Abstract
The primary role of apolipoprotein E (apoE) is to mediate the cellular uptake of lipoproteins. However, a new role for apoE as a regulator of bone metabolism in mice has recently been established. In contrast to mice, the human APOE gene is characterized by three common isoforms APOE ε2, ε3 and ε4 that result in different metabolic properties of the apoE isoforms, but it remains controversial whether the APOE polymorphism influences bone traits in humans. To clarify this, we investigated bone phenotypes of apoE knock-in mice, which express one human isoform each (apoE2 k.i., apoE3 k.i., apoE4 k.i.) in place of the mouse apoE. Analysis of 12 week-old female knock-in mice revealed increased levels of biochemical bone formation and resorption markers in apoE2 k.i. animals as compared to apoE3 k.i. and apoE4 k.i., with a reduced OPG/RANKL ratio in apoE2 k.i., indicating increased turnover with prevailing resorption in apoE2 k.i.. Accordingly, histomorphometric and μCT analyses demonstrated significantly lower trabecular bone mass in apoE2 than in apoE3 and apoE4 k.i. animals, which was reflected by a significant reduction of lumbar vertebrae maximum force resistance. Unlike trabecular bone, femoral cortical thickness, and stability was not differentially affected by the apoE isoforms. To extend these observations to the human situation, plasma from middle-aged healthy men homozygous for ε2/ε2, ε3/ε3, and ε4/ε4 (n=21, n=80, n=55 respectively) was analyzed with regard to bone turnover markers. In analogy to apoE2 k.i. mice, a lower OPG/RANKL ratio was observed in the serum of ε2/ε2 carriers as compared to ε3/ε3 and ε4/ε4 individuals (p=0.02 for ε2/ε2 vs ε4/ε4). In conclusion, the current data strongly underline the general importance of apoE as a regulator of bone metabolism and identifies the APOE ε2 allele as a potential genetic risk factor for low trabecular bone mass and vertebral fractures in humans.
Keywords: apolipoprotein E, OPG, RANKL, bone mass, genetic risk factor
Introduction
Apolipoprotein E (apoE), which is a major apolipoprotein of several lipoprotein classes, mediates the cellular uptake of lipoproteins by binding to hepatic endocytotic cell surface lipoprotein receptors.(1–4) The human APOE gene is characterized by three common isoforms APOE ε2, ε3 and ε4, which arise from two single nucleotide polymorphisms and result in different metabolic properties of the apoE isoforms, contributing to the association of the polymorphism with plasma lipid levels and cardiovascular and neurodegenerative disease.(5–7) The precise cellular mechanisms which are responsible for the differences between apoE2, apoE3 and apoE4 are not fully understood,(6,8) but lipoproteins containing apoE2 bind less well to the low-density lipoprotein receptor (LDLR), while apoE4 has a higher affinity to lipoprotein receptors, which results in different plasma lipoprotein profiles as well as different intracellular degradation and recycling pathways of the isoforms.(9) Recently a new role for apoE has emerged as a regulator of bone metabolism. Numerous papers have been published on the question of whether or not apoE isoforms are differentially associated with bone mineral density and fractures. These studies have yielded controversial results, at least at superficial analysis.(10) Part of the reason for this apparent controversy is different study designs with regard to cohort size, gender, ethnicity, end points and confounding parameters.
One major additional point that renders human clinical studies difficult is the asymmetric allele frequency of APOE ε2, ε3 and ε4 (about 0.05–0.1 vs 0.7–0.8 vs 0.1–0.2 in Caucasians),(11,12) leading to the requirement of very large patient cohorts in order to be able to compare adequate numbers of APOE ε2/ε2 versus ε3/ε3 versus ε4/ε4 individuals.(10) More recently, genetic mouse models with apoE deficiency have been used to shed further light on its role as a regulator of bone metabolism. As such, we and others have previously described that Apoe deficiency results in a high bone mass phenotype, and that the lack of apoE modulates the effect of a high-fat diet and of chronic renal failure on bone in mice.(13–15) Human APOE deficiency by contrast is an extremely rare condition and thus impossible to study in a clinical setting. From the very few individuals that have been described in the literature, there is no information available on potential bone phenotypic abnormalities.(16,17)
Given the clear impact of apoE on bone biology in mice, and in light of the putative isoform-specific effects of apoE on bone in humans, the current study was designed to clarify the issue of whether or not the human apoE2, apoE3 and apoE4 isoforms differentially affect bone metabolism. To address this question, we investigated bone turnover, structure and biomechanical stability of transgenic apoE knock-in mice, which exclusively express one human isoform each (apoE2 k.i., apoE3 k.i., apoE4 k.i.) instead of the murine apoE.(18–21) We also determined serum levels of bone turnover markers in samples from healthy men whose APOE genotype was known.
Material and Methods
Biochemical reagents
All biochemical reagents were purchased from Sigma-Aldrich (St. Louis) unless indicated otherwise.
Animals
Transgenic mice, homozygous for targeted replacement of the mouse endogenous Apoe gene with the human APOE2, APOE3 and APOE4 on a C57BL/6 background were purchased from Taconic (www.taconic.com). All animals were kept at the UT Southwestern animal facility on a 12h light cycle and fed a standard rodent chow diet (Diet 7001, Harlan Teklad, Madison, WI) with access to water ad libitum. At 12 weeks, female mice were fasted for 4h at a standardized time of the day prior to collection of urine. Animals were euthanized with isoflurane, exsanguinated and the skeletons were freed of soft tissue attachments. Upon sacrifice, mouse skeletons were fixed in 4% PBS-buffered paraformaldehyde (Electron Microscopy Sciences) over night at 4°C followed by an overnight incubation in 70% ethanol, and finally stored in 80% ethanol. All procedures were performed in accordance with the protocols approved by the Institutional Committee for Use and Care of Laboratory Animals of the University of Texas Southwestern Medical Center at Dallas (IACUC). Calcein label (Merck) was intraperitoneally injected 9 and 2 days before sacrifice according to a standard double-labeling protocol.(22)
Mouse serum and urine analysis
Mouse serum osteocalcin (OCN) was determined by an immunoradiometric assay (Immutopics) according to the manufacturer’s instructions and serum alkaline phosphatase (ALP) activity by the para-nitrophenyl phosphate (pNPP) method. The concentration of deoxypyridinoline (DPD) cross-links in urine was measured using an ELISA (Quidel, San Diego) and normalized to urinary creatinine levels determined by an alkaline picrate assay (Quidel, San Diego) according to manufacturer’s instructions. Mouse serum osteoprotegerin (OPG) and serum receptor activator of NFkB ligand (RANKL) were determined by quantitative sandwich enzyme immunoassays (R&D Systems) according to manufacturer’s instructions.
