Abstract
Objectives
Early onset of heroin use during adolescence might increase chances of later drug addiction. Prior work from our laboratory suggests, however, that adolescent male rats are actually less sensitive than adults to some enduring effects of heroin self-administration. In the present study, we tested two likely correlates of sensitivity to behavioral reinforcement in rats: physical withdrawal and locomotor sensitization.
Methods
Adolescent (35 days old at start) and adult (79 days old) male Sprague-Dawley rats were administered escalating doses of heroin, increasing from 1.0 to 8.0 mg/kg (i.p.) every 12 hr, across 13 days. Somatic signs of spontaneous withdrawal were scored 12 and 24 hr after the last injection, then every 24 hr for 5 days; locomotion was recorded concurrently. Challenge injections of heroin (1 mg/kg i.p.) were given at 4 points: as the first of the escalating doses (day 1), at days 7 and 13 during the escalating regimen, and after 12 days of forced abstinence. Body mass and food intake were measured throughout experimentation.
Results
A heroin withdrawal syndrome was not observed among adolescents as it was among adults, including somatic signs as well as reduced locomotion, body mass, and food intake. On the other hand, heroin-induced locomotor sensitization did not differ across ages.
Conclusion
Reduced withdrawal is consistent with the attenuated reinforcing effects of heroin among adolescent male rats that we reported previously. Thus, it is possible that adolescent rats could reveal important neuroprotective factors for use in treatment of heroin dependence.
Introduction
Drug abuse is prevalent in the U.S. and is typically initiated in adolescence (Johnston et al. 2010; SAMHSA 2009). In fact, rates of adolescent heroin use have held steady for decades, while rates of synthetic opioid abuse by adolescents are on the rise. Also, early onset of drug abuse has been hypothesized to increase the risk of later drug addiction (Anthony and Petronis 1995; Clark et al. 1998; Kandel et al. 1992; Palmer et al. 2009). Whether or not these trends could be attributed to biological vulnerability associated with adolescence is unclear (Shram et al. 2008a), and calls for research using animal models of adolescent drug exposure. Although adolescent sensitivity to psychostimulants and alcohol receives attention (e.g. Frantz et al. 2007; Schramm-Sapyta et al. 2011; Spear and Varlinskaya 2010), experimentation on adolescent sensitivity to opiates lags behind.
Aversive withdrawal may contribute to drug-seeking and addiction through the process of negative reinforcement (Kenny et al. 2006; Koob et al. 1992; Solomon and Corbit 1974). In opiate addicts, the withdrawal syndrome is well characterized and follows a precise time course, consisting of dysphoria, anxiety, irritability, and intense somatic withdrawal signs which may drive continued drug use and/or help to trigger reinstatement of drug seeking after abstinence (reviewed in Frenois et al. 2005; O'Connor and Fiellin 2000). Mounting evidence suggests that adolescent subjects exhibit less aversive withdrawal than adults, after cessation of exposure to several drugs of abuse. For example, adolescent mice exhibit less affective withdrawal from morphine compared to adult mice, as measured in forced swim and locomotor tests (Hodgson et al. 2009). Adolescent male rats also exhibit fewer physical and affective signs of nicotine withdrawal (Infurna and Spear 1979; Natividad et al. 2010; O'Dell et al. 2006; O'Dell et al. 2007; Shram et al. 2008b; Wilmouth and Spear 2004) and fewer “hangover-like” effects of ethanol (Doremus et al. 2003; Varlinskaya and Spear 2004). A drop in extracellular levels of dopamine in the nucleus accumbens during precipitated withdrawal from nicotine is also attenuated in adolescents (Natividad et al. 2010). The severity of withdrawal from heroin has not been compared across age groups, so the first aim of the present study was to test for age differences in a classic set of somatic signs of withdrawal after repeated heroin injections in adolescent vs. adult male rats. We used an experimenter-administered, escalating heroin dose regimen intended to produce dependence and to reveal somatic signs of spontaneous withdrawal upon cessation of injections (Antonilli et al. 2005; Ventayol et al. 1997; Yang et al. 2006; Zhou et al. 2008). Classic signs of opiate withdrawal (Bläsig et al. 1973; Gellert and Holtzman 1978), along with locomotor activity, body mass, and food intake were all measured as indicators of heroin withdrawal. Our hypothesis was that adolescent male rats experience less withdrawal from heroin than adults, and we predicted that escalating doses of heroin would produce fewer somatic, motor, and physiological signs of spontaneous withdrawal in the younger rats. Beyond our main goal of testing for age differences in somatic signs of opiate withdrawal, we added a test of heroin-induced locomotor sensitization in the same rats. Although no common neurobiological mechanism has been identified, locomotor sensitization can correlate with some aspects of drug reward and reinforcement (Robinson and Berridge 1993; Vanderschuren and Pierce 2010). In adult rats, locomotor sensitization is observed after repeated injections of morphine (reviewed in Vanderschuren and Kalivas 2000) or heroin (Paolone et al. 2007; Pontieri et al. 1997; Ranaldi et al. 2009). Compared to adults, adolescent male rats exhibit heightened morphine-stimulated motor activity and greater locomotor sensitization after repeated morphine injections (Spear et al. 1982; White and Holtzman 2005; White et al. 2008). Heroin-induced sensitization has not been reported for adolescent rats. Thus, we inserted locomotor activity tests after four challenge doses of heroin (1 mg/kg) into the present escalating dose regimen used for observations of withdrawal. Given the differential age effects for morphine vs. heroin we reported previously (Doherty and Frantz 2012; Doherty et al. 2009), we did not assume that adolescents would exhibit more heroin-stimulated activity than adults, as occurred with morphine. Yet based on heightened acute and sensitized locomotor activation by morphine in adolescent vs. adult male rats (Spear et al. 1982; White and Holtzman 2005; White et al. 2008), we did hypothesize that adolescent male rats would exhibit heightened heroin-induced locomotor sensitization compared to adults. Our overarching goal for this data set was to explore the effect of age-at-onset of drug exposure on a physiological/behavioral state associated with negative reinforcement (withdrawal) as well as a measure sometimes associated with positive reinforcement (sensitization), in the same subjects. We interpret the present results in the context of the age differences in cue-induced reinstatement of heroin-, morphine-, or cocaine-seeking that we reported previously (Doherty and Frantz 2012; Doherty et al. 2009; Li and Frantz 2009), i.e. adolescent-onset of i.v. drug self-administration is associated with lower rates of cue-induced reinstatement after 1–90 days of abstinence, compared with adult-onset of self-administration.
