptide boosted memory OT-I cells (M4) generated by different adjuvants in Fig. 1A were transferred into new B6 recipients at 105 per mouse, and were subsequently challenged with LM-OVA at 104 CFU/mouse. Six days after challenge, spleens were harvested for analysis. (A) Comparison of OT-I expansion. Each group was compared to naïve OT-I transferred group. The number beneath each group represents the mean of fold changes. Fold change was calculated by comparing the number of OT-I in the spleen with the starting number. Overnight parking rate for transferred cells was 10% based on previous publications [56,57], so the starting number was calculated as 104. (B) Comparison of IFNγ production. Each group was compared to M1. (C) Comparison of Granzyme B production, which is directly reflected by Mean Fluorescence Intensity (MFI). Each group was compared to LPS group.
Discussion
Repetitive intravenous boosting with peptide and LPS in live vector-primed animals progressively enhances functional memory CTLs (Smyth et al. Accepted by BBRC). Whether this result is strictly dependent on activation of TLR4 or can be similarly achieved by signaling through other TLRs is of practical interest in vaccine development but has yet to be determined. Compounds that trigger other TLRs, such as TLR3 and TLR9, are promising adjuvants for enhancing cellular immunity. Poly IC is a synthetic analog of double-stranded RNA that binds to TLR3 [18]. TLR3 is unique in that it does not signal through the MyD88-dependent pathway but instead utilizes the adaptor molecule TRIF. The TLR3 pathway facilitates antigen cross-presentation, allowing CD8 T cells to be primed by exogenous antigen presented by MHC I molecules [18]. TLR9 recognizes unmethylated CpG motifs, which are relatively common in bacterial and viral DNA but are rare in mammals [43]. Stimulation of TLR9, located within the endosomal compartments of numerous cells of the immune system, produces an immune response geared toward eliminating intracellular pathogens by promoting the production of IL-12, IFN-γ, and TNF-α [18].
Here, we present evidence that peptide boosting with LPS, CpG, or Poly IC can progressively enhance memory CTLs to high numbers (Fig. 1A). Peptide boosting with LPS, CpG or Poly IC results in CTLs of effector memory phenotype (Fig. 2A) with reduced IFNγ production and enhanced GZB production (Fig. 2B and 2D). That this phenotype remains stable for more than six months (Smyth et al. Accepted by BBRC) and is unaltered by adjuvant implies that memory CTLs generated by intravenous peptide boosts are trapped in this effector phenotype. Interestingly, induction of a large number of effector memory CTLs is considered an important strategy for vaccination against HIV [10,44,45,46].
Although differences in expansion are observed among CTLs from boosting with different adjuvants (Fig. 4A), these CTLs achieve similar protection against LM-OVA challenge regardless of the adjuvant applied (Fig. 3). Repeated stimulation induces replicative senescence in human CD8 T cells in vitro, which is characterized by ceased division, resistance to apoptosis, and production of TNFα [36,37,39,40,47]. Immune senescence, which has been attributed to the Hayflick limit, is also apparent in a mouse model employing adoptive transfer [29]. In the present study, a large number of memory CTLs generated by sequential peptide boosts in the same animals (Fig. 1A) was able to expand continuously, albeit at a lower level than the naïve CTLs (Fig. 4A) and produced IFNγ and GZB (Fig.4B–C). Thus, repeated peptide boosting with TLR agonists does not lead to senescence, and concerns about the functional limit of memory CD8 T cells can be laid to rest.
While differences in CTL phenotype and function were minimal, CpG and Poly IC did out-perform LPS in one particular aspect. After the first and second boosts, CpG and Poly IC generated more robust immune responses, as evidenced by significantly greater percentages of OT-I T cells out of total CD8 T cells, compared to LPS (Fig. 1A). This has potentially profound implications for adjuvant selection in vaccine design as it indicates that a protective level of CTLs might be achieved with fewer boosts when CpG or Poly IC is utilized. Although the underlying cellular and molecular mechanisms are not clear, we suspect that dendritic cells (DCs), critical regulators of adaptive immunity, are involved. Stimulation of TLR3/4/9 induces similar phenotypic DC maturation, including upregulation of CD80, CD86, CD40 and MHC II [48,49] and drives DC polarization to Th1 [50], resulting in production of IL-12 and IFNα [51,52,53]. This is consistent with the adjuvant effects on memory CTLs in this report. Whereas TLR3 and TLR9 are present on fresh isolated DCs, TLR4 is undetectable [53,54,55]. Therefore, stimulation of TLR3/9 with Poly IC/CpG may act directly on DCs. Administration of LPS, on the other hand, may exert its effects indirectly—through non-DC or DC precursors [48]. Future research into the molecular mechanisms by which each adjuvant influences the cytokine milieu of the immune response and directly or indirectly alters the biologically relevant functions of memory CTLs may reveal new options for enhancing the immunogenicity of vaccines.
Highlights.
Peptide boosting with TLR-ligand adjuvants induces large numbers of memory CTLs.
Boosting with peptide plus CpG, Poly IC, or LPS generates CTLs of similar phenotype.
CpG and Poly IC may induce more robust immune responses, compared to LPS.
The resultant memory CTLs from boosting with different TLR agonists as adjuvant are not immune senescent.