Bone histomorphometry and histological analysis
For comparative quantitative histomorphometry, undecalcified lumbar spines (L2–L4) were embedded in methylmethacrylate after dehydration and 5 μm sections were cut in sagittal plane on a rotation microtome (Cut 4060E, MicroTech) as described.(22) Sections were stained with toluidine blue and with modified von Kossa/van Gieson. Analysis of bone volume (BV/TV; %), trabecular thickness (Tb.Th; μm), trabecular number (Tb.N; mm−1), trabecular separation (Tb.Sp; μm), osteoblast number per bone perimeter (N.Ob./B.Pm; mm−1), osteoclast number per bone perimeter (N.Oc./B.Pm; mm−1), and trabecular bone formation rate (BFR/BS; μm3/μm2/y) was performed on L3 and L4 according to standardized protocols of the American Society for Bone and Mineral Research (23) using the Osteomeasure histomorphometry system (Osteometrics) in a blinded fashion. For the assessment of dynamic histomorphometric indices, mice were injected twice with calcein label as described above. Fluorochrome measurements were made on two nonconsecutive 12 μm thick sections per sample that were mounted unstained in Fluoromount (Electron Microscopy Sciences).
Micro CT and biomechanical testing
In order to analyze the bone microstructure and bone mineralization, the femora and lumbar vertebrae were scanned (55 kV/145 μA) in a micro computer tomograph μCT40 (Scanco Medical) with an isometric voxel size of 12 μm. Five samples were scanned simultaneously using a specially-built sample-holder with an integrated mineralization phantom which enables immediate bone mineral density evaluation. For further histomorphometric analyses the raw data were manually segmented and analyzed with the μCT Evaluation Program V4.4A (Scanco Medical). Thus data for trabecular BV/TV (%) and cortical thickness (Ct.Th; mm) of the femur diaphysis were obtained in the scanned samples.(24)
Biomechanical stability of the bone samples was determined using a commercial high precision instrument (Z2.5/TN 1S Zwick, Ulm Germany). For femoral testing three-point-bending assays were used. In brief, the ends of the bone were supported on two fulcra separated by 7 mm. With the posterior aspect of the femur resting on the fulcra, a load was applied from above to the anterior diaphysis midway between the two fulcra, at a constant speed of 3 mm/min to failure. Accordingly, vertebral strength was assessed using compression testing. Lumbar vertebrae (L5, L6) were mounted axially between the flat ends of two collinearly aligned aluminium cylinders by filling the gaps with sufficient amount of light-curing dental adhesive. These cylinders served as compression pins in subsequent biomechanical tests as force was applied axially from above at a constant speed of 3 mm/min to failure. Biomechanical failure was defined as a reduction of 25% of maximum force or a displacement of more than 10 mm. A chart recorder was used to generate a force-deformation curve. Maximum force (Fmax, N) and energy absorption when reaching maximum force (N x mm) were evaluated.
Quantitative backscattered electron imaging
BMD distribution (BMDD) measurements were performed on methylmethacrylate-embedded biopsies using quantitative backscattered electron imaging (qBEI). The application of this method and our technical set-up have been reported previously.(25–27) The biopsies were grinded coplanar, polished and carbon-coated. The scanning electron microscope (LEO 435 VP, Leo Electron Microscopy Ltd., England) was operated at 20 kV and 580 pA at a constant working distance of 20 mm (BSE Detector, Type 202, K.E. Developments Ltd., England). The signal amplification was calibrated during the entire procedure using carbon and aluminium standards (MAC Consultants Ltd., England). Grey level histograms of the bone specimens were obtained (Image J 1.42, National Institute of Health, USA) and converted into Ca weight. For statistical analysis Ca mean, Ca peak and Ca weight were evaluated as described elsewhere.(25) The accuracy of the method was ensured by EDX measurements of previously qBEI-measured bone biopsies (EDAX DX-4, EDAX Inc., U.S.A).
Determination of human bone turnover markers in healthy subjects
The prospective Second Northwick Park Heart Study (NPHS II) commenced in 1989. A sample of 3052 middle-aged men (50–61 years) were recruited from nine general medical practices in the UK. Methods have been described elsewhere.(28,29) NPHSII has a total of 3052 participants of which 3012 are Caucasian. 2775 of these had DNA taken and of these 2685 have the APOE genotype determined. The study was ethically approved by the local MRC institutional review committee. Participants attended examination in a non-fasting state, but were asked to refrain from smoking, vigorous exercise, or heavy meals from the midnight prior to the appointment. Height to the nearest 0.1 cm below and weight to the nearest 0.1 kg below were measured using a stadiometer and balance scale, respectively, in order to ascertain body mass index (BMI, kg/m2). Smoking habits were assessed by questionnaires. A 5 ml sample of venous blood was collected into a glass tube by Vacutainer technique (Becton Dickenson, Cowley, Oxford), and serum stored at −40°C prior to analysis in plastic screwcap vials (Nunc).
Human bone turnover markers were determined using commercially available assays according to the respective manufacturer’s instructions: osteoprotegerin and bone specific alkaline phosphatase from Quidel, RANKL (ampli-sRANKL) from Biomedica, osteocalcin from Immunotopics and NTX from Teco Medical.
Human Genotyping
DNA was extracted using the salting-out method from nucleated blood cells, and APOE genotype was determined by PCR and restriction enzyme analysis as described.(12,30) Independent observers unaware of the status of the participants read and cross checked all genotypes, and genotype was obtained in 2685/2775 (96.8%) of subjects.
Statistical analysis
For mouse data, a one-way ANOVA test including a Tukey for post-hoc analysis was used to assess statistical significance between groups for the respective mouse data (Figures 1–3). Statistical analysis for the human data was performed using STATA (Intercooled STATA Version 11.2, STATA Corporation, Texas). Baseline characteristics were compared by analysis of variance, continuous variables being presented as means and standard errors of the mean. Non-normally distributed variables were log-transformed before analysis with results presented as geometric means and approximate standard errors of the mean. For pairwise comparisons ε2ε2 and ε4ε4 groups were compared using unpaired t-tests. As each ε2ε2 and ε4ε4 was individually matched to a ε3ε3 participant, comparisons with the ε3ε3 group were made within matched pairs using paired t-tests. Smoking status was compared by chi-squared test. Stars in the figures are used to indicate levels of significance *p<0.05, **p<0.005, *** p<0.001.