Methods
Subjects
Forty male Sprague-Dawley rats (Charles River, Raleigh NC) arrived in the laboratory at postnatal day (P) 29 or P72 for adolescent or adult age groups, respectively. Rats were housed in a temperature and humidity controlled vivarium (targeted at 20–22°C and 50% humidity) in groups of two (one cage housed three adults from day 10 of the escalating dose regimen and onward, due to loss of a cagemate during heroin treatment; see below). Rats were maintained on a reversed 12-hr light/dark cycle, with lights off at 0700 hr. All behavioral testing occurred at approximately the same time every day. Food and water were available ad libitum in home cages. Principles of laboratory animal care were followed; all procedures complied with the NIH Guide for Care and Use of Laboratory Animals (7th Ed., 1998) and were approved by the Institutional Animal Care and Use Committee of Georgia State University.
Drugs
Heroin HCl (gift from NIDA) was dissolved in sterile saline and filtered through a 25 µm syringe filter (Fisher Scientific, Pittsburgh, PA) before intraperitoneal (i.p.) administration.
Drug administration
As depicted in the experimental timeline (Fig. 1), rats received daily injections of saline or heroin, were observed for somatic and locomotor signs of spontaneous withdrawal, and tested for heroin-induced locomotor activity in the following treatment groups: adolescent-saline (n=10), adolescent-heroin (n=10), adult-saline (n=10), adult-heroin (n=9; 1 rat died from an apparent overdose of heroin on the 10th day of the 13-day escalating dose regimen). Testing began at P35 for the adolescent groups and P79 for the adult groups. Treatment groups (saline or heroin) were counterbalanced by levels of baseline locomotor activity (see below). All injections (i.p.; 1 ml/kg), observations of withdrawal, and locomotor activity tests occurred in the same testing room under red light illumination. Absolute body mass and food intake were recorded daily throughout the experiment and used to assess the effects of daily heroin administration as well as withdrawal from heroin. To measure food intake, chow in each hopper and any observable spillage in the cage were weighed and then subtracted from the amount placed in the hopper the previous day. To allow comparison of the present results with previous data from our laboratory, rats were pair housed (although one adult cage had three subjects, see above) and food consumption per rat was calculated as the total amount of food consumed per day per cage divided by the number of rats per cage, with all rats in a cage receiving the same daily drug treatment (saline or heroin).
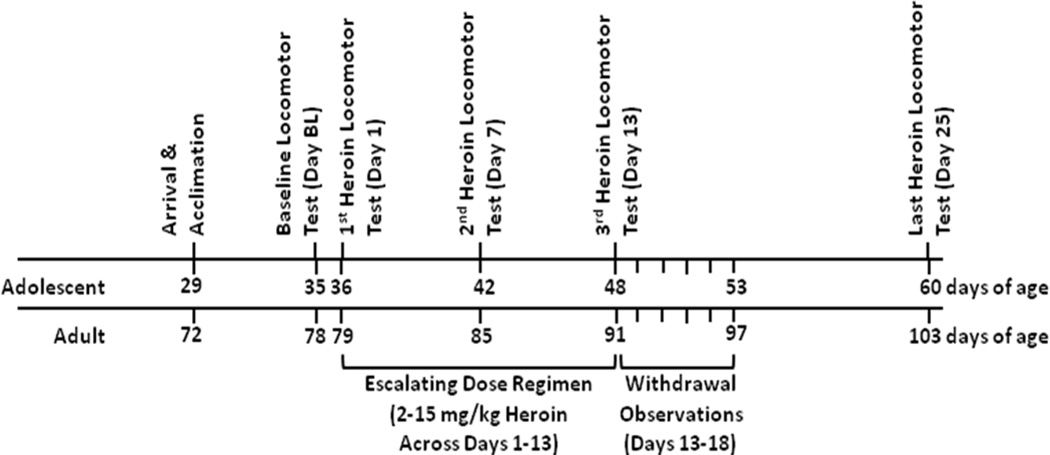
Fig. 1.
Timeline of experimentation.
For the escalating dose regimen (days 1–13), rats received injections of either saline or heroin every 12 hr for 13 days (progression of total daily heroin doses: 2, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14, 15 mg/kg, and then 8.0 mg/kg more on the morning of the 13th day). Injections were given at approximately 09:00 and 21:00 hr, and rats were placed individually in holding cages for 10 min after each injection. This dosing schedule and route of administration (i.p.) was expected to produce dependence on heroin as measured by observable signs of withdrawal on abrupt cessation of drug administration (Antonilli et al. 2005; Ventayol et al. 1997; Yang et al. 2006; Zhou et al. 2008).
Observations of withdrawal
Two classes of withdrawal signs were measured: counted somatic signs and observed body mass loss. Following a method modified from Glover and Davis (2008), Maldonado et al. (1992), and Gellert and Holtzman (1978), experimenters blind to treatment groups were first trained by an experienced observer (E. Glover, cited above) and then monitored rats for the following behaviors: chewing, teeth chatter, eye twitching, head shakes, genital grooming, abdominal spasms, forepaw shaking, audible vocalizations, abnormal posture, jumping, wet dog shakes, and piloerection. For each rat, behaviors were counted in 5 min blocks for 45 min, starting 12-hr and continuing at 24-hr intervals over 5 days after the last heroin injection. Each withdrawal sign was quantified by assigning a score of “1” if it occurred at all during a 5 min block, resulting in a maximum score of “9” for each sign over the 45 min observation period. As an additional measure of withdrawal severity, body mass lost since the last observation period was included in the withdrawal score (expressed as percent change in a 12–24 hr period; Gellert and Holtzman 1978; Azar et al. 2004). Locomotor activity during each withdrawal observation was also measured. (See below for the locomotor testing procedure.)
Locomotor test
After six days of acclimation to our vivarium and daily handling, rats underwent a baseline locomotor test after saline injection (day BL). (Saline vs. heroin treatment groups were counterbalanced by behavior in this test.) At four subsequent time points, locomotor activity induced by a challenge dose of heroin (1 mg/kg i.p., or saline control) was measured: the first heroin injection (day 1), at days 7 and 13 in the escalating dose regimen, and after 12 days of abstinence (day 25). Rats were acclimated to the testing room for 20 min prior to injections. Locomotor activity was tested for 45-min in clean Plexiglas chambers (53 L×29 W×20 H cm; with a small amount of corncob bedding spread on the floor). Activity was videotaped by ceiling-mounted cameras and later quantified as crossings over three evenly spaced matrix zones marked with tape on the wire top of the Plexiglas chamber. Immediately after each 45-min locomotor test, rats were injected with the appropriate dose of heroin or saline to continue the escalating dose regimen (see above).
Data analysis
Withdrawal scores were analyzed using Kruskal-Wallis and Mann-Whitney U non-parametric analyses. We did not observe eye twitching or forepaw shaking in any rat regardless of age or treatment group, so data for these signs are not reported. Locomotor activity during withdrawal, absolute body mass, food intake as percent pre-heroin baseline, and sensitized locomotor activity expressed as percent day 1 were each analyzed using three-way mixed-measures analyses of variance (ANOVAs), with age and treatment as between-subjects factors and time point as a within-subjects repeated measure. Daily food intake was measured for each cage of two subjects (one adult cage had three subjects; see above), and divided by the number of subjects. Total food intake (in grams) from experimental days 1–13 (during heroin treatment) was summed and analyzed using a two-way ANOVA with age and treatment as the between-subjects factors, as was food intake during withdrawal days 1–5. Targeted pairwise comparisons between age and treatment groups were made for each time point in withdrawal, given our main focus on this experimental phase. Follow-up ANOVAs, pairwise multiple comparison Tukey’s tests, and paired or unpaired two-sided t-tests with Bonferroni’s correction were used for post-hoc comparisons, as appropriate. Results were significant if p<0.05.