Acknowledgements
We thank Drs. MF Mescher and SC Jameson from the University of Minnesota for providing reagents. The authors declare no conflict of interest. This work was supported by National Institutes of Health Grants R21AI095715A (to X. Z.) and Startup (to X. Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kindt TJ, Goldsby RA, Osborne BA, Kuby J. Kuby Immunology. 6 ed. New York: W.H. Freeman and Company; 2007. [Google Scholar]
- 2.Kalia V, Sarkar S, Ahmed R. CD8 T-cell memory differentiation during acute and chronic viral infections. Adv Exp Med Biol. 2010;684:79–95. doi: 10.1007/978-1-4419-6451-9_7. [DOI] [PubMed] [Google Scholar]
- 3.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 5.Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annual Review of Immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 6.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Ramshaw IA, Ramsay AJ. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today. 2000;21:163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 10.Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265:125–137. doi: 10.1111/j.1365-2796.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- 11.Tizard IR. Veterinary Immunology: An Introduction. 8 ed. St. Louis: Saunders Elsevier; 2009. [Google Scholar]
- 12.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 13.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Celis E. Toll-like receptor ligands energize peptide vaccines through multiple paths. Cancer Res. 2007;67:7945–7947. doi: 10.1158/0008-5472.CAN-07-1652. [DOI] [PubMed] [Google Scholar]
- 15.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;131:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2010;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Garcia K, Sun Z, Xiao Z. Temporal Regulation of Rapamycin on Memory CTL Programming by IL-12. PLoS ONE. 2011;6:e25177. doi: 10.1371/journal.pone.0025177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Z, Curtsinger JM, Prlic M, Jameson SC, Mescher MF. The CD8 T cell response to vaccinia virus exhibits site-dependent heterogeneity of functional responses. Int Immunol. 2007;19:733–743. doi: 10.1093/intimm/dxm039. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. J Exp Med. 2007;204:2667–2677. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M, Levine SJ. Toll-like receptor 3, RIG-I-like receptors and the NLRP3 inflammasome: Key modulators of innate immune responses to double-stranded RNA viruses. Cytokine Growth Factor Rev. 2011;22:63–72. doi: 10.1016/j.cytogfr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner H. Toll meets bacterial CpG-DNA. Immunity. 2001;14:499–502. doi: 10.1016/s1074-7613(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 28.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 30.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonjic S. Functional plasticity and robustness are essential characteristics of biological systems: lessons learned from KLRG1-deficient mice. Eur J Immunol. 2010;40:1241–1243. doi: 10.1002/eji.201040506. [DOI] [PubMed] [Google Scholar]
- 32.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 34.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol. 2011;46:135–140. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perillo NL, Walford RL, Newman MA, Effros RB. Human T lymphocytes possess a limited in vitro life span. Exp Gerontol. 1989;24:177–187. doi: 10.1016/0531-5565(89)90009-0. [DOI] [PubMed] [Google Scholar]
- 37.Perillo NL, Naeim F, Walford RL, Effros RB. The in vitro senescence of human T lymphocytes: failure to divide is not associated with a loss of cytolytic activity or memory T cell phenotype. Mech Ageing Dev. 1993;67:173–185. doi: 10.1016/0047-6374(93)90121-7. [DOI] [PubMed] [Google Scholar]
- 38.Adibzadeh M, Mariani E, Bartoloni C, Beckman I, Ligthart G, Remarque E, Shall S, Solana R, Taylor GM, Barnett Y, Pawelec G. Lifespans of T lymphocytes. Mech Ageing Dev. 1996;91:145–154. doi: 10.1016/0047-6374(96)01783-6. [DOI] [PubMed] [Google Scholar]
- 39.Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol. 1999;34:633–644. doi: 10.1016/s0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 40.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 41.Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirth TC, Harty JT, Badovinac VP. Modulating numbers and phenotype of CD8(+) T cells in secondary immune responses. Eur J Immunol. 2010;40:1916–1926. doi: 10.1002/eji.201040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Belyakov IM, Berzofsky JA. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 2004;20:247–253. doi: 10.1016/s1074-7613(04)00053-6. [DOI] [PubMed] [Google Scholar]
- 45.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 46.West EE, Youngblood B, Tan WG, Jin HT, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, Haining WN, Ahmed R. Tight Regulation of Memory CD8(+) T Cells Limits Their Effectiveness during Sustained High Viral Load. Immunity. 2011 doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Effros RB. Telomere/telomerase dynamics within the human immune system: Effect of chronic infection and stress. Exp Gerontol. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 49.Singh-Jasuja H, Thiolat A, Ribon M, Boissier MC, Bessis N, Rammensee HG, Decker P. The mouse dendritic cell marker CD11c is down-regulated upon cell activation through Toll-like receptor triggering. Immunobiology. 2012 doi: 10.1016/j.imbio.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Chen K, Xiang Y, Yao X, Liu Y, Gong W, Yoshimura T, Wang JM. The active contribution of Toll-like receptors to allergic airway inflammation. Int Immunopharmacol. 2011;11:1391–1398. doi: 10.1016/j.intimp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 52.Popovic PJ, DeMarco R, Lotze MT, Winikoff SE, Bartlett DL, Krieg AM, Guo ZS, Brown CK, Tracey KJ, Zeh HJ., 3rd High mobility group B1 protein suppresses the human plasmacytoid dendritic cell response to TLR9 agonists. J Immunol. 2006;177:8701–8707. doi: 10.4049/jimmunol.177.12.8701. [DOI] [PubMed] [Google Scholar]
- 53.Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, Wang Y, Venzon D, Epstein SL, Segal DM. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentationm CTL responses, and antiviral protection. J Immunol. 2011;186:2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 55.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O'Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]