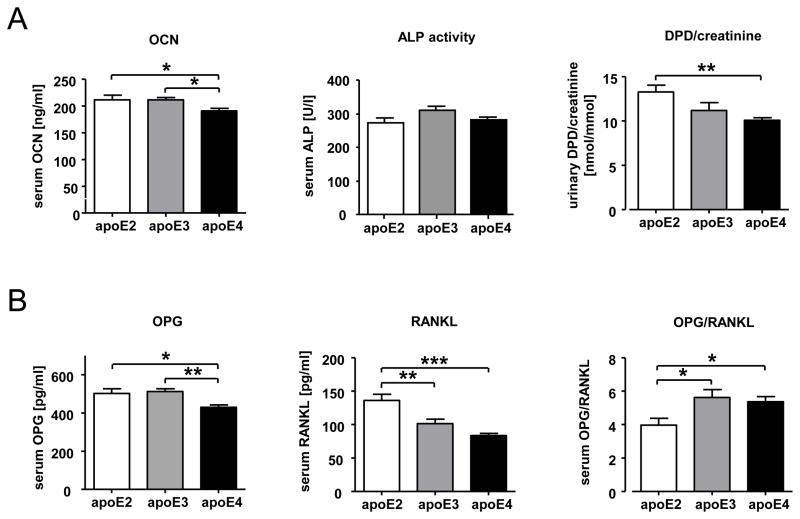
Figure 1. Differences in bone turnover markers in apoE2, apoE3, and apoE4 k.i. mice.
Female transgenic apoE2, apoE3, and apoE4 k.i. mice (12 weeks of age) were fasted for 4 hours prior to serum and urine collection upon sacrifice at a standardized time of day (1 p.m.). Alkaline phosphatase (ALP) enzyme activity, osteocalcin (OCN), osteoprotegerin (OPG), and RANK ligand (RANKL) concentrations were determined from serum (A,B) DPD and creatinine from urine (B). The bars represent mean values +/− SEM (n≥10 animals per group). Statistical differences were calculated by one-way ANOVA including a Tukey for post-hoc analysis. *p<0.05, **p<0.01, ***p<0.001
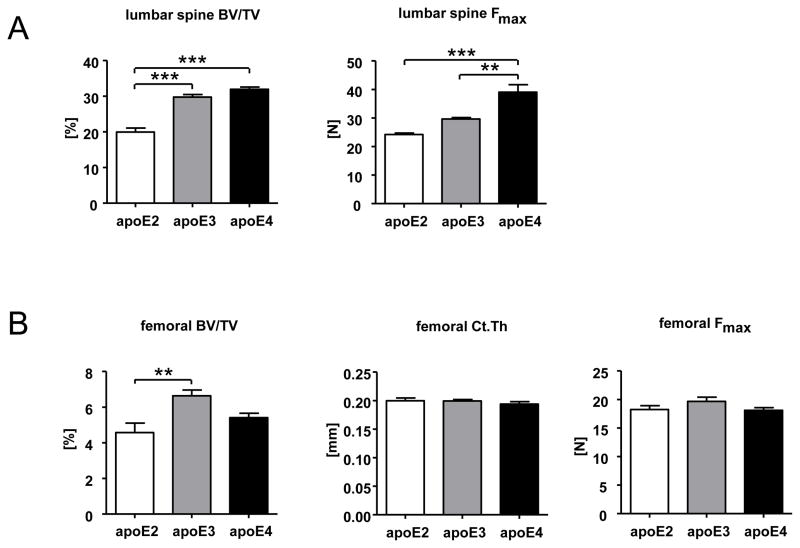
Figure 3. Reduced biomechanical stability of apoE2 k.i. lumbar vertebrae.
Female transgenic apoE2, apoE3, and apoE4 k.i. mice (12 weeks of age) were sacrificed, the skeleton was fixed and the lumbar spine (L2–L4) as well as one femur per animal was dissected out for μCT analysis and biomechanical testing. Lumbar trabecular bone mass (BV/TV [%]) and maximal force endurance (Fmax [N]) was determined in two vertebrae of each animal (n=5 mice per genotype) (A). Femoral trabecular bone mass (BV/TV [%]) was determined in the distal femur, cortical thickness [mm] in the mid-diaphysis and maximum force resistance (Fmax [N]) by three-point bending tests (n=10 femurs per genotype) (B). The bars represent mean values +/− SEM. Statistical differences were calculated by one-way ANOVA including a Tukey for post-hoc analysis. *p<0.05, **p<0.01, ***p<0.001
Results
Bone turnover markers and OPG/RANKL differ significantly between apoE2, apoE3 and apoE4 knock-in mice
As a first approach to test in vivo whether the human apoE isoforms apoE2, apoE3 and apoE4 may differentially affect bone metabolism, we analyzed biochemical bone turnover markers in serum and urine as well as the serum OPG and RANKL concentrations in 12 week-old female animals of each genotype (apoE2 k.i., apoE3 k.i., apoE4 k.i.; n ≥ 13 per group). Overall, total differences in the serum concentration of the bone formation markers osteocalcin and alkaline phosphatase (not bone specific), as well as the urinary resorption marker desoxypyridinoline (DPD), were small between the groups of mice with different genotypes, but significant when comparing apoE2 k.i. with apoE4 k.i. mice (OCN p<0.05, DPD p<0.005). Higher OCN and DPD concentrations in apoE2 k.i. than in apoE 4 k.i. indicated a relatively high bone turnover status in apoE2 k.i. animals and a relatively low turnover status in apoE4 k.i. mice, with apoE3 k.i. values in between the other two genotypes (Figure 1A). This finding was further supported by significantly higher serum OPG (p<0.05) and RANKL (p<0.001) serum concentrations in apoE2 k.i. mice with the OPG/RANKL ratio being significantly lower in apoE2 k.i. animals as compared to apoE3 k.i. and apoE4 k.i. (p<0.05, Figure 1B), suggesting a high bone turnover state with prevailing resorption in apoE2 k.i. mice.