Results
Somatic signs of spontaneous withdrawal
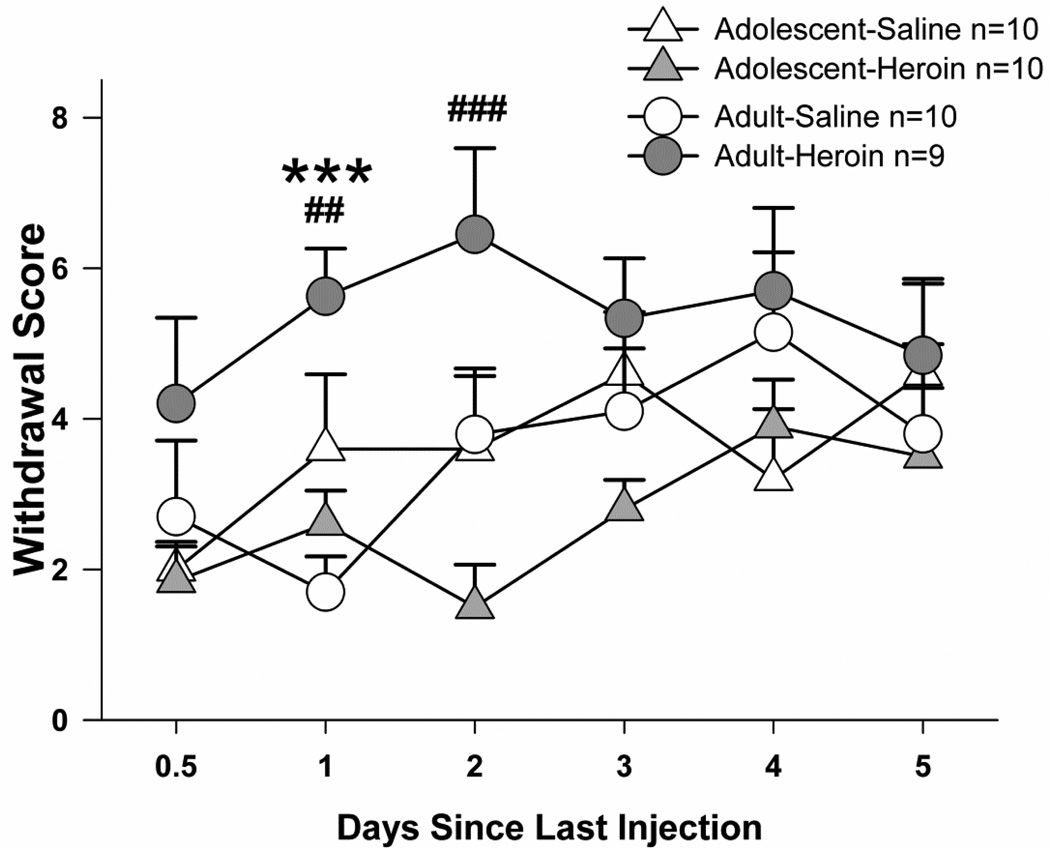
Adolescents exhibited fewer somatic signs of spontaneous withdrawal than adults after an escalating dose regimen of heroin injections (Fig. 2 and Table 1). When withdrawal scores were compared at each time point (Fig. 2), significant treatment effects were observed at days 1 and 2 since last injection (Kruskal-Wallis day 1: H(3)=15.1, p<0.005; day 2: H(3)=10.5, p<0.05). Follow-up analyses confirmed that heroin induced somatic signs of withdrawal in adults; the adult-heroin group displayed more somatic signs of withdrawal than their saline controls at one day since the last injection (U=12.2 p<0.0005), but no significant differences were observed between adolescent-heroin vs. -saline groups on individual test days. Moreover, the adolescent-heroin group displayed fewer somatic signs of spontaneous withdrawal than the adult-heroin group at both one and two days since the last injection (day 1: U=8.9, p<0.005; day 2: U=10.8, p<0.01).
Fig. 2.
Somatic signs of spontaneous withdrawal from repeated systemic heroin from 0.5–5 days since the last injection. Treatment effects within the adult age group are shown (***p<0.001), as are age effects within the heroin treatment groups (## p<0.01, ### p<0.001). All points represent mean +/− SEM.
Table 1.
Somatic signs of withdrawal summed across all time points (±SEM).
| Treatmen | Chewing | Teeth chatter | Head shakes | Genital grooming | Abdominal spasms | Vocalizations | Abnormal posture | Jumping | Wet dog shakes | Piloerection | Body mass lost % | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adolescent | Saline | 0.1±0.1 | 0.8±0.5 | 0.3±0.2 | 4.8±1.2 | 0.1±0.1 | 0.6±0.3 | 0.0±0.0 | 7.7±2.4 | 7.2±1.7 | 0.0±0.0 | 0.0±0.0 | 21.6±4.0 |
| Heroin | 0.1±0.1 | 1.9±0.9 | 0.6±0.3 | 3.3±0.9 | 0.3±0.2 | 0.5±0.3 | 0.0±0.0 | 3.5±1.3 | 5.7±1.0 | 0.0±0.0 | −3*±0.1 | 16.2±2.5 | |
| Adult | Saline | 0.3±0.2 | 3.0±1.3 | 0.5±0.3 | 2.7±0.4 | 0.4±0.3 | 0.6±0.2 | 0.0±0.0 | 5.2±1.5 | 8.3±2.4 | 0.0±0.0 | −0.2±0.2 | 21.2±4.1 |
| Heroin | 0.6±0.2 | 3.8±1.8 | 1.1±0.5 | 5.6±1.2 | 0.3±0.2 | 1.1±0.3 | 0.9±0.6 | 3.0±1.4 | 12.1#±1.5 | 1.5*#±0.6 | −2.3 *#±0.4 | 32.2#±3.0 |
Effect of treatment, within age groups
Effect of age, between heroin-treated groups.