Low lumbar trabecular bone mass and bone formation rate in apoE2 knock-in mice
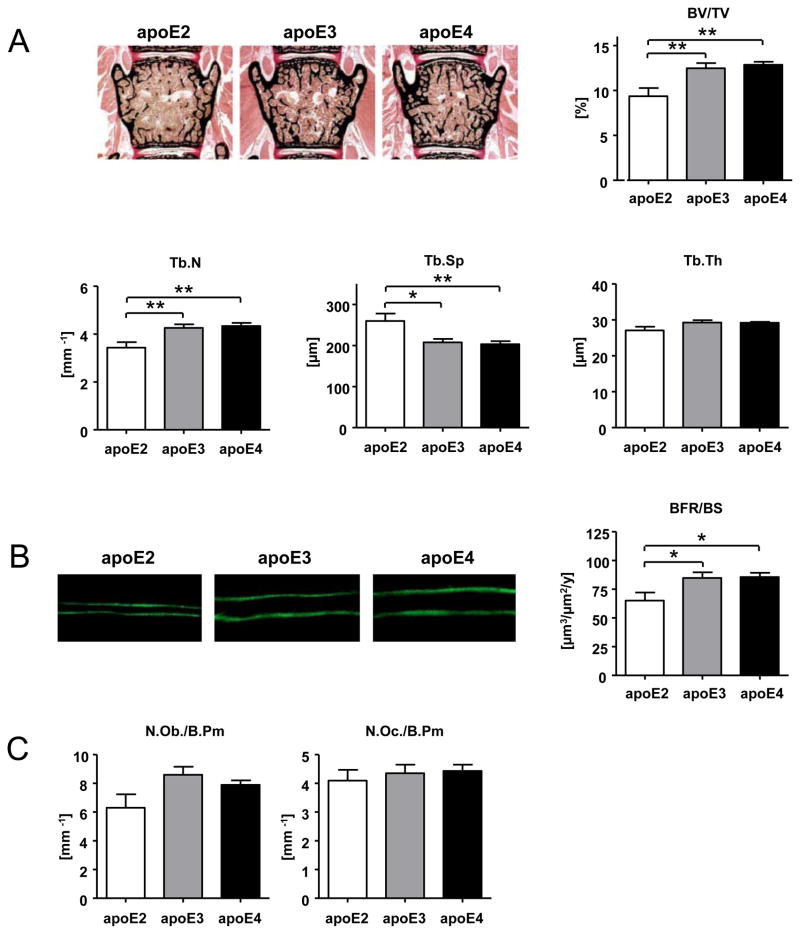
We next analyzed whether the apoE isoform-specific differences in bone turnover markers and serum OPG/RANKL ratio corresponded to apoE isoform-specific differential effects on bone architecture. Histomorphometric analysis of lumbar vertebrae of eight (apoE2, apoE3) or nine (apoE4) animals per genotype revealed a significantly lower trabecular bone mass (BV/TV) in apoE2 k.i. versus apoE3 k.i. and apoE4 k.i. mice (p<0.005) with according changes in trabecular numbers (Tb.N) and trabecular separation (Tb.Sp) (Figure 2A), corresponding well to the biochemical indicators of high bone turnover, with prevailing resorption in apoE2 k.i. animals (Figure 1). Bone formation rate (BFR), as assessed by the calcein-labeling technique, showed a similar pattern between the three apoE isoforms, with significantly lower BFR in apoE2 k.i. in comparison to apoE3 k.i. and apoE4 k.i. (p<0.05, Figure 2B). Osteoblast and osteoclast cell numbers were not significantly different in the lumbar vertebrae of mice with different genotypes (Figure 2C).
Figure 2. Low trabecular bone mass in apoE2 k.i. mice.
Female transgenic apoE2, apoE3, and apoE4 k.i. mice (12 weeks of age). Histomorphometric analysis of lumbar (L3 and L4) trabecular bone structure (bone volume (BV/TV)), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp) (A), trabecular bone formation rate (BFR/BS) (B) and histomorphometric determination of bone cell numbers (osteoblast number (N.Ob/B.Pm) and osteoclast number (N.Oc/B.Pm) (C). The bars represent mean values +/− SEM (n≥8 animals per group). Statistical differences were calculated by one-way ANOVA including a Tukey for post-hoc analysis. *p<0.05, **p<0.01, ***p<0.001
Reduced biomechanical stability of the lumbar spine, but not the femur in apoE2 knock-in mice
We assessed the microarchitecture and stability of lumbar vertebrae and femoral cortex in the transgenic apoE k.i. animals by micro CT and biomechanical stress tests, in order to understand whether the apoE isoforms have a compartment-specific effect on trabecular and cortical bone. The histomorphometric findings of reduced vertebral trabecular bone mass (Figure 2B) was confirmed by the μCT analyses, which demonstrated significantly lower BV/TV in apoE2 k.i. mice than in apoE3 k.i. and apoE4 k.i. mice for the lumbar vertebrae (p<0.001) as well as the distal femur (apoE2 k.i. vs apoE3 k.i. only, p<0.005) (Figure 3A, B). The lower vertebral trabecular bone mass in apoE2 k.i. mice did result in a corresponding significantly reduced biomechanical stability of the lumbar vertebrae as assessed by the maximum force endurance in an axial compression loading test in comparison to apoE4 k.i. (p<0.005 Figure 3A). In contrast to the clear differences in trabecular BV/TV and vertebral stability, the femoral cortical thickness in the mid-shaft region was not different between mice with different genotypes (Figure 3B). Accordingly, the maximum force resistance of the femora in a three-point bending test did not differ between mice with different apoE isoforms (Figure 3B).
Bone mineral density distribution in apoE knock-in mice
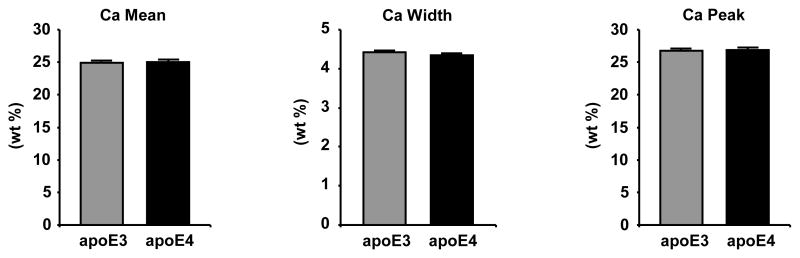
Since there was an apparent disconnect between the lumbar trabecular BV/TV and lumbar vertebral stability between the apoE3 k.i. and apoE4 k.i. mice (Figure 3A) which suggests underlying differences beyond bone microarchitecture between the genotypes that contribute to biomechanical stability, we investigated the bone mineral density distribution (BMDD) in the mineralized phase of the bone matrix. However, BMDD as assessed by quantitative backscattered electron imaging (qBEI) did not reveal differences between the mean calcium content or the peak and width of the calcium distribution between apoE3 and apoE4 lumbar vertebral trabecular bone of L3 and L4 (Figure 4).
Figure 4. No differences in bone mineral density distribution between apoE3 k.i. and apoE4 k.i. lumbar vertebrae.