With all withdrawal signs summed over time (data not shown), targeted pairwise comparisons revealed that the effect of heroin to elevate somatic signs of withdrawal among adults just missed significance (U=3.2, p=0.072), and no differences between adolescent treatment groups were observed. The adolescent-heroin group did display significantly fewer signs of withdrawal than the adult-heroin group (U=9.6, p<0.005). When individual withdrawal signs were summed separately over time (Table 1), significant treatment effects occurred within the measures of piloerection (H(3)=18.5, p<0.0005) and % body mass lost (H(3)=25.2, p<0.00005), and just missed significance for wet dog shakes (H(3)=7.6, p=0.055). Individual comparisons confirmed that the adolescent-heroin group displayed fewer somatic signs of spontaneous withdrawal than the adult-heroin group with regard to piloerection (U=6.9, p<0.01), % body mass lost (U=12.5, p<0.0005), and wet dog shakes (U=8.3, p<0.005). Moreover among adolescents, the heroin group lost more body mass than their saline controls (U=6.2, p<0.05). Also among adults, the heroin group displayed more piloerection (U=6.9, p<0.01) and lost more body mass than their saline controls (U=11.8, p<0.001).
Locomotion during spontaneous withdrawal
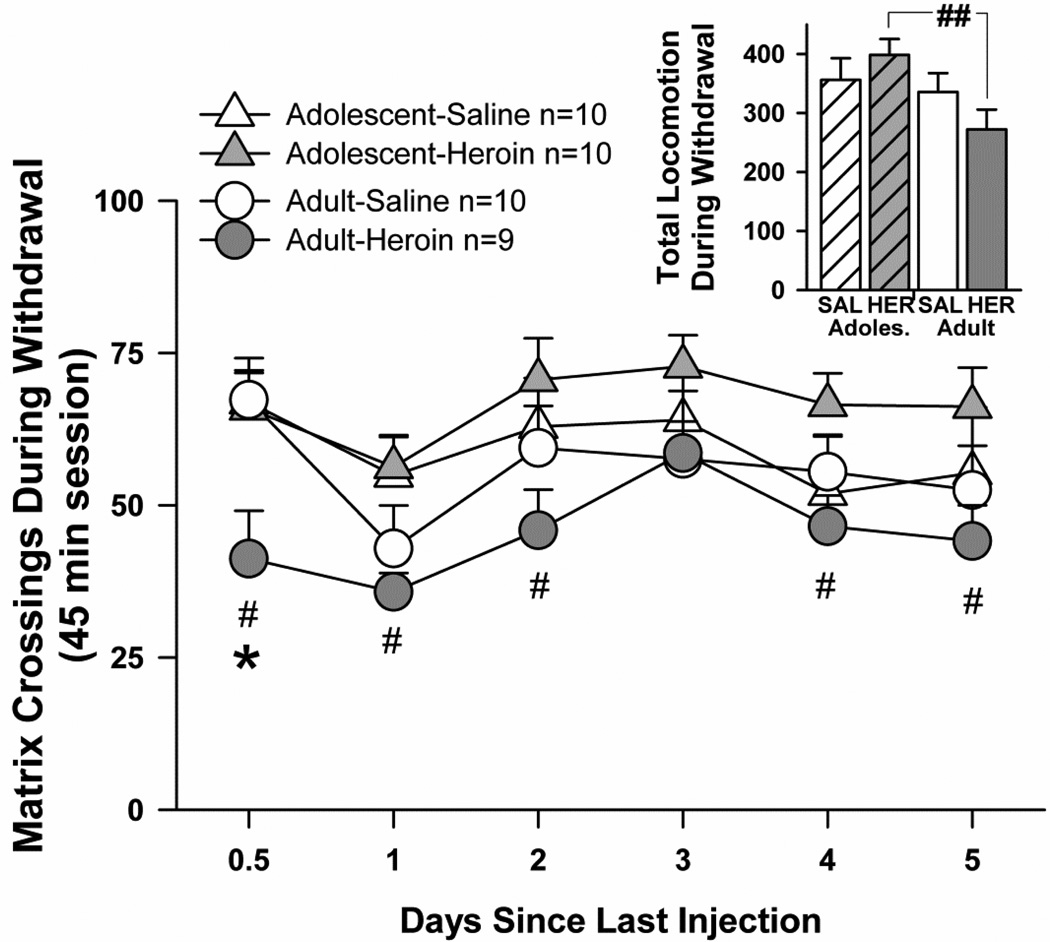
Locomotion during withdrawal testing was not affected in the adolescent-heroin group compared to their saline counterparts, but locomotion in the adult-heroin group was significantly lower compared to their saline counterparts shortly after the last heroin injection (Fig. 3). Generally, adolescents were more active than adults during withdrawal tests, regardless of treatment, as suggested by a main effect of age (F(1,35)=5.08, p<0.05). No main effect of heroin treatment on locomotion during withdrawal occurred, nor were there any interactions, although matrix crossings differed according to a main effect of time point (F(5,175)=5.72, p<0.001). Pre-planned t-tests at each time point confirmed the lack of effect of heroin withdrawal on locomotion among adolescents, i.e. matrix crossings during withdrawal tests did not differ in adolescent-heroin vs. adolescent-saline groups at any time point tested. Among adults, locomotion was decreased by heroin treatment early in heroin withdrawal, i.e. the adult-heroin group was less active than their saline controls at the 12-hr time point (t=2.5, df=17, p<0.025). Across age groups, the adult-heroin group was also less active than the adolescentheroin group at 12-hr (t=2.5, df=17, p<0.025), 1 day (t=3.3, df=17, p<0.01), 2 days (t=2.6, df=17, p<0.025), 4 days (t=2.8, df=17, p<0.025), and 5 days since the last heroin injection (t=2.5, df=17, p<0.025). Finally, when matrix crossings during withdrawal tests were summed across all time points, the adult-heroin group displayed significantly less activity than the adolescent-heroin group (t=3.5, df=17, p<0.01). (Fig. 3 inset).
Fig. 3.
Locomotor signs of spontaneous withdrawal from repeated systemic heroin. For adults, a treatment effect (saline vs. heroin groups) is shown (* p<0.05). For heroin groups, age differences were observed at 0.5–2 days, and days 4 and 5 since the last heroin injection (# p<0.05). Inset. Total locomotion summed over all time points. For heroin groups, an age difference was observed (## p<0.01). SAL=saline treatment, HER=heroin treatment. All points and bars represent mean +/− SEM.