Quantification of the frequency distribution of calcium within the mineralized phase of lumbar vertebrae L3 and L4 of female transgenic apoE3 and apoE4 k.i. mice (12 weeks of age) by quantitative backscattered electron imaging as assessed by the average calcium content (Ca mean), the heterogeneity of calcium distribution (Ca width) and the most frequently occurring calcium concentration (Ca peak). The bars represent mean values +/− SD (n≥8 animals per group).
Reduced OPG/RANKL ratio in the serum of human homozygous APOE ε2/ε2 individuals
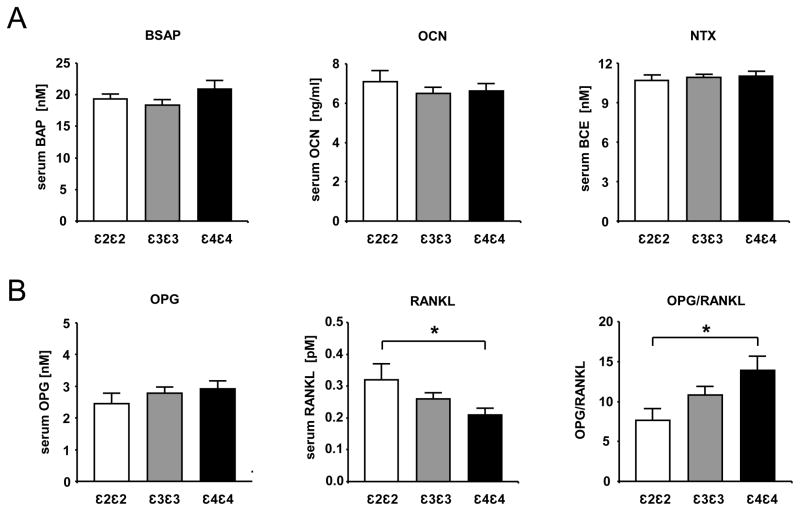
In a first attempt to transfer the above findings in the animal model to humans, we have analyzed biochemical turnover markers as well as OPG and RANKL concentrations in the serum of healthy, male individuals homozygous for the APOE ε2, ε3 and ε4 alleles, respectively. The serum samples were selected according to genotype from the Northwick Park Heart Study II (NPHS II), which comprised a total 2685 healthy middle-aged Caucasian UK males. The basic characteristics of this cohort have been described previously.(12) Because of its comparably low allele frequency, the APOE ε2 allele was the limiting factor for the purpose of the current study, allowing for the analysis of serum samples of n=21 homozygous APOE ε2/ε2 men and n=55 homozygous APOE ε4/ε4 men that were matched (on age +/− 3 years) to n=80 APOE ε3/ε3 (Table 1). Baseline characteristics with an influence on bone mass, such as age, BMI and smoking status did not show any differences between the groups. In contrast to the transgenic mice, the serum bone formation markers bone-specific alkaline phosphatase (BSAP) and osteocalcin as well as the serum bone resorption marker N-terminal telopeptide of collagen type 1 (NTX) did not display any significant differences between the three APOE genotype groups (Figure 5A). However, serum concentrations of RANKL and OPG did show a pattern that was reflecting the isoform-specific differences of the apoE knock-in mice: APOE ε2/ε2 individuals displayed a significantly higher RANKL serum concentration, resulting in a significantly lower OPG/RANKL ratio than APOE ε4/ε4 individuals, with APOE ε3/ε3 values intermediate between the two other groups (Figure 5B).
Table 1.
APOEgenotype distribution, age, BMI and smoking status in the NPHS II subgroup analyzed in this study.
| APOE genotype distribution | |||
|---|---|---|---|
| ε2ε2 (n=21) | ε3ε3 (n=80) | ε4ε4 (n=55) | |
| age (years) | 54.9 (2.6) | 55.3 (3.5) | 55.4 (3.7) |
| BMI (kg/m2) | 26.0 (3.0) | 26.0 (3.5) | 26.5 (3.5) |
| current smoking (%(N)) | 33.3 (7.0) | 31.3 (25.0) | 27.3 (15.0) |
Figure 5. Reduced OPG/RANKL serum ratio in healthy APOE ε2ε2 males.
The concentrations of the bone turnover markers bone-specific alkaline phosphatase (BSAP), osteocalcin (OCN), N-terminal telopeptide of collagen type 1 (NTX) (A) as well as osteoprotegerin (OPG) and RANK ligand (RANKL) (B) were determined with standard procedures from serum specimens of fasted male individuals from the NPHSII study (compare Table 1). The bars represent the geometric mean values +/− SD. (APOE ε2ε2 n=21; APOE ε3ε3 n=80; APOE ε4ε4 n=55). Statistical differences were calculated by paired or unpaired t-test *p<0.05, **p<0.01, ***p<0.001
Discussion
Here we report that the human apoE2, apoE3 and apoE4 isoforms, when expressed instead of the murine apoE in a transgenic knock-in mouse model, differentially affect bone turnover, trabecular bone mass and biomechanical stability of trabecular bone. ApoE2 was associated with a higher bone turnover, with prevailing resorption over formation and consequently a lower trabecular bone mass with reduced stability as compared to apoE3 and apoE4. Conversely, apoE4 is characterized by a relatively lower bone turnover, higher bone mass, and higher biomechanical stability. Of note, the significantly lower OPG/RANKL ratio in apoE2 k.i. mice was mirrored in healthy middle-aged men homozygous for APOE ε2/ε2, who also displayed a decreased ratio of OPG/RANKL serum concentrations when compared to APOE ε3/ε3 and ε4/ε4 individuals.