Body mass
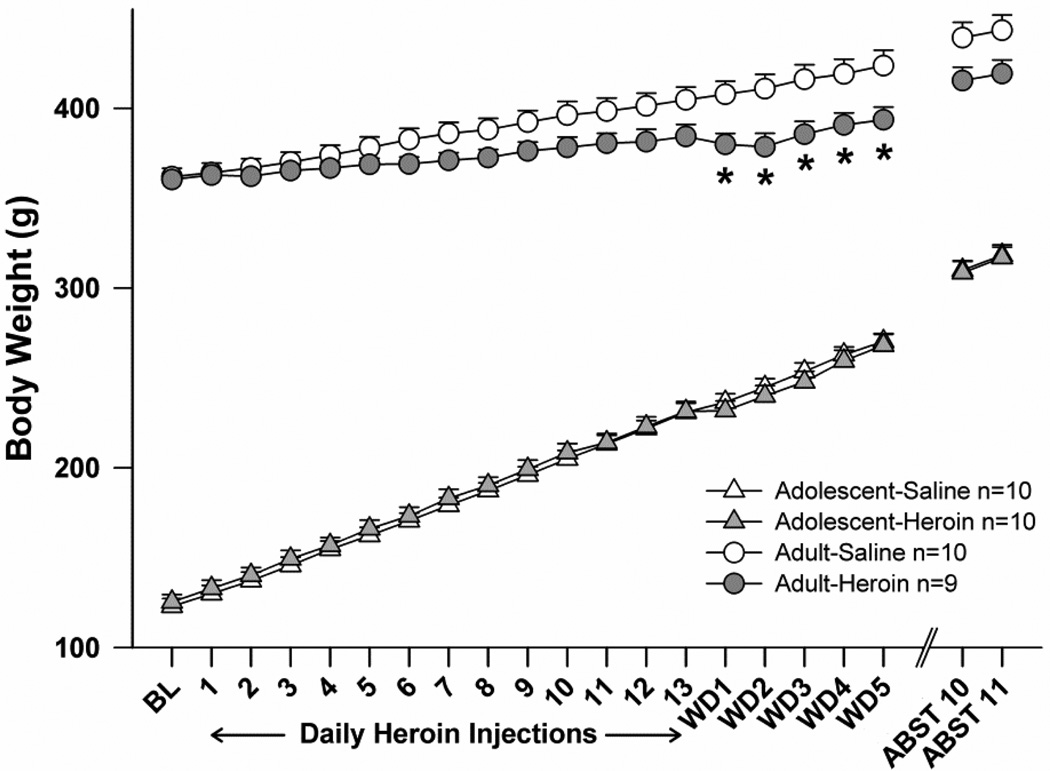
Heroin treatment did not appear to alter body mass among adolescent subjects, although it reduced body mass among adults (Fig. 4). With regard to acute effects of heroin during days 1–13, initial analysis revealed significant main effects of age (F(1,35)=1552.5, p<0.001), time point (F(12,216)=1552.5, p<0.001), as well as significant interactions between time point and age (F(12,420)=220.7, p<0.001), time point and treatment (F(12,420)=5.6, p<0.001), and an overall three-way interaction (F(12,420)=3.9, p<0.001). Follow-up two- and one-way ANOVAs revealed that body mass in adolescents increased steadily but was not influenced by heroin treatment, according to a main effect of time point (F(12,324)=1825.2, p<0.001). Among adults, a significant treatment×time point interaction (F(12,204)=6.0, p<0.001) was not robust enough to reveal significant results of pairwise comparisons between heroin and saline treatment groups on each day, given Bonferroni’s corrections. With regard to body mass during withdrawal, we performed targeted and uncorrected t-tests for treatment effects on body mass at each withdrawal day, given that this experimental phase was the focus of our study. The adult-heroin group had significantly lower body mass than the adult-saline group on each day (p<0.025). By 11 days abstinence from heroin (ABST 11), body mass differences between adult treatment groups were no longer significant.
Fig. 4.
Effects of repeated systemic injections of heroin and withdrawal on body mass (g). The adult-heroin group gained less than their saline counterparts during withdrawal (* p<0.025). BL=baseline, WD=withdrawal, ABST=abstinence. All points represent mean +/− SEM.
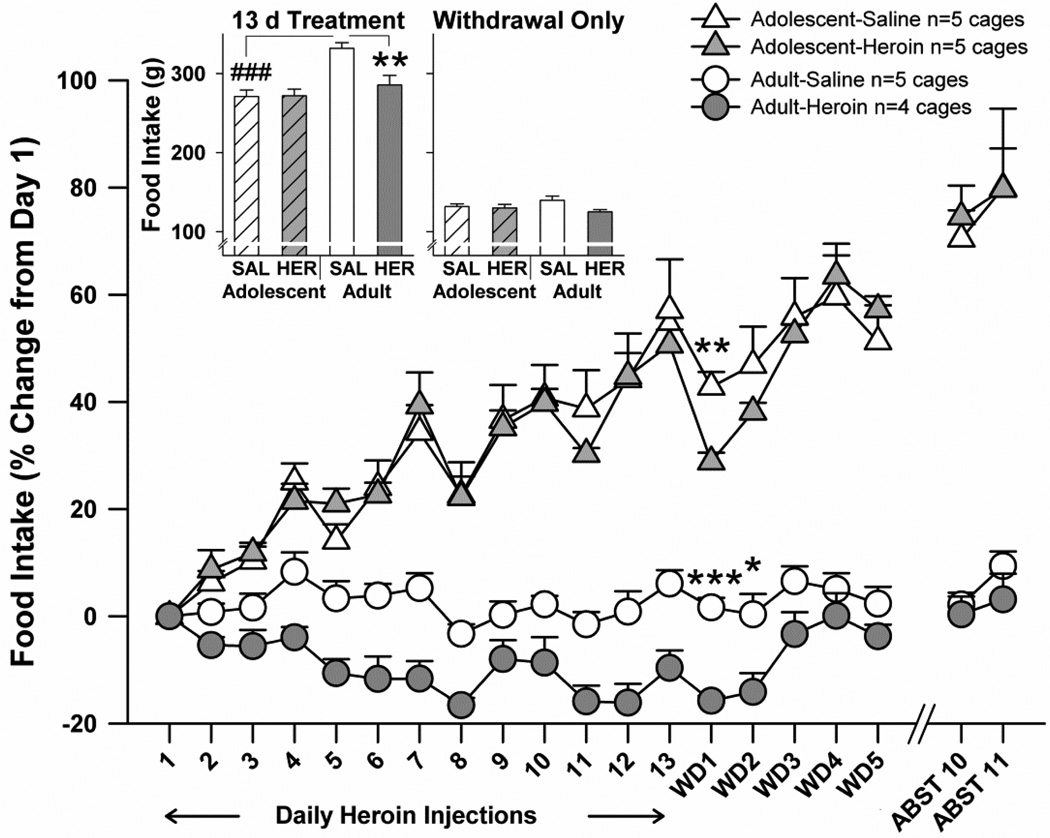
Food intake
Effects of heroin to decrease food intake were attenuated in adolescents, compared to adults (Fig. 5). Rats were group housed by age, and food intake is reported as an average per cage with all rats in a cage receiving the same daily treatment with saline or heroin (baseline intake: adolescents 16.9 ± 0.5 g; adults 26.6 ± 0.7 g). Analyzing only the acute effects of heroin (days 2–13), an initial three way age×treatment×time point ANOVA on food intake per cage as a percent of pre-heroin baseline (day 1) revealed significant main effects of age (F(1,15)=135.0, p<0.001), treatment (F(1,15)=5.2, p<0.05), and time point (F(11,165)=21.0, p<0.001), as well as a significant interaction of time point with age (F(11,165)=25.6, p<0.001). When summed over days 1–13 (Fig 5 inset), food intake (in grams) differed according to main effects of age (F(1,15)=17.9, p=0.001) and treatment (F(1,15)=6.7, p<0.05), as well as an age×treatment interaction (F(1,15)=7.2, p<0.05). Follow-up testing revealed that the adult-heroin group consumed less than their saline controls, whereas no such treatment effect was observed among adolescents. Rats in the younger saline-control group also consumed less than their adult saline-treated counterparts. The focus of our study was disruptions in food intake during testing for withdrawal, and we thus performed targeted unpaired t-tests for treatment effects on food intake at each withdrawal day. Withdrawal-related decreases in food intake extended through withdrawal day 2 for adults, whereas only on withdrawal day 1 did the adolescent-heroin group consume significantly less food than the adolescent-saline group. Of important note, we did not record any cases in which body mass changes suggested dominance of one cagemate over another in terms of food consumption.
Fig. 5.
The effects of repeated systemic heroin on food intake, expressed as percent pre-heroin baseline. During withdrawal, differences in saline vs. heroin treatment groups are shown (*p,0.05, ** p<0.01, *** p<0.001). Inset. Total food intake (g) summed over heroin treatment (days 1–13; left) or over the withdrawal period (days WD1–5; right). During heroin treatment, the significant difference between adult treatment groups is shown (** p<0.01), along with a significant difference across saline-treated age groups (### p<0.001). WD=withdrawal, ABST=abstinence, SAL=saline treatment, HER=heroin treatment. All points and bars represent mean +/− SEM.
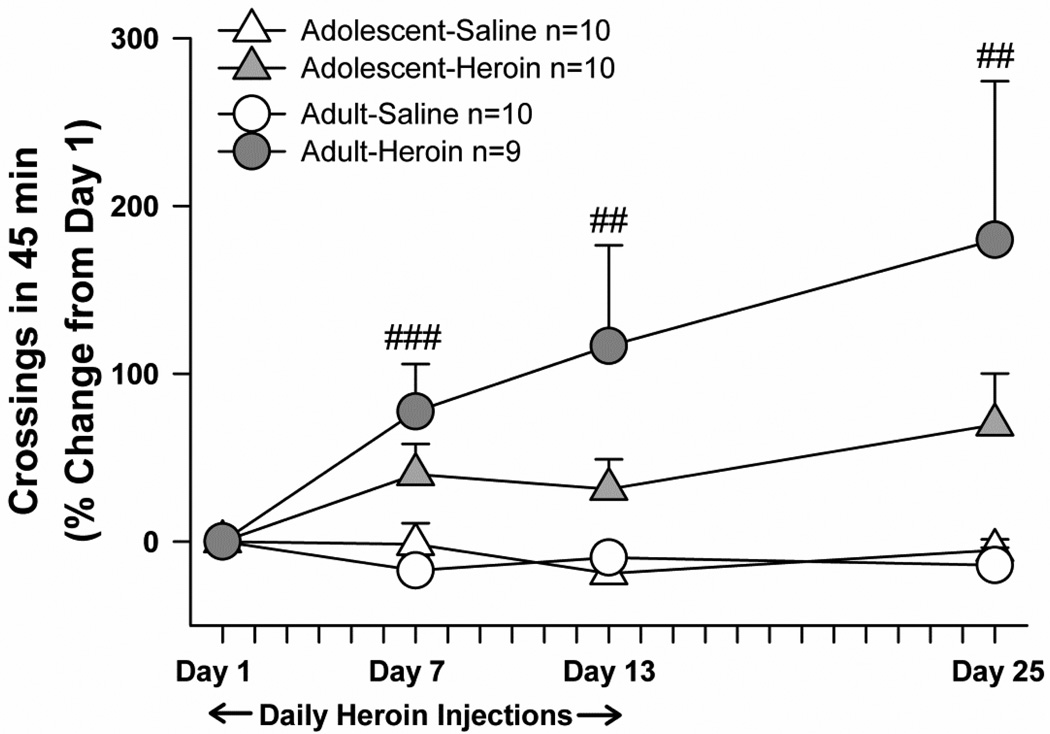
Heroin-induced locomotor sensitization
Adolescents and adults exhibited similar levels of heroin-induced locomotion and locomotor sensitization (Fig. 6), with the higher variability in the adult-heroin group explained by one subject raising the adult-heroin group mean at days 13 and 25, and a second subject contributing to the high mean at day 25. At baseline testing before any drug treatment began, matrix crossings did not differ by age (adolescent mean+/−SEM: 92.75+/− 5.26; adult mean+/−SEM: 97.31+/−8.47), nor did they differ by age or treatment group on the first day of heroin treatment (day 1). Analyzed as a percent change from day 1, matrix crossings did not differ by a main effect of age, nor were there any interactions with age. Heroin did produce locomotor sensitization, according to main effects of treatment (F(1,35)=12.72, p<0.001) and test day (F(3,105)=3.93, p<0.05) and a treatment×test day interaction (F(3,105)=5.49, p<0.025). With data collapsed over age groups, follow-up t-tests with Bonferroni’s corrections revealed that heroin-treatment groups exhibited more matrix crossings at days 7 (t=−3.76, df=37, p=0.001), day 13 (t=−2.83, df=37, p=0.007), and day 25 (t=−2.79, df=37, p<0.008), compared to day 1.
Fig. 6.
Heroin-induced locomotor sensitization, expressed as percent change from day 1. With data collapsed over age groups, significant differences in locomotor activity after heroin injection on days 7, 12, and 25, compared with day 1, are shown (## p<0.01, ###p<0.001). All points and bars represent mean +/− SEM.
Discussion
Adolescent male rats treated with an escalating dose regimen of heroin did not exhibit the somatic signs of spontaneous drug withdrawal observed in heroin-treated adults. They also failed to show heroin-related disruptions in spontaneous locomotor activity, body mass, and food intake during withdrawal. On the other hand, adolescents and adults exhibited similar levels of locomotor sensitization after repeated injections of a challenge dose of heroin during and after the escalating dose regimen. Together these findings reveal that some, but not all, behaviors related to repeated exposure to drugs of abuse are attenuated in younger subjects.
The absence of measurable withdrawal from heroin in adolescent male rats adds to a growing body of literature on attenuated signs of withdrawal from various drugs in adolescent rodents. Our data set is consistent with Hodgson et al. (2009) in which adolescent mice displayed less affective withdrawal from morphine than adults, as measured during forced swim and locomotor tests. It is also consistent with less intense somatic withdrawal among adolescent rats after nicotine (O'Dell et al. 2007) or alcohol (Doremus et al. 2003; Varlinskaya and Spear 2004). Perhaps the most convincing evidence of attenuated withdrawal among adolescents is the fact that brain reward thresholds are not elevated in adolescent rats after nicotine exposure as they are among adults (O'Dell et al. 2006). In terms of neurochemistry, a drop in extracellular levels of dopamine in the NAcc during precipitated nicotine withdrawal is attenuated in adolescents vs. adults (Natividad et al. 2010). Less somatic and affective withdrawal among adolescents may suggest that negative reinforcement (alleviation of negative states of withdrawal by return to drug intake) contributes less to drug dependence among younger subjects compared with older subjects.