The present study was initiated to gain more insight into the presumed, but controversial role of APOE genetics in bone metabolism. In the literature, numerous reports have investigated whether the human APOE ε4 allele is associated with inferior bone quality (for review, see (10)). The first report that was published specifically on this question 15 years ago (31) was based on two arguments. First, the observation that vitamin K levels correlate with plasma triglyceride-rich apoE-containing lipoproteins and via this pathway could affect osteoblast biology.(32) Second, the observation that apoE4 in particular is associated with other involutional diseases, such as cardiovascular and Alzheimer’s disease,(33,34) on the basis of which the authors predicted a negative influence of apoE4 on bone. Their actual finding of a negative APOE ε4 gene dose effect on BMD in postmenopausal women has subsequently triggered a series of currently more than 25 publications. These studies have focused on effects ε3 versus ε4, either of which allele is present in the majority of the general population (>90%),(11) or on ε4 carriers versus non-carriers.(10) Considering the data that we present here, looking for a ε4 gene-dose effect on bone traits while neglecting the presence or absence of ε2 in the respective haplotypes, may have been a decade of research missing the crucial point. As such, it is possible that the ε2 allele was only another confounding parameter which was uncontrolled for in a very complex analysis that is subject to a multitude of interacting factors, including age, sex, weight, smoking, menopausal status, physical activity, medication and baseline BMD. To our knowledge, there is only one published report that has specifically looked at bone traits in ε2 carriers.(35) These authors did not find an association of either APOE allele with BMD at baseline or after adjustment for multiple confounders in early postmenopausal women. But for the purpose of their analysis, the ε2/ε2 homozygotes (n=16) were grouped together with heterozygotes ε2/ε3 (n=301) and compared to ε3/ε3 (n=1664) in a second and ε3/ε4 (n=619) plus ε4/ε4 (n=50) in a third group. This way of analyzing the data may have masked a potential differential effect of homozygous genotypes, in particular of the rare ε2 variant, on BMD.(35)
It has been clearly established that apoE regulates bone mass in mice (13–15) and therefore it is very likely that apoE also contributes to the regulation of bone mass in humans. To reduce the complexity of the human situation and to circumvent the problems associated with the asymmetric allele frequency of the human APOE gene, we used a well-defined model system that has been developed before by gene targeting to replace the murine Apoe gene with the three respective human APOE alleles.(18,20,21) These mice retain the murine Apoe regulatory sequences and have been carefully characterized to display apoE mRNA tissue distribution and expression levels comparable to wildtype mice and to each other.(18–21) Thus we consider these apoE knock-in mice a valuable tool to study the influence of the human genetic APOE polymorphism on bone in vivo whilst being able to control for any other confounding factor that renders clinical or epidemiological studies so difficult. Theoretically, body weight could be a confounding factor in this mouse model. It has previously been described that on chow diet, total body weights of adult apoE knock-in mice display slight but non-significant differences between apoE2, E3 and E4 in the order of E3>E2>E4 (20.2 ± 1.1 vs 19.9 ± 2.6 vs 19.3 ± 2.6 g).(36) Similarly, we have observed a small relative increase of body weight in 12 week-old female apoE3 (21.43 ± 0.38 g) as compared to apoE2 (20.01 ± 0.32 g) and apoE4 k.i. mice (20.51 ± 0.34 g). Although it cannot be ruled out that these differences in body weight may influence bone turnover and bone mass in this model, we consider it to be very unlikely that they are a relevant causative factor in mediating the 25% relative reduction of lumbar vertebral BV/TV in apoE2 k.i. mice as compared to the other two genotypes (Figure 2A) or any other significant difference on bone traits that we describe here. In addition, although differences in locomotor activity between apoE3k.i. and apoE4 k.i. mice have been described,(37) it is unlikely that these small differences contribute to the observed phenotype, especially since apoE4 k.i. mice have been reported to move less and thus would be expected to have a reduced bone mass phenotype. One of the major difficulties in the attempt to reproduce the key findings of the mouse model in humans is to find cohorts large and homogenous enough to be able to include a sufficiently large number of homozygous ε2/ε2 carriers while minimizing confounding factors. We therefore selected a subgroup of individuals from a study population of healthy, middle-aged males (NPHSII cohort) (12) because this cohort provided us with the advantage of ruling out the influences of skeletal growth versus old age, the influence of serious co-morbidities and, most importantly, the influence of menopause. The NPHSII cohort examined includes only UK white European Caucasian individuals and we have previously ruled out the possibility of significant population structure as an influence that might confound the relationship between APOE genotype and plasma traits.(38) In addition, BMI and smoking status as two further potentially confounding factors have been controlled for and were found to be of comparable incidence within the three groups of ε2/ε2, ε3/ε3, and ε4/ε4 carriers (Table 1) and thus are highly unlikely to influence the observed differences in OPG/RANKL serum concentrations. The observation that the OPG/RANKL ratio in the ε2/ε2 men compared to ε3/ε3 and ε4/ε4 carriers is similarly decreased as in the mouse model is indicating that the apoE system is likely to play an important role in human bone biology: APOE ε2 may in fact be the forgotten but most relevant variant with regard to bone traits in humans. In order to further confirm this concept, this finding needs to be reproduced in other cohorts and to be corroborated by direct measurements of bone qualities such as BMD and fracture incidence in further studies, in particular also in female populations as well as younger and older cohorts.
At present, we can only speculate about the underlying mechanisms for the profound differential effect of the human apoE isoforms on bone. One possible explanation could be differential modulation of signal transduction pathways by the three apoE isoforms in bone cells. For instance, it is possible that there is a differential effect on canonical Wnt signaling,(39) PDGF signaling (40) or nitric oxide signaling,(41) since all of these pathways are known to be involved in bone cell regulation and have been described to be modulated in apoE isoform-specific manner in other cell types.(39–41) Another explanation includes impaired lipoprotein-associated vitamin K delivery in apoE2 k.i. mice to target cells, i.e. osteoblasts, because of the reduced binding affinity of apoE2 to cell surface lipoprotein receptors.(32,42,43) Such a scenario would imply that there is a lower degree of carboxylation of osteoblast-derived gla-proteins which in turn would have to contribute to a higher bone turnover and development of a lower trabecular bone mass. Currently such a hypothesis is difficult to include or rule out, because the role of carboxylation of osteocalcin and matrix gla-protein for the regulation of bone mass is still not fully understood. In addition, it has been described recently that apoE4 k.i. mice display higher serum 25-(OH) vitamin D levels than apoE3 k.i. mice.(44) This difference in vitamin D could influence the bone mineral density distribution (BMDD) in the mineralized phase of the bone matrix and thereby contribute to the otherwise unexplained differences in lumbar vertebral stability of apoE3 k.i. and apoE4 k.i. mice in this study (Figure 3A). The apparent disconnect between lumbar trabecular bone mass with almost equal BV/TV in apoE3 k.i and apoE4 k.i. but concomitant significantly higher biomechanical stability of apoE4 k.i. lumbar vertebrae (Figure 3A) is intriguing. In general, differences in biomechanical stability can be due to bone mass and bone material properties. The inorganic mineralized phase, the organic un-mineralized phase and the relative composition of mineral to matrix ratio each contribute to the material properties of the composite tissue.(45,46)
In a first attempt to explain the discrepancy between bone mass and stability of the lumbar vertebral trabecular bone, we have focused on the mineral content and have quantified the respective distribution of the calcium content in the bone matrix of apoE3 k.i. and apoE4 k.i. mice (25–27) but did not find differences between the mean calcium content, nor the peak and width of the calcium distribution between apoE3 and apoE4 lumbar vertebrae (Figure 4). Thus, the genotype-specific differences in the mechanical stability of the lumbar vertebrae are more likely to be explained by differences in the organic bone matrix than by the frequency distribution of the calcium content within the bone matrix. A recently published paper reports of differences in collagen IV levels and other extracellular proteins between apoE3 and apoE4 k.i. mice in cerebral vascular basement membranes, which are sheets of highly specialized extracellular matrix.(47) These data suggest that, in principle, the apoE alleles may also differentially affect the composition of the organic bone matrix, e.g. by regulating collagen levels. At present, these intriguing questions remain open but since they have potential clinical importance they will have to be addressed further in future studies.
We have observed previously that Apoe deficiency in mice affects not only trabecular bone mass but femur cortical architecture as well.(15) In addition, numerous reports in the literature have discussed the question of whether human apoE4 was associated with not only increased incidence of vertebral but also of extremity fractures.(10) It is interesting that the isoform-specific differences in this study are apparent in trabecular bone, but not in cortical bone. This finding could imply that the APOE genotype in humans may predispose primarily to vertebral, but not peripheral fractures and should be kept in mind for future study designs with human cohorts.
In conclusion, the present study strongly underlines the general importance of apoE as a regulator of bone metabolism and indicates that the APOE ε2 allele is a candidate genetic risk factor for low trabecular bone mass and vertebral fractures in humans, calling for further large scale studies in different human populations that may ultimately lead to the integration of the APOE ε2 allele into diagnostic and therapeutic algorithms either in specified subpopulations or even in the general population.
Acknowledgments
We thank Walter Tauscher for excellent technical assistance and Joerg Heeren for critical reading of the manuscript. The work was supported by the DFG grant Ni 637/2-3 and the BMBF grant ANCYLOSS (to AN), a fellowship by the Boehringer Ingelheim Stiftung (to MD) and a postdoctoral fellowship award by the European Atherosclerosis Society (to AB). BM was supported by the DFG GRK 1459. NPHSII was supported by the British Medical Research Council, the US National Institute of Health (grant NHLBI 33014) and Du Pont Pharma, Wilmington, USA. JC and SEH are supported by the British Heart Foundation (PG08/008). JH is supported by grants from the NIH, AHAF, the Consortium for Frontotemporal Dementia Research and the Lupe Murchison Foundation.
MD performed the mouse experiments, helped to design the study and to write the manuscript. FTB performed the histomorphometric analyses. BM and AB measured biochemical bone turnover markers. RPM performed μCT and biomechanical tests. TK performed qBEI/BMDD analyses. MA, WR and JH were involved in the conceptual design and coordination of the study. JAC and SHE have recruited, genotyped and maintained the NHPSII cohort and collected serum. AN designed the study, coordinated the project, analyzed all data and wrote the manuscript. All authors have read and revised the manuscript.
This work was supported by the DFG grant Ni 637/2-3 and the BMBF grant ANCYLOSS (to AN), a fellowship of the Boehringer Ingelheim Stiftung (to MD) and a post-doctoral fellowship award by the European Atherosclerosis Society (to AB).
Footnotes
Conflict of Interest: All authors have no conflict of interest.
Contributor Information
Marco Dieckmann, Email: dieckmann.marco@UTsouthwestern.edu.
F. Timo Beil, Email: t.beil@uke.de.
Brigitte Mueller, Email: br.mueller@uke.de.
Alexander Bartelt, Email: abartelt@uke.uni-hamburg.de.
Robert P. Marshall, Email: r.marshall@uke.de.
Till Koehne, Email: t.koehne@uke.de.
Michael Amling, Email: amling@uke.de.
Wolfgang Ruether, Email: ruether@uke.uni-hamburg.de.
Jackie A. Cooper, Email: jackie.cooper@ucl.ac.uk.
Steve E. Humphries, Email: s.humphries@ucl.ac.uk.
Joachim Herz, Email: joachim.herz@utsouthwestern.edu.
Andreas Niemeier, Email: niemeier@uke.uni-hamburg.de.
Reference List
- 1.Beisiegel U. Receptors for triglyceride-rich lipoproteins and their role in lipoprotein metabolism. Curr Opin Lipidol. 1995;6:117–22. doi: 10.1097/00041433-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Herz J, Willnow TE. Lipoprotein and receptor interactions in vivo. Curr Opin Lipidol. 1995;6:97–103. doi: 10.1097/00041433-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–30. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 4.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 5.Heeren J, Beisiegel U, Grewal T. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:442–8. doi: 10.1161/01.ATV.0000201282.64751.47. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y. Mechanisms linking apolipoprotein E isoforms with cardiovascular and neurological diseases. Curr Opin Lipidol. 2010;21:337–45. doi: 10.1097/MOL.0b013e32833af368. [DOI] [PubMed] [Google Scholar]
- 7.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 8.Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heeren J, Grewal T, Laatsch A, Becker N, Rinninger F, Rye KA, Beisiegel U. Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J Biol Chem. 2004;279:55483–92. doi: 10.1074/jbc.M409324200. [DOI] [PubMed] [Google Scholar]
- 10.Niemeier A, Schinke T, Heeren J, Amling M. The role of apolipoprotein E in bone metabolism. Bone. 2012;50:518–24. doi: 10.1016/j.bone.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Humphries SE, Talmud PJ, Hawe E, Bolla M, Day IN, Miller GJ. Apolipoprotein E4 and coronary heart disease in middle-aged men who smoke: a prospective study. Lancet. 2001;358:115–9. doi: 10.1016/S0140-6736(01)05330-2. [DOI] [PubMed] [Google Scholar]
- 13.Bartelt A, Beil FT, Schinke T, Roeser K, Ruether W, Heeren J, Niemeier A. Apolipoprotein E-dependent inverse regulation of vertebral bone and adipose tissue mass in C57Bl/6 mice: modulation by diet-induced obesity. Bone. 2010;47:736–45. doi: 10.1016/j.bone.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Nikolov IG, Joki N, Nguyen-Khoa T, Ivanovski O, Phan O, Lacour B, Drueke TB, Massy ZA, Dos Reis LM, Jorgetti V, Lafage-Proust MH. Chronic kidney disease bone and mineral disorder (CKD-MBD) in apolipoprotein E-deficient mice with chronic renal failure. Bone. 2010;47:156–63. doi: 10.1016/j.bone.2010.04.600. [DOI] [PubMed] [Google Scholar]
- 15.Schilling AF, Schinke T, Munch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20:274–82. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- 16.Ghiselli G, Schaefer EJ, Gascon P, Brewer HB., Jr Type III hyperlipoproteinemia associated with apolipoprotein E deficiency. Science. 1981;214:1239–41. doi: 10.1126/science.6795720. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer EJ, Gregg RE, Ghiselli G, Forte TM, Ordovas JM, Zech LA, Brewer HB., Jr Familial apolipoprotein E deficiency. J Clin Invest. 1986;78:1206–19. doi: 10.1172/JCI112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–86. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N. Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res. 2009;50:178–82. doi: 10.1194/jlr.R800070-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–80. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Invest. 1998;102:130–5. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–7. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 24.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 25.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–66. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Roschger P, Plenk H, Jr, Klaushofer K, Eschberger J. A new scanning electron microscopy approach to the quantification of bone mineral distribution: backscattered electron image grey-levels correlated to calcium K alpha-line intensities. Scanning Microsc. 1995;9:75–86. [PubMed] [Google Scholar]
- 27.Seitz S, Koehne T, Ries C, De Novo Oliveira A, Barvencik F, Busse B, Eulenburg C, Schinke T, Puschel K, Rueger JM, Amling M, Pogoda P. Impaired bone mineralization accompanied by low vitamin D and secondary hyperparathyroidism in patients with femoral neck fracture. Osteoporos Int. 2012 doi: 10.1007/s00198-012-2011-0. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Miller GJ, Bauer KA, Barzegar S, Cooper JA, Rosenberg RD. Increased activation of the haemostatic system in men at high risk of fatal coronary heart disease. Thromb Haemost. 1996;75:767–71. [PubMed] [Google Scholar]
- 29.Miller GJ, Bauer KA, Barzegar S, Foley AJ, Mitchell JP, Cooper JA, Rosenberg RD. The effects of quality and timing of venepuncture on markers of blood coagulation in healthy middle-aged men. Thromb Haemost. 1995;73:82–6. [PubMed] [Google Scholar]
- 30.Bolla MK, Haddad L, Humphries SE, Winder AF, Day IN. High-throughput method for determination of apolipoprotein E genotypes with use of restriction digestion analysis by microplate array diagonal gel electrophoresis. Clin Chem. 1995;41:1599–604. [PubMed] [Google Scholar]
- 31.Shiraki M, Shiraki Y, Aoki C, Hosoi T, Inoue S, Kaneki M, Ouchi Y. Association of bone mineral density with apolipoprotein E phenotype. J Bone Miner Res. 1997;12:1438–45. doi: 10.1359/jbmr.1997.12.9.1438. [DOI] [PubMed] [Google Scholar]
- 32.Saupe J, Shearer MJ, Kohlmeier M. Phylloquinone transport and its influence on gamma-carboxyglutamate residues of osteocalcin in patients on maintenance hemodialysis. Am J Clin Nutr. 1993;58:204–8. doi: 10.1093/ajcn/58.2.204. [DOI] [PubMed] [Google Scholar]
- 33.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 34.Wilson PW, Myers RH, Larson MG, Ordovas JM, Wolf PA, Schaefer EJ. Apolipoprotein E alleles, dyslipidemia, and coronary heart disease. The Framingham Offspring Study. JAMA. 1994;272:1666–71. [PubMed] [Google Scholar]
- 35.Macdonald HM, McGuigan FE, Lanham-New SA, Fraser WD, Ralston SH, Reid DM. Vitamin K1 intake is associated with higher bone mineral density and reduced bone resorption in early postmenopausal Scottish women: no evidence of gene-nutrient interaction with apolipoprotein E polymorphisms. Am J Clin Nutr. 2008;87:1513–20. doi: 10.1093/ajcn/87.5.1513. [DOI] [PubMed] [Google Scholar]
- 36.Huang ZH, Maeda N, Mazzone T. Expression of the human apoE2 isoform in adipocytes: altered cellular processing and impaired adipocyte lipogenesis. J Lipid Res. 2011;52:1733–41. doi: 10.1194/jlr.M017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193:174–82. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Chen XH, Rodriguez S, Hawe E, Talmud PJ, Miller GJ, Underhill P, Humphries SE, Day IN. Evidence of admixture from haplotyping in an epidemiological study of UK Caucasian males: implications for association analyses. Hum Hered. 2004;57:142–55. doi: 10.1159/000079245. [DOI] [PubMed] [Google Scholar]
- 39.Caruso A, Motolese M, Iacovelli L, Caraci F, Copani A, Nicoletti F, Terstappen GC, Gaviraghi G, Caricasole A. Inhibition of the canonical Wnt signaling pathway by apolipoprotein E4 in PC12 cells. J Neurochem. 2006;98:364–71. doi: 10.1111/j.1471-4159.2006.03867.x. [DOI] [PubMed] [Google Scholar]
- 40.Zeleny M, Swertfeger DK, Weisgraber KH, Hui DY. Distinct apolipoprotein E isoform preference for inhibition of smooth muscle cell migration and proliferation. Biochemistry. 2002;41:11820–3. doi: 10.1021/bi026202k. [DOI] [PubMed] [Google Scholar]
- 41.Zhang KJ, Zhang HL, Zhang XM, Zheng XY, Quezada HC, Zhang D, Zhu J. Apolipoprotein E isoform-specific effects on cytokine and nitric oxide production from mouse Schwann cells after inflammatory stimulation. Neurosci Lett. 2011;499:175–80. doi: 10.1016/j.neulet.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 42.Niemeier A, Kassem M, Toedter K, Wendt D, Ruether W, Beisiegel U, Heeren J. Expression of LRP1 by human osteoblasts: a mechanism for the delivery of lipoproteins and vitamin K1 to bone. J Bone Miner Res. 2005;20:283–93. doi: 10.1359/JBMR.041102. [DOI] [PubMed] [Google Scholar]
- 43.Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PC, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43:230–7. doi: 10.1016/j.bone.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Huebbe P, Nebel A, Siegert S, Moehring J, Boesch-Saadatmandi C, Most E, Pallauf J, Egert S, Muller MJ, Schreiber S, Nothlings U, Rimbach G. APOE epsilon4 is associated with higher vitamin D levels in targeted replacement mice and humans. FASEB J. 2011;25:3262–70. doi: 10.1096/fj.11-180935. [DOI] [PubMed] [Google Scholar]
- 45.Burr DB. The contribution of the organic matrix to bone’s material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 46.Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. Journal of Materials Chemistry. 2004;14:2115–2123. [Google Scholar]
- 47.Hawkes CA, Sullivan PM, Hands S, Weller RO, Nicoll JA, Carare RO. Disruption of Arterial Perivascular Drainage of Amyloid-beta from the Brains of Mice Expressing the Human APOE epsilon4 Allele. PLoS One. 2012;7:e41636. doi: 10.1371/journal.pone.0041636. [DOI] [PMC free article] [PubMed] [Google Scholar]