We did not observe a particularly robust withdrawal syndrome in either age group in the present experiment, an effect with at least three possible explanations. We intentionally used a heroin dose regimen on the lower end of the total heroin dosing amount given in previous studies (Antonilli et al. 2005; Ventayol et al. 1997; Yang et al. 2006; Zhou et al. 2008); our aim was to come close to aligning heroin exposure levels with our previous study on heroin self-administration in adolescent vs. adult rats (Doherty and Frantz 2012). As a result, the present doses may have been too low to induce a strong spontaneous withdrawal syndrome. We also chose to quantify spontaneous, rather than precipitated, withdrawal signs. This approach was taken in order to avoid problems in choosing appropriate antagonist doses across age groups. Yet spontaneous withdrawal is difficult to measure in rodents because of individual variability in the time course of withdrawal response, perhaps related to rates of clearance of the opiate (Garrido et al. 1999). Finally, the dosing regimen we used, with injections every 12 hr rather than constant infusion, could be considered an ‘intermittent’ schedule that is more likely to produce sensitization than tolerance, dependence, and its associated withdrawal syndrome (Stewart and Badiani 1993). Nonetheless, our data do align with other reports on the amount and time course of spontaneous somatic signs of opiate withdrawal among adult rats (Cicero et al. 2002; Papaleo and Contarino 2006). With regard to the occurrence of some withdrawal signs in saline-treated controls, it is not uncommon to observe so-called indices of withdrawal in drug-naïve rats, given that all of these behaviors are part of the general repertoire in rodents. For example, our scoring procedure was based on Glover and Davis (2008), and we saw similar levels of ‘withdrawal signs’ in controls. Even when using a more robust scoring method counting behavioral frequencies rather than interval occurrence, saline-treated control rats of both adolescent and adult age groups exhibited an average of one ‘withdrawal sign’ per minute (O'Dell et al. 2006).
To complement our observations of classic signs of opiate withdrawal, we also measured locomotor activity during withdrawal, under the assumption that less locomotion reflects more malaise and discomfort. Indeed, the same age differences were observed in the locomotor assay as in the withdrawal syndrome; younger rats failed to show reductions in locomotion during heroin withdrawal, whereas heroin-treated adults were less active than their age-matched saline controls shortly after heroin treatment ended. Similarly, adolescents failed to show any significant loss of body mass during withdrawal, and their reduction in food intake endured through only the first withdrawal day; this contrasts adult effects of reduced body mass over five days and reduced food intake over two days in withdrawal. It is possible that the demand for growth during adolescence opposes the ability of heroin to produce malaise, anorexia or alterations in energy balance. The role of these physiological effects in age-dependent heroin-related behavior remains to be explored.
Adolescents and adults exhibited statistically similar patterns of heroin-induced locomotor sensitization. At face value, these results contrast reports on greater sensitization to repeated morphine injections among younger subjects (White and Holtzman 2005; White et al. 2008), and point out that heroin and morphine effects often differ for as yet unknown reasons, as we have noted previously (Doherty et al. 2009, Doherty and Frantz 2012). These results also conflict with our previous report on attenuated behavioral sensitization after repeated cocaine injections among adolescent male rats, compared with adults (Frantz et al. 2007). As with the present withdrawal signs, the locomotor sensitization we observed was not robust. Again this could be explained by the dosing regimen. Locomotor sensitization is most robust after a series of drug injections given intermittently with several days between injections, rather than with only 12 hr between injections. Coupled with our note above regarding lack of robust spontaneous withdrawal, we could conclude that the present dosing regimen created a mixed condition characterized by low rates of both tolerance and sensitization. We are also compelled to note that locomotor sensitization among rats in the present adult-heroin group was more variable than in the adolescent-heroin group, with one or two rats driving up the averages. Future tests with different dosing regimens may help to clarify the parameters that determine age-dependence in the effects of heroin and other drugs.
Whether or not locomotor sensitization is related to vulnerability to drug-taking or drugseeking has been debated extensively (e.g. Vanderschuren and Pierce 2010). Some evidence suggesting that sensitization correlates with drug intake focuses on the reinforcing efficacy of drugs in the self-administration model, such that an intermittent drug pretreatment schedule that produces motor sensitization also increases break points on a progressive ratio schedule of reinforcement, as does prior drug self-administration (e.g. Liu et al. 2007; Lorrain et al. 2000; Vezina et al. 1999, Ward et al. 2006). In this context, the present lack of age differences in locomotor sensitization aligns with our prior report that break points in responding maintained by heroin did not differ by age (Doherty and Frantz 2012). With regard to reinstatement, however, the present lack of age differences in sensitization does not suggest correlation between the ontological development of sensitization and reinstatement. This data set instead joins numerous reports on the discrepancy between procedures that produce sensitization vs. those that trigger reinstatement (e.g. Lenoir and Ahmed 2007).
A major point of consideration for all studies in behavioral pharmacology is pharmacokinetics. Notably, brain levels of morphine do not differ between adolescent, late adolescent, and adult mice after i.p. injection of a range of doses, and plasma levels of the drug are marginally higher in the youngest group (Koek et al. 2012). Moreover, relevant liver enzyme function does not differ in adolescent vs. adult rats (MacLeod et al. 1972). These data undermine the argument that pharmacokinetics explain the age differences in heroin effects reported herein. Yet age differences in production and function of opioid metabolites remain to be considered. In adult rats, heroin is metabolized first to 6-mono-acetyl-morphine, then to morphine, and then to morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G), with the balance in this last step shifting toward M6G after repeated heroin injections (Antonilli et al. 2003). Few reports exist on the metabolic breakdown of heroin or even morphine in developing rats, but one study, implies that it is not until some point between P21 and P90 that repeated morphine injections shift the final breakdown product from exclusively M3G to the combination of M3G and M6G (Wang et al. 2005). Given that M6G is an opioid receptor agonist (whereas M3G is not), the developmental rise in M6G could be relevant for tolerance, dependence, somatic signs of withdrawal, and/or locomotor sensitization. Furthermore, in terms of pharmacodynamics, variants of the mu-opioid receptor exist in adult rats and influence the behavioral effects of opiates (Pan et al. 2009), but expression levels and patterns of these variants across ontogeny are unknown. We cannot rule out some influence of these types of factors on the present data set.
On the whole, the attenuated heroin withdrawal among adolescent rats we report at present suggests that heroin produces less dependence in younger rats. Thus, lower rates of reinstatement of heroin-, morphine-, or cocaine-seeking after forced abstinence (Doherty et al. 2009, Doherty and Frantz 2012) could be explained by age differences in negative reinforcement, particularly related to the absence of a robust withdrawal syndrome. Although the validity of these animal paradigms as models of drug use, abuse, dependence, and relapse among humans has been questioned (Epstein et al. 2006), this line of research might suggest that human adolescent drug addicts seeking treatment will respond better than adults to strategies aimed at alleviating the motivational effects of withdrawal on relapse.
Acknowledgements
The authors would like to thank Chen Li, Bonnie Williams, Patrick Dunigan and Adria Lee for their excellent technical assistance. This research was supported in part by a National Institute on Drug Abuse B/START grant to KJF (1 RO3 DA020110-01), the Center for Behavioral Neuroscience NSF Science & Technology Center (IBN-9876754), and a seed grant from the Brains & Behavior program at Georgia State University.
References
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Antonilli L, Petecchia E, Caprioli D, Badiani A, Nencini P. Effect of repeated administrations of heroin, naltrexone, methadone, and alcohol on morphine glucuronidation in the rat. Psychopharmacology (Berl) 2005;182:58–64. doi: 10.1007/s00213-005-0030-7. [DOI] [PubMed] [Google Scholar]
- Antonilli L, Suriano C, Paolone G, Badiani A, Nencini P. Repeated Exposures to Heroin and/or Cadmium Alter the Rate of Formation of Morphine Glucuronides in the Rat. J Pharmacol Exp Ther. 2003;307:651–660. doi: 10.1124/jpet.103.055467. [DOI] [PubMed] [Google Scholar]
- Azar MR, Ahmed SH, Lintz R, Gutierrez T, Stinus L, Koob GF. A non-invasive gating device for continuous drug delivery that allows control over the timing and duration of spontaneous opiate withdrawal. J Neurosci Methods. 2004;135:129–135. doi: 10.1016/j.jneumeth.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bläsig J, Herz A, Reinhold K, Zieglgänsberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacology (Berl) 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav. 2002;72:691–697. doi: 10.1016/s0091-3057(02)00740-2. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Doherty J, Frantz K. Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology (Berl) 2012;219:763–773. doi: 10.1007/s00213-011-2398-x. [DOI] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92:164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Epstein D, Preston K, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Frenois F, Le Moine C, Cador M. The motivational component of withdrawal in opiate addiction: role of associative learning and aversive memory in opiate addiction from a behavioral, anatomical and functional perspective. Rev Neurosci. 2005;16:255–276. doi: 10.1515/revneuro.2005.16.3.255. [DOI] [PubMed] [Google Scholar]
- Garrido MJ, Valle M, Calvo R, Troconiz IF. Altered plasma and brain disposition and pharmacodynamics of methadone in abstinent rats. J Pharmacol Exp Ther. 1999;288:179–187. [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- Glover EM, Davis M. Anxiolytic-like effects of morphine and buprenorphine in the rat model of fear-potentiated startle: tolerance, cross-tolerance, and blockade by naloxone. Psychopharmacology (Berl) 2008;198:167–180. doi: 10.1007/s00213-008-1112-0. [DOI] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2009. NIH Publication No. 10-7583. 2010
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France C, Javors M. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology (Berl) 2012;219:1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 2009;204:725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Liu Y, Morgan D, Roberts DC. Cross-sensitization of the reinforcing effects of cocaine and amphetamine in rats. Psychopharmacology (Berl) 2007;195:369–375. doi: 10.1007/s00213-007-0909-6. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- MacLeod SM, Renton KW, Eade NR. Development of hepatic microsomal drug-oxidizing enzymes in immature male and female rats. J Pharmacol Exp Ther. 1972;183:489–498. [PubMed] [Google Scholar]
- Maldonado R, Negus S, Koob GF. Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. Neuropharmacology. 1992;31:1231–1241. doi: 10.1016/0028-3908(92)90051-p. [DOI] [PubMed] [Google Scholar]
- Natividad L, Tejeda H, Torres O, O'Dell L. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor PG, Fiellin DA. Pharmacologic treatment of heroin-dependent patients. Ann Intern Med. 2000;133:40–54. doi: 10.7326/0003-4819-133-1-200007040-00008. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11- associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci U S A. 2009;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Conversi D, Caprioli D, Del Bianco P, Nencini P, Cabib S, Badiani A. Modulatory effect of environmental context and drug history on heroin-induced psychomotor activity and fos protein expression in the rat brain. Neuropsychopharmacology. 2007;32:2611–2623. doi: 10.1038/sj.npp.1301388. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Contarino A. Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res. 2006;170:110–118. doi: 10.1016/j.bbr.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Calò L, Di Grezia R, Orzi F, Passarelli F. Functional correlates of heroin sensitization in the rat brain. Eur J Pharmacol. 1997;335:133–137. doi: 10.1016/s0014-2999(97)01195-3. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Egan J, Kest K, Fein M, Delamater A. Repeated heroin in rats produces locomotor sensitization and enhances appetitive Pavlovian and instrumental learning involving food reward. Pharmacol Biochem Behav. 2009;91:351–357. doi: 10.1016/j.pbb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Depart. of Health and Human Services OoAS, editor. SAMHSA. Substance Abuse and Mental Health Services Administration, results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2009. [Google Scholar]
- Schramm-Sapyta N, Cauley M, Stangl D, Glowacz S, Stepp K, Levin E, Kuhn C. Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology (Berl) 2011;215:493–504. doi: 10.1007/s00213-011-2216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008a;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu ECK, Li Z, Tyndale RF, Lê AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 2008b;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: 1. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: Implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization Processes in Drug Addiction. Curr Top Behav Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Ventayol P, Busquets X, Garcia-Sevilla JA. Modulation of immunoreactive protein kinase C-alpha and beta isoforms and G proteins by acute and chronic treatments with morphine and other opiate drugs in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:491–500. doi: 10.1007/pl00004974. [DOI] [PubMed] [Google Scholar]
- Vezina P, Pierre PJ, Lorrain DS. The effect of previous exposure to amphetamine on drug-induced locomotion and self-administration of a low dose of the drug. Psychopharmacology (Berl) 1999;147:125–134. doi: 10.1007/s002130051152. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mitchell J, Moriyama K, Kim KJ, Sharma M, Xie GX, Palmer PP. Age-dependent morphine tolerance development in the rat. Anesth Analg. 2005;100:1733–1739. doi: 10.1213/01.ANE.0000152192.23851.40. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Lack C, Morgan D, Roberts DC. Discrete-trials heroin self-administration produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl) 2006;185:150–159. doi: 10.1007/s00213-005-0288-9. [DOI] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- White D, Michaels C, Holtzman S. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89:188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Yang YD, Zhang JZ, Sun C, Yu HM, Li Q, Hong M. Heroin affects purine nucleotides catabolism in rats in vivo. Life Sci. 2006;78:1413–1418. doi: 10.1016/j.lfs.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of Arginine Vasopressin and V1b Receptor in Heroin Withdrawal and Heroin Seeking Precipitated by Stress and by Heